Abstract
Worldwide wastewater treatment plants generate enormous amounts of sewage sludge, and their further disposal depends on the treatment technologies applied and spontaneously occurring microbiological processes. From different ages urban sewage sludge, 12 strains of bacteria with simultaneous tolerance to two or more trace elements: Co, Ni, Cu, Zn, Cd and Pb at concentration of 3-5 mmol were isolated and identified by PCR of target genes and Sanger sequencing methods. The isloated metal(loids) tolerant strains belong to the species, i.e., Serratia fonticola, Rhodococcus qingshengii, Pseudomonas fragi, Pseudomonas extremaustralis, Pseudomonas cedrina, Stenotrophomonas maltophilia, Serratia liquefaciens and Citrobacter freundii. The ecological features of the isolated strains were studied. The optimal growth temperatures for most strains was 15–30°C at pH range of 5–9, although some strains grew at 7°C (Pseudomonas fragi SS0-4, Serratia fonticola SS0-9 and Serratia fonticola SS12-11). Satisfactory growth of two strains (Serratia fonticola SS0-1and Citrobacter freundii SS60-12) was noted in an acidic medium at pH 4. Most of the strains grew in the NaCl concentration range of 1–5%. The isolated bacteria resistant to high concentrations of trace elements can be used for the effective mineralization of sewage sludge and for the decontamination of wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the rapid growth of the world's population and its urbanization, humanity is facing a serious problem of disposal of liquid and solid domestic waste. Over the past decades, the population of the planet has grown faster than ever before. In 1950 there were 2.5·109 people in the world; and in 2022, the planet had 8·109 people. The latest forecasts by the United Nations suggest that population of the Earth could grow to 8.5·109 in 2030, 9.7·109 in 2050 and 10.4·109 in 2100 (United Nations 2022). Most of the population lives in town and cities than in countryside. Approximately 55% of the world’s population residing in urban areas by 2018, and it could increase 68% by 2050 (United Nations 2018).
Sewage sludge (SS) is a byproduct of wastewater treatment and is produced worldwide. In the scientific literature, municipal or urban SS is defined as final solid component from the municipal wastewater treatment process (Grobelak et al. 2019; Nuamah et al. 2012). SS is a complex mixture of organic and inorganic substances of biological and mineral origin. It is a product of physical (after primary treatment), biological (trickling filters, or rotating biological contractors, activated sludge), and physio-chemical (precipitation with lime, alum or ferric chloride) treatment of wastewater (Singh et al. 2022). The main stages of SS processing are thickening, digestion, dewatering and disposal. After wastewater decontamination, the sludge contains large volumes of water. Sludge thickening reduces the volume of water making it suitable for further operations. There are two main strategies for municipal SS management: reuse, including agriculture or landscaping purposes, or final disposal. There are many approaches to reuse SS but also many restrictions on its application (Kacprzak et al. 2017). Anaerobic sludge digestion is a microbiological process where the organic solids in the sludge are transformed to liquids and gases. Final dewatering is done before disposal of sludge that is used as organic fertilizers, sanitary landfill or is incinerated (Roychoudhury et al. 2022).
The sewage treatment plants produce an enormous quantity of SS. Its mass and content are related to the origin of the wastewater and applied technological systems of treatment. The quantity of SS is estimated to be, by mass, on average 3% of the wastewater passing through the treatment plant (Buta et al. 2021). Currently, a production of SS in the world is about 45·106 tons (dry matter) annually (Zhang et al. 2017). North America, Europe and East Asia are main world`s producers of SS (Shaddel et al. 2019). According to the data for 2016, around 30·106 tons of wet sludge with a high moisture content (up to 80%) are produced only in China (Zhang et al. 2017). Urban SS production and disposal for number of European countries in 2018 is shown in Figure 1 (EUROSTAT n.d.) Fig. 1.
Sewage sludge production and disposal from urban wastewater (thousand tons in dry substance) for some European countries in 2018 (EUROSTAT n.d.)
SS is characterized by a huge microbial diversity that may affect sludge mineralization, agricultural soil quality at their use as fertilizers and possible contamination of agricultural crops and adjacent environments (Nascimento et al. 2018; Li et al. 2021). Biodiversity of SS may change depending on wastewater origin (industrial or domestic) and conditions of treatment (Cydzik-Kwiatkowska and Zielinska 2016). Chemical features of sludge, such as pH, redox condition, macronutrient content, presence of inorganic and organic pollutants and biological composition of activated sludge can directly impact bacterial communities’ structure (Nascimento et al. 2018). Significant differences in bacterial communities were identified in the samples of inflow and effluent wastewater. Dominant operational taxonomic units varied both spatially and temporally. For example, groups of pathogenic bacteria were sufficiently reduced in effluent water by the treatment process, except for Leptospira and Legionella species, which demonstrated a percentage rise from inflow to effluent water (Numberger et al. 2019).
The number of bacterial species in activated sludge at large wastewater treatment plant may varied from 3860 to 4868 (Begmatov et al. 2022; Wu et al. 2019). Communities of wastewater and SS microorganisms include bacteria of different taxonomic (gram-positive and gram-negative), biochemical (nitrification, denitrification, sulfur oxidation, nitrogen fixation, etc.) and physiological (autotrophic, heterotrophic, aerobic, anaerobic) groups, which provide functional benefits for water decontamination, such as removal of different nutrients and pollutants (Ye and Zhang 2013; McLellan et al. 2010). During digestion of sludge by microorganisms (mainly bacteria) organic matter converts into simpler organic substances. Moreover, bacteria are capable of converting inorganic pollutants, such as heavy metals and metalloids, from solution into less mobile organic compounds (own biomass and organic complexes), as well as less soluble inorganic compounds during bioaccumulation, biosorption and biotransformation (Perelomov and Chulin 2014; Priya et al. 2022). So, various microorganisms in sludge significantly reduce the environmental threat from pollutants posed by wastewater (Xie et al. 2021). In addition, microbiological processes largely determine the further use of SSe, which under certain conditions is a valuable organic fertilizer. Sludge microbial communities contain microorganisms of human and animal origin, which also may interact with microorganisms of natural environments including enriching them with genes for resistance to pollutants and antibiotics (Cai et al. 2014). All these microorganisms could deactivate organic and inorganic toxicants only if they are tolerant to high these pollutant concentrations.
From an environmental point of view, microorganism strains that are members of activated and SS, tolerant to high concentrations of pollutants, especially heavy metals and metalloids, are of great interest (Perelomov et al. 2022). They are capable of mineralization of SS organic matter and thus reduce their toxicity. Moreover, interaction of metal-tolerant bacteria and plants can play an important role in adaptation of vegetation to soils polluted by metal(loid)s. Different bacteria able to promote plant growth by providing important substances, minimizing the harmful effects of metal(loid)s, as well as boosting tolerance to them (Narayanan and Ma 2023). Various metabolites of plant-associated bacteria (phytohormones such as abscisic acid, cytokinins, jasmonic, ethylene, and gibberellic acids, organic acids, aminoacids, siderophores etc. involve in many processes occurring in the rhizosphere, such as nutrient acquisition, post-embryonic root elongation, metal detoxification and alleviation of biotic/abiotic stress in plants (Rajkumar et al. 2012). Different direct and indirect mechanisms of effects of plant growth-promoting bacteria (PGPB) on the bioremediation of metal-contaminated soils are summarized in Fig. 2 (Wang et al. 2022).
Mchanisms of effects of plant growth-promoting bacteria on the bioremediation of metal-contaminated soils (accoding to Wang et al. 2022)
In this investigation, we studied the systematic belonging of some strains of bacteria tolerant to high concentration of trace elements isolated from uneven-aged SS of the urban wastewater treatment plant and ecological conditions (temperature, pH, salinity) for their normal activity.
Results and discussion
Isolated and identified bacterial strains in sewage sludge
The humidity of the collected sludge samples was noted between12-18%. The elemental composition (H, C, N, S) of SS of different ages is presented in Table 1.
Undoubtedly, the elemental composition of SS depends on the initial composition of wastewater and the features of the technological process of treatment, which is more reflected in sludge up to 1 year old. However, there is a trend towards a decrease in the content of N, C, H during long-term storage of sediments, and the C/N ratio also decreased (Table 1). The decrease in the content of nitrogen and organic carbon in sludge of five years of age indicates its active mineralization. At the same time, sulfur compounds are relatively stable and accumulate in SS over time.
The content of total heavy metals (Co, Ni, Cu, Zn, Cd, Pb) in SS was given in Table 2.
The metal concentrations in bold font are those exceeding the maximum permissible concentrations (MPC) for SS of the group I and relating this sludge to the group II. In the Russian Federation, according to State standards, all SS is divided into two groups according to the concentration of trace elements and, accordingly, to the possible nature of use in agriculture. For each group, there are MPC of trace elements (Table 2) (State Standard 2001).
If the content of at least one of the normalized elements exceeds its MPC for group I, then sludge is classified as group II. The SS of group I is used for all types of crops, except for vegetables, mushrooms, greens and strawberries. The sludge of group II is used for cereals, legumes, grain fodder and industrial crops.
The sludge of groups I and II are used in industrial floriculture, green building, forest and decorative nurseries, for biological reclamation of disturbed lands and solid waste landfills. Doses of sludge for agricultural crops in each case are calculated taking into account the actual content of normalized pollutants in sludge and soil. If the soil contains any of the normalized contaminants at a concentration of more than 0.8 MPC, the application of sludge as a fertilizer is prohibited. Thus, the studied SS, starting from 6 months old, can be attributed to the second group due to the excess Zn concentration, and five-year-old sludge - due to the Cd concentration (Table 2).
The number of colony-forming units (CFU) in SS of different ages was: 1.2 × 107 cells/g in fresh SS, 3.1 × 107 cells/g in 6-month-old SS, 3.8 × 106 cells/g in 1-year-old SS, 2.8 × 107 cells/g in 5-year-old SS.
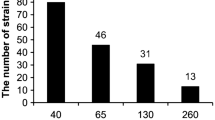
Based on the results of inoculation on the medium with trace elements, bacteria with medium (3 mM) and high (5 mM) resistance to Co, Ni, Cu, Zn, Pb and Cd were isolated (Table 3). Zinc and Pb resistant bacteria were present in all SS samples. As for bacteria resistant to Cd and Co, they were rare (Table 3). Some bacteria were resistant simultaneously to two and more elements, for example, Zn and Cd, Pb and Zn. Such multi-resistant bacteria were of interest to us.
When cultivated on a medium with high trace elements concentrations, a number of bacterial colonies acquired a specific color (Fig. 3).
There are different views as to why microbial pigments form in response to the presence of trace elements. It is possible that the production of a number of pigments can protect microorganisms from photooxidative damage (Armstrong 1994). However, there is often no linear relationship between the color intensity and the metal concentration in the medium, and pigmentation appears only in a certain range of element concentrations (Lima de Silva et al. 2012). Some scientific publications have argued that the ability to produce pigment can be directly related to metal(loids) tolerance. For example, Fujimore et al. (1996) observed that the red pigment-deficient white mutant of the Pseudomonas K-62 strain was more sensitive to Hg2+ than the original reddish wild-type strain. Melanin acts as a protective agent against oxidizing agents and trace elements (Nosanchuk and Casadevall 2003). A metabolically engineered bacterium was employed to produce blue-purple pigment violacein responsive to toxic Cd2+. Visual and quantifiable signals could be captured after a 1.5-h Cd2+ exposure (Hui et al. 2022).
The large number of publications were also indicated a great diversity and variation in the structure of bacterial communities in SS. In the conditions of Brazil (Sao Paulo), Clostridium was the dominant genera of SS, followed by genera Treponema, Propionibacterium, Syntrophus, and Desulfobulbus (Nascimento et al. 2018). The SS of Navarra region (Spain) contains in concentrations between 105 and 107 CFU g−1 of total coliforms, i.e., Escherichia coli, Staphylococcus aureus, Enterococcus sp., Pseudomonas sp. and total mesophilic bacteria and it does not contain Salmonella sp. (Miguel et al. 2020). Under the climatic conditions of Croatia, a study of 10 wastewater treatment plants produced results showing that eight groups (Anaerolineaceae, Methanosaeta, Trichococcus, Acinetobacter, Romboutsia, Clostridium, Turicibacter, Intestinibacter) were present in all studied urban SS.
Characteristics of bacterial isolates and their significance
In our research molecular genetic methods have identified 12 metal-tolerant strains of bacteria resistant to two and more trace elements (in concentration 3 mmol, 5 mmol) simultaneously in SS of different ages (Table 4). The isolated metal(loid)-tolerant strains belong to different genera of both gram-negative and gram-positive bacteria.
The Rhodococcus qingshengii SS60-2 strain resistant to Co was isolated from 5-year-old sediments with the maximum concentration of this element (309 mg/kg). Rhodococcus qingshengii SS6-3 strain, resistant to Ni, was isolated from SS 6 months old with the maximum concentration of Ni (72.3 mg/kg), although slightly exceeding the concentrations in other SS. Zn-resistant Pseudomonas cedrina SS60-7 and Serratia liquefaciens SS60-8 strains were isolated from 5-year-old SS with the maximum Zn concentration (2360.8 mg/kg). The Citrobacter freundii SS60-12 strain, resistant to Pb, was isolated from 5-year-old SS with the highest metal content (60 mg/kg). Thus, the metal tolerance of isolated strains of bacteria in almost half of the cases correlates with the maximum content of trace elements in their habitat.
The question of the factors that determine the composition and structure of the bacterial community in SS is debatable. Authors, using weighted UniFrac distance-based redundancy analysis, found that a group of 5 trace elements (Cr, Cu, Ni, Pb, Zn) and a pair of organic contaminants (hexabromocyclododecane and tribromodiphenyl ethers) were significantly associated with the bacterial community structure in mesophilic anaerobic stabilized samples. Altogether, 85% of the variance in bacterial community structure could be ascribed to these pollutants (Stiborova et al. 2020). Major et al. (2022) points out that the presence of some trace elements (iron, lead, mercury or zinc) in SS samples seems to be associated with particular microbial community. At the same time, the Nascimento et al. (2018) discovered that inorganic toxicants, such as heavy metals, had little impact on the structure of microbial community in the sludge (Nascimento et al. 2018). The sequencing results of Bhat et al. (2020) also revealed that the bacterial diversity showed evident variations under heavy metal stress. Cluster analysis and Principal component analysis showed that the microbial community in SS with high Cu(II) concentration was different from the sludge samples with high Cd(II) and Pb(II) concentrations. Obviously, the influence of heavy metals and metalloids on the structure of the microbial community depends on various factors, including the concentrations of pollutants and the initial composition of the community. It was also noted that the composition of the microbial community of SS does not correlate with the method of biosolids stabilization (aerobic processing, aerobic plus anaerobic stabilization; and no stabilization and with lime addition) and on the number of residents served by wastewater treatment plants (Major et al. 2022). The high contents of Fe and S were important modulators of microbial community structure for some sludge after anaerobic treatments and with relatively low N and P contents (Nascimento et al. 2018). At the same time high content of these elements was important factor for municipal, aerobically treated sludge with low Fe and Al concentrations. In addition, there can be a substantial seasonal variation in the composition of the microbial community, described in the literature (Numberger et al. 2019).
A detailed examination of the genera and species of strains identified by us shows that all of them were either found earlier in SS or active sludge treatment plants, or are extremophiles.
Serratia are facultative anaerobes, catalase-positive, and motile with peritrichous flagella. The bacteria can use many different compounds as carbon sources in medium containing ammonium sulfate as the nitrogen source (Batt and Robinson 2014). Serratia fonticola found in a wide array of environments, including drinking water, soil and sewage. For example, Serratia fonticola IB4r (NCTC 13193) was isolated from pilot-scale sewage bed (Ashelford et al. 2001).
Serratia liquefaciens can be found typically in soil, wastewaters, sewage, but also in food or in the intestinal tract of humans or animals (Liu et al. 2015; Lepesova and Krahulcova 2019). Isolated from contaminated soil bacterium Serratia liquefaciens producing lignin peroxidase is able to degrade effluent with substantial reduction of lignin (58%) and phenol (95%) (Haq et al. 2016). The metal tolerance of strains of Serratia liquefaciens has been shown in the works of Han et al. (2018), Ahmed et al. (2020) and others.
Fast growth and high adaptability to different ecological conditions (oxidative, nutritional, etc.) allows Pseudomonas genus one of the most diverse and ubiquitous group (Peix et al. 2009). Members of Pseudomonas are able to mineralization of various organic compounds (aromatic hydrocarbons, chloro- and nitro-organic compounds, pesticides) and play an important role in the bioremediation and detoxification of contaminated soils and grounds. Pseudomonas spp. are also key participant of bacterial community active in wastewater treatment processes. In the studied of SS, we found strains that can be attributed to three species: Pseudomonas fragi, P. cedrina and P. extremaustralis.
The discovery by us of a strain of the P. extremaustralis species requires further confirmation by analyzing the full genome of the isolated strain because this species is very rare (López et al. 2017). However, a strain of this species was isolated from wastewater by Spanish researchers (Vargas-Ordóñez et al. 2023). This species isolated from a season pond in Antarctica presents high levels of resistance to oxidative stress and low temperature. It is also resistant to hydrocarbons and able to them degradation (Cai et al. 2014). P. extremaustralis resisted to 4 mM Cu2+ in a rich microbial medium such as LB (Colonnella et al. 2019). This Cu resistance mechanism includes the presence of the cus and cop operons together with other efflux systems and porins located in a single region in P. extremaustralis genome (López et al. 2017). Cu2+ negatively affected on degradation of diesel despite the fact that Cu enhanced bacterial attachment to hydrocarbons. At the same time, when a small amount of glucose (0.05% w/v) was added to the bacteria, the presence of Cu2+ intensified degradation of alkanes (Colonnella et al. 2019).
Pseudomonas fragi, psychrotrophic bacterium, which has high proteolytic potential (Liu et al. 2015), can produce several types of enzymes, including lipases and proteases. These enzymes are responsible for the spoilage of meat, fish, vegetables, dairy products and other products of animal origin.
Pseudomonas cedrina is a rod-shaped bacterium isolated from Lebanese spring waters (Dabboussi et al. 1999). It has been placed in the P. fluorescens group, and showed that the species was resistant to high concentrations of NaCl (Tirry et al. 2021).
Stenotrophomonas maltophilia is an aerobic, nonfermentative bacillus that is closely related to the Pseudomonas species (Calza et al. 2003). Its name means "a unit feeding on few substrates". "Maltophilia" translates from Greek as "affinity, love for malt". It is frequently isolated from soil, water, plant matter, animals, and hospital equipment (Denton and Kerr 1998). This species is found in effluents and rivers that receive sewage water of pig farms (Kim et al. 2018). Strains of Stenotrophomonas maltophilia was isolated from anaerobic sludge from sewage treatment plant (Feng et al. 2015). From soil of polluted wasteland novel resistant to Cu bacteria S. maltophilia PD2 was isolated (Ghosh and Saha 2013). Pb, Zn and Ni resistant bacterial strains of S. maltophilia were isolated from a wastewater treatment plant in Poland (Wierzba 2015).
Rhodococcus spp. are isolated from many polluted areas; dominate in hydrocarbon-contaminated ecosystems (van der Geize and Dijkhuizen 2004). Rhodococci are capable of utilizing a wide range of organic compounds, including toxic ones (Kawagoe et al. 2019). They are able to metabolize a wide range of pollutants including petroleum hydrocarbons, polychlorinated biphenyls, pharma pollutants, pesticides, explosives, flame retardants, plasticizers, defoliants, dyes, and microplastics. Moreover, the bacteria are resistant to multiple stresses; are able to maintain high metabolic activities under adverse conditions (Krivoruchko et al. 2019; Krivoruchko et al. 2023).
Most strains of Rhodococcus have been found to have very high levels of metal resistance. These bacteria are not only capable of metabolizing various organic pollutants in the presence of co-contaminating trace elements, but they able to biosorption and/or bioconversion of various metals and metalloids. Bacterial strains belonging to the Rhodococcus genus exploit different mechanisms enabling them to highly tolerate metal(loid) compounds (Pavel et al. 2013). For instance, the reduction of cellular sensitivity, the intracellular sequestration of metal ions and oxyanions, their complexation with siderophores, the alteration of the membrane permeability, mutations, and repairing mechanisms of the DNA responsible for both plasmid and chromosomal DNA stability are some of the mechanisms implemented by bacteria to tolerate and/or resist to metalloids’ toxicity (Stillman and Irwim 1995; Garbisu and Alkorta 2003; Figueira et al. 2006; Presentato et al. 2019).
The species Rhodococcus qingshengii was first isolated from the soil contaminated by carbendazim. Although this species is a typical soil inhabitant, it has been found in different habitats such as sludge, sawdust, and seawater (Peng et al. 2023).
Citrobacter freundii is a soil microorganism, but can also be found in water, food, the intestinal tracts of animals and humans and sewage (Kus and Burrows 2008). The strain C. freundii JPG1, isolated from gold mining tailing in China was cross-tolerate to several trace elements: Ag+, Cd2+, Co2+, Cr6+, Cu2+ and Ni2+ and was able to biosorption and bioaccumulation of Cu under both aerobic and anaerobic conditions (Wang et al. 2018). The strain C. freundii SRS1 from Bangladesh could tolerate up to 3 mmol/ L Pb(NO3)2, 2.5 mmol/ L CoCl2, 2.5 mmol/ L Cd(CH3COO)2, and 2.5 mmol/ L CrCl3. The genome of C. freundii SRS1 czcA, czcD, cbiN, and cbiM genes for Co resistance; chrA and chrB genes for Cr resistance; and zntA gene for Pb and Cd resistance (Uddin et al. 2022).
Thus, numerous literature data confirm the presence of the species identified by us in SS, as well as their metal tolerance.
Optimal environmental conditions (temperature, pH, salinity) for isolated metal-tolerant strains
An interesting question is whether the isolated trace element tolerant strains are also extremophiles in relation to the most important environmental factors - temperature, pH, salinity, or whether their adaptation to high concentrations of trace elements is due to a specific mechanism. That's why for isolated from SS metal-tolerant species of bacteria, the features of the conditions for their growth were studied – the optimal values of temperature, acidity and salinity of the environment.
The range of optimal growth temperatures for most strains for 48 hours was 15–30°C (Table 5). After 2 days of cultivation, the growth of 3 strains was noted at a temperature of 7°C: Pseudomonas fragi SS0-4, S. fonticola SS0-9 and S. fonticola SS12-11. The same bacterial strains S. fonticola SS0-9 and S. fonticola SS12-11 as well as strain Stenotrophomonas maltophilia SS0-10 showed growth at 37°C. Species of Serratia are known to give optimum growth from 20-37°C (Giri et al. 2004). However, some strains of S. liquefaciens, S. odorifera, S. plymuthica, and S. ficaria can grow at 4–5°C; other strains of S. odorifera, S. marcescens, and S. rubidaea can grow at 40°C. At the same time, the strain of bacterial species P. extremaustralis, which was mostly isolated from places with a cold climate, did not show psychrophilicity in our experiment.
Cultivation of the studied strains in a liquid LB medium at temperature of 28°C with shaking for 18 h followed by determination of optical density revealed that all strains grew in the pH range of 5–9. Only two strains, Serratia fonticola SS0-1and Citrobacter freundii SS60-12, grew in an acidic medium at pH 4. After the digesters, wastewater usually has a slightly alkaline reaction. The SS, we have selected was slightly alkaline or close to neutral pH (Table 1). At the same time, with age, the pH of SS is somewhat acidified, changing towards the acidity of zonal soils. It is logical that among the studied bacterial strains there are practically no acidophilic ones.
During cultivation for 2 days at 28°C on LB agar medium with different NaCl salt concentrations in Petri dishes, colony formation was observed for the following strains (Table 6).
Most of the strains grew in the NaCl concentration range of 1–5%, strains (Serratia fonticola SS0-1, Serratia fonticola SS12-11, Citrobacter freundii SS60-12) grew at 6% salt, two strains of them (Serratia fonticola SS0-1, Citrobacter freundii SS60-12) grew at 7% and one (Citrobacter freundii SS60-12) grew at 8% NaCl weakly. It is interesting to note that 7% sodium chloride resistant strains Serratia fonticola SS0-1 and Citrobacter freundii SS60-12 also grow at low pH (pH 4), and 6% salt resistant strain Serratia fonticola SS12-11 also grows successfully at low temperatures (7 ºC). Thus, it is possible that they have resistance genes not to individual adverse environmental factors, but to stress in general.
Conclusions
The identification of the trace elements tolerant strains from urban sewage sludge showed that strains belong to the different species of gram-positive (Rhodococcus qingshengii) and gram-negative (Serratia fonticola, Pseudomonas fragi, Stenotrophomonas maltophilia, Pseudomonas extremaustralis, Pseudomonas cedrina, Serratia liquefaciens and Citrobacter freundii) bacteria. The metal tolerance bacterial strains correlated with the maximum content of metal(loids) in their habitat in a substantial number of cases. Our data demonstrate that the isolated trace element tolerant bacteria are, for the most part, not extreme in relation to basic environmental factors, such as temperature, pH and salinity. Thus, it can be assumed, that adaptation to chemical pollutants is a specific, rather than a stressor, mechanism. Wastewater treatment plants and sewage sludge can be hotspots for heavy metals and metalloids resistant genes and for the spread of bacteria into the environment objects. The presence of metal(loids) tolerant bacteria also increases the potential risk of gene transfer to non-resistant bacteria. The problem of disposal of sewage sludge is largely determined by the microbiological processes of decomposition of organic matter and the transformation and detoxification of pollutants that occur in them. The composition of the microbial community in sewage sludge must be adapted to extreme habitat conditions, primarily to high concentrations of trace elements and antibiotics. Thus, the microbial community present in sewage sludge especially metal(loids) tolerant can be used to create modern technologies for the effective mineralization of sewage sludge and for the decontamination of industrial and domestic wastewater.
Materials and methods
Sample collection and chemical analysis
In June 2022 different age’s mixed SS samples from Tula`s sewage treatment plant (capacity of 450·103 m3/day) were taken from the silt storage sites for chemical and microbiological analysis.
The age of the selected sludge (according to the documentation of the management of the treatment facilities of the city of Tula) was 1 month, 6 months, 1 year (12 month), 5 years (about 60 month). Fresh sludge, just unloaded from the digester after dehydration, was also collected. From the sites for storing sludge of the appropriate age, using a special sampler from a layer of 20 cm using the envelope method (from five points), point samples weighing about 200 g were taken. By mixing the point samples, a combined sample was formed, which was examined. In order to prevent secondary contamination, samples were taken under aseptic conditions: with a sterile instrument, mixed on a sterile surface, and placed in a sterile plastic container. Samples sampled for chemical analysis were air dried to constant mass. The salts, acidity, content of nitrogen, carbon and hydrogen, and the concentration of total trace elements were determined in the collected SS samples.
Determination of the concentration of organic carbon (Corg), total nitrogen (N), sulfur (S) and hydrogen (H) in SS samples was performed using an automatic HCNS analyzer Elementar Vario El III (Germany) at the Center of Common Facilities of the Institute of Physicochemical and Biological Problems in Soil Science of Russian Academy Sciences. The total concentration of metals and metalloids in the solutions obtained after microwave acid decomposition of SS in a mixture of HNO3 + HClO4 was measured by atomic absorption spectrometer with flame atomization of samples Analytik Jena contrAA® 800 (Germany) at the Laboratory of Biogeochemistry, Tula State Lev Tolstoy Pedagogical University, Tula, Russian Federation.
Bacteria cultivation
Bacterial strains were isolated from the collected different ages SS samples, extracts were diluted 7 ten-fold dilutions by physiological solution and inoculated on the LB agar medium with following content: yeast extract—5 g/L, tryptone—10 g/L, NaCl—10 g/L, agar—15 g/L. Amount of colony-forming units (CFU) were determined as described in Standard Methods for Examination of Water and Wastewater (Standard Methods… 2022). The 100 µL of bacterial cell suspension was distributed over the surface of the agar medium in Petri dishes by a glass spatula. Dishes were placed for 24–48 h at 28℃ in a thermostat. The amount of CFU was estimated by direct counting of colonies and expressed as a quantity of bacteria in 1 g of the SS sample. Microorganisms were tested for tolerance to high concentrations of six trace elements: Co, Ni, Cu, Zn, Cd and Pb. The elements stock solutions were prepared from nitrate salts of the elements as described by Cai et al. (2014). The 100 µL of the bacterial suspension by a sterilized pipet was spread on Petri dishes with twice diluted LB medium (to prevent of trace elements complexes precipitation) with metal concentrations: 2 mmol (low tolerance), 3 mmol (middle tolerance), 5 mmol (high tolerance). Bacteria resistant to heavy metals and metalloids were isolated from SS by direct seeding on selective media. The study of bacterial resistance to six trace elements (Co, Ni, Cu, Zn, Cd, Pb) was carried out by visual assessment of their growth on a medium containing water-soluble salts of these metals at concentrations of 2 mmol (low resistance), 3 mmol (medium resistance), 5 mmol (high resistance). After incubation of dishes at 28℃ for 24 h in an incubator the bacterial colonies with different morphology, including size, edge appearance and color of colony, were visually assessed for isolation and purification.
The marked colonies were seeded by glass stick in Petri dishes with LB medium containing metal concentration 2, 3, and 5 by mmol/l. The Petri dishes were incubated in a thermostat at conditions described above, and the sowing and incubation were repeated until a single specific colony was formed. Bacterial strains were harvested from the LB medium and stored with sterilized glycerol at 5℃. From the isolated bacteria, 12 strains with different phenotype properties were selected based on their tolerance to two or three trace elements simultaneously in concentrations 3 and 5 mmol. Genomic DNA was isolated from the chosen tolerant bacterial strains using the Quick-DNA Miniprep Kit (Zymo Research, USA).
Sequencing and identification of bacterial isolates
The isolated bacterial strains were identified using polymerase chain reaction (PCR) analysis of target genes and sequenced using Sanger sequencing method. The isolated DNA was used to amplification of the 16S rRNA gene. PCR was carried out with the two primers: forward - 27f 5'-AGAGTTTGATCCTGGCTCAG-3' and reverse - 1492r 5'-GGTTACCTTGTTACGACTT-3'. Amplification of the 16S rRNA gene was performed on GeneAmp PCR System 9700 device (Applied Biosystems, Foster City, CA, USA) at the following parameters: primary denaturation: 95℃ for 5 min; then 30 cycles: 95℃ for 30 s, 55℃ for 30 s (temperature of primer annealing), 72℃ for 40 s; final elongation stage—72℃ for 5 min.
The PCR product size was 1465 bp. The products of reaction were divided by electrophoresis in agarose gel (1.0%) at a voltage of 10 V/cm. Gel staining was performed by ethidium bromide solution (5 µg/mL) and photographed under UV light by a gel documentation system (Gel DocTMXR, Bio-Rad, Hercules, USA). PCR components, taqDNApolymerase, concentrated electrophoresis buffer, and GeneRuler 1kb DNA Ladder marker (SM0311) were produced by Fermentas (Lithuania). Products of PCR were purified by DNA Clean & Concentrator (Zymo Research, USA) according to the instructions of manufacturer.
Sequencing of the 16S rRNA gene of the bacterial strains was performed with PCR products using the described above primers. Sequencing was carried out on an automatic sequencer ABI Prism 373 3130XL (Applied Biosystems, USA). Preliminary phylogenetic screening for the similarity of nucleotide sequences of the 16S rRNA gene was received from the GenBank database of National Center for Biotechnology Information (NCBI) using the Basic Local Alignment Search Tool (BLAST) software package. To more precise study of the phylogenetic position of the investigated strain, the sub-sequent 16S rRNA gene sequence was aligned with the corresponding sequences of the closest bacterial species using the CLUSTAL W program.
Determination of optimal environmental conditions for the growth of strains
To study the optimal growth temperatures, the isolated strains were transferred by the replica method onto LB agar medium and grown in a thermostat at different temperatures: 7, 15, 24, 30, 37, 42°C for 2–7 days, depending on temperature. The incubation time depended on the temperature, since at low temperatures strains grow longer. At temperatures of 24, 30, 37 and 42°C they were incubated for 48 hours. At a temperature of 15°C - 5 days and at 7°C - 7 days. The growth of the strains was assessed by the presence of colonies on the plate.
To determine the optimal pH for bacterial growth, individual colonies of strains from the agar medium were inoculated in test tubes with 5 ml of liquid LB medium and cultivated at 28°C for 18 h on a shaker at 160 cycles/min. Then the strains were inoculated into test tubes with LB medium with different pH values - from 4 to 9 with a step of 1 unit. The tubes were cultured at 28°C on a shaker at 160 cycles/min for 18 h. Next, the growth of strains was assessed by the optical density (OD) of the culture on a Shimadzu UV-160A spectrophotometer at λ = 260 nm.
The growth of strains at different concentrations of NaCl in the medium was assessed as follows: the isolated strains were transferred by the replica method to LB agar medium with different NaCl salt concentrations, % (1, 2, 3, 4, 5, 6, 7, 8, 9, 10). The dishes were incubated in a thermostat at 28°C for 2 days. The growth of strains was assessed by the presence of colonies on a Petri dish.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at the Tula State Lev Tolstoy Pedagogical University.
Abbreviations
- SS:
-
Sewage sludge
- CFU:
-
Colony-forming units
- PCR:
-
Polymerase chain reaction
- MPC:
-
Maximum permissible concentration
References
Ahmed S, Aktar S, Zaman S, Jahan RA, Bari ML (2020) Use of natural bio-sorbent in removing dye, heavy metal and antibiotic-resistant bacteria from industrial wastewater. Appl Water Sci 10:107. https://doi.org/10.1007/s13201-020-01200-8
Armstrong GA (1994) Eubacteria show their true colors: genetics of carotenoid pigment biosynthesis from microbes to plants. J Bacteriol 176(16):4795–4802. https://doi.org/10.1128/jb.176.16.4795-4802.1994
Ashelford KE, Learner MA, Fry JC (2001) Gene transfer and plasmid instability within pilot-scale sewage filter beds and the invertebrates that live in them. FEMS Microbiol Ecol 35(2):197–205. https://doi.org/10.1111/j.1574-6941.2001.tb00804.x
Batt CA, Robinson RK (2014) Encyclopedia of Food Microbiology. Academic Press. p 3248
Begmatov S, Dorofeev AG, Kadnikov VV, Beletsky AV, Pimenov NV, Ravin NV, Mardanov AV (2022) The structure of microbial communities of activated sludge of large-scale wastewater treatment plants in the city of Moscow. Sci Rep 12:3458. https://doi.org/10.1038/s41598-022-07132-4
Bhat SA, Cui G, Li W, Wei Y, Li F (2020) Effect of heavy metals on the performance and bacterial profiles of activated sludge in a semi-continuous reactor. Chemosphere 241:125035. https://doi.org/10.1016/j.chemosphere.2019.125035
Buta M, Hubeny J, Zieliński W, Harnisz M, Korzeniewska E (2021) Sewage sludge in agriculture – the effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops – a review. Ecotoxicology and Environmental Safety 214:112070. https://doi.org/10.1016/j.ecoenv.2021.112070
Cai L, Ju F, Zhang T (2014) Tracking human sewage microbiome in a municipal wastewater treatment plant. Appl Microbiol Biotechnol. 98:3317–3326. https://doi.org/10.1007/s00253-013-5402-z
Calza L, Manfredi R, Chiodo F (2003) Stenotrophomonas (Xanthomonas) maltophilia as an emerging opportunistic pathogen in association with HIV infection: a 10-year surveillance study. Infection 31(3):155–161. https://doi.org/10.1007/s15010-003-3113-6
Colonnella MA, Lizarraga L, Rossi L, Díaz Peña R, Egoburo D, López NI, Iustman LJR (2019) Effect of copper on diesel degradation in Pseudomonas extremaustralis. Extremophiles 23:91–99. https://doi.org/10.1007/s00792-018-1063-2
Cydzik-Kwiatkowska A, Zielinska M (2016) Bacterial communities in full-scale wastewater treatment systems. World J Microbiol Biotechnol 32:66. https://doi.org/10.1007/s11274-016-2012-9
Dabboussi F, Hamzé M, Elomari M, Verhille S, Baida N, Izard D, Leclerc H (1999) Taxonomic study of bacteria isolated from Lebanese spring waters: proposal for Pseudomonas cedrella sp. nov and P. orientalis sp. nov.. Research in Microbiology 150(5):303–316. https://doi.org/10.1016/s0923-2508(99)80056-4
Denton M, Kerr KG (1998) Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev 11(1):57–80. https://doi.org/10.1128/cmr.11.1.57
EUROSTAT. (n.d.) Sewage sludge production and disposal from urban wastewater (in dry substance (d.s)): https://ec.europa.eu/eurostat/databrowser/view/TEN00030/default/bar?lang=en&category=env.env_wat.env_nwat Accessed on 25.07.2023.
Feng W, Song P, Zhang Y et al (2015) Stenotrophomonas maltophilia having decolorization capability of azo dye isolated from anaerobic sludge. In: Advances in Applied Biotechnology. Lecture Notes in Electrical Engineering, Zhang, T.C.; Nakajima, M., Eds. Springer, Berlin, Heidelberg. Volume 333, pp. 109-116. https://doi.org/10.1007/978-3-662-46318-5_12
Figueira JR, Greco S, Ehrogott M et al (2006) Multiple criteria decision analysis. State of the art surveys. Springer: NY, USA, 1048 p. https://doi.org/10.1007/b100605
Fujimori H, Kiyono M, Nobuhara K, Pan‐Hou H (1996) Possible involvement of red pigments in defense against mercury in Pseudomonas K-62. FEMS Microbiol Lett 135(2–3):317–321. https://doi.org/10.1016/0378-1097(95)00472-6
Garbisu C, Alkorta I (2003) Basic Concepts on heavy metal soil bioremediation. European Journal of Mineral Processing and Environmental Protection 3:58–66
Ghosh A, Saha PD (2013) Optimization of copper bioremediation by Stenotrophomonas maltophilia PD2. Journal of Environmental Chemical Engineering 1(3):159–163. https://doi.org/10.1016/j.jece.2013.04.012
Giri AV, Anandkumar N, Muthukumaran G, Pennathur G (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol 4:11. https://doi.org/10.1186/1471-2180-4-11
Grobelak A, Czerwińska K, & Murtaś A (2019) General considerations on sludge disposal, industrial and municipal sludge. In: Prasad MNV, Paulo Jorge de Campos Favas PJ, Vithanage M, S. Mohan V (eds)Industrial and Municipal Sludge. Emerging Concerns and Scope for Resource Recovery. Elsevier Inc, Butterworth-Heinemann, pp. 135–153. https://doi.org/10.1016/b978-0-12-815907-1.00007-6.
Han H, Sheng X, Hu J, He L, Wang Q (2018) Metal-immobilizing Serratia liquefaciens CL-1 and Bacillus thuringiensis X30 increase biomass and reduce heavy metal accumulation of radish under field conditions. Ecotoxicol Environ Saf 161:526–533. https://doi.org/10.1016/j.ecoenv.2018.06.033
Haq I, Kumar S, Kumari V et al (2016) Evaluation of bioremediation potentiality of ligninolytic Serratia liquefaciens for detoxification of pulp and paper mill effluent. Journal of Hazardous Materials 305:190–199. https://doi.org/10.1016/j.jhazmat.2015.11.046
Hui CY, Guo Y, Li H, Gao CX, Yi J (2022) Detection of environmental pollutant cadmium in water using a visual bacterial biosensor. Sci Rep 12:6898. https://doi.org/10.1038/s41598-022-11051-9
Kacprzak M, Neczaj E, Fijałkowski K, Grobelak A, Grosser A, Worwag M, Rorat A, Brattebo H, Almås Å, Singh BR (2017) Sewage sludge disposal strategies for sustainable development. Environmental Research. 156:39–46. https://doi.org/10.1016/j.envres.2017.03.010)
Kawagoe T, Kubota K, Araki KS, Kubo M (2019) Analysis of the alkane hydroxylase gene and long-chain cyclic alkane degradation in Rhodococcus. Advances in Microbiology 9(3):151–163. https://doi.org/10.4236/aim.2019.93012
Kim YJ, Park JH, Seo K-H (2018) Presence of Stenotrophomonas maltophilia exhibiting high genetic similarity to clinical isolates in final effluents of pig farm wastewater treatment plants. Int J Hyg Environ Health 221(2):300–307. https://doi.org/10.1016/j.ijheh.2017.12.002
Krivoruchko A, Kuyukina M, Ivshina I (2019) Advanced Rhodococcus biocatalysts for environmental biotechnologies. Catalysts 9(3):236. https://doi.org/10.3390/catal9030236
Krivoruchko A, Kuyukina M, Peshkur T, Cunningham CJ, Ivshina I (2023) Rhodococcus strains from the specialized collection of alkanotrophs for biodegradation of aromatic compounds. Molecules 28(5):2393. https://doi.org/10.3390/molecules28052393
Kus JV, Burrows LL (2008) Infections due to Citrobacter and Enterobacter. In: Enna,SJ, Bylund DB (eds) xPharm. The Comprehensive Pharmacology Reference. Elsevier, pp. 1–12. https://doi.org/10.1016/B978-008055232-3.60868-2
Lepesova K, Krahulcova M (2019) Sewage sludge as a source of triclosan-resistant bacteria. Acta Chemica Slovaca 12(1):34–40. https://doi.org/10.2478/acs-2019-0006
Li Y, Sun B, Deng T, Lian P, Chen J, Peng X (2021) Safety and efficiency of sewage sludge and garden waste compost as a soil amendment based on the field application in woodland. Ecotoxicol Environ Saf 222:112497. https://doi.org/10.1016/j.ecoenv.2021.112497
Lima de Silva AA, de Carvalho MA, de Souza SA, Dias PM, da Silva Filho RG, de Meirelles Saramago CS, de Melo Bento CA, Hofer E (2012) Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz J Microbiol 43(4):1620–1631. https://doi.org/10.1590/s1517-838220120004000047
Liu YJ, Xie J, Zhao LJ, Qian YF, Zhao Y, Liu X (2015) Biofilm formation characteristics of Pseudomonas lundensis isolated from meat. J Food Sci 80(12):2904–2910. https://doi.org/10.1111/1750-3841.13142
López G, Diaz-Cárdenas C, Shapiro N, Woyke T, Kyrpides NC, David Alzate J, González LN, Restrepo S, Baena S (2017) Draft genome sequence of Pseudomonas extremaustralis strain USBA-GBX 515 isolated from Superparamo soil samples in Colombian Andes. Stand in Genomic Sci 12:78. https://doi.org/10.1186/s40793-017-0292-9
Major N, Jechalke S, Nesme J, Goreta Ban S, Černe M, Sørensen SJ, Ban D, Grosch R, Schikora A, Schierstaedt J (2022) Influence of sewage sludge stabilization method on microbial community and the abundance of antibiotic resistance genes. Waste Manag 154:126–135. https://doi.org/10.1016/j.wasman.2022.09.033
McLellan SL, Huse SM, Mueller-Spitz SR, Andreishcheva EN, Sogin ML (2010) Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ Microbiol 12:378–392. https://doi.org/10.1111/j.1462-2920.2009.02075.x
Miguel N, Sarasa J, López A, Gómez J, Mosteo R, Ormad MP (2020) Study of evolution of microbiological properties in sewage sludge-amended soils: a pilot experience. Int J Environ Res Public Health 17(18):6696. https://doi.org/10.3390/ijerph17186696
Narayanan M, Ma Y (2023) Mitigation of heavy metal stress in the soil through optimized interaction between plants and microbes. J. Environ. Manage 345:118732. https://doi.org/10.1016/j.jenvman.2023.118732
Nascimento AL, Souza AJ, Andrade PAM, Andreote FD, Coscione AR, Oliveira FC, Regitano JB (2018) Sewage sludge microbial structures and relations to their sources, treatments, and chemical attributes. Front Microbiol 9:1462. https://doi.org/10.3389/fmicb.2018.01462
Nosanchuk JD, Casadevall A (2003) The contribution of melanin to microbial pathogenesis. Cell Microbiology 5:203–233. https://doi.org/10.1046/j.1462-5814.2003.00268.x
Nuamah AA, Malmgren G, Riley LE (2012) In: Ali Sayigh(ed) 5.05 - Biomass Co-Firing. Comprehensive Renewable Energy. Elsevier, pp 55–73. https://doi.org/10.1016/B978-0-08-087872-0.00506-0.
Numberger D, Ganzert L, Zoccarato L, Mühldorfer K, Sauer S, Grossart HP, Greenwood AD (2019) Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-length 16S rRNA sequencing. Sci Rep 9:9673. https://doi.org/10.1038/s41598-019-46015-z
Pavel V, Sobariu DL, Diaconu M, Stătescu F, Gavrilescu M (2013) Effects of heavy metals on Lepidium sativum germination and growth. Environmental Engineering and Management Journal 12(4):727–733. https://doi.org/10.30638/eemj.2013.089
Peix A, Ramirez-Bahena M-H, Velázquez E (2009) Historical evolution and current status of the taxonomy of genus Pseudomonas. Infect Genet Evol 9(6):1132–1147. https://doi.org/10.1016/j.meegid.2009.08.001
Peng M, Zhang D, Wang C, Jiang Z, Huang X, Zhou F, Huang F, Wang Z (2023) Complete genome sequence of Rhodococcus qingshengii strain PM1, isolated from a selenium-rich mine in China. Microbiol Resour Announc 12(1):0100722. https://doi.org/10.1128/mra.01007-22
Perelomov LV, Chulin AN (2014) Molecular mechanisms of interaction of microelements with microorganisms in the environment. Direct biological transformation of microelement compounds. Biol Bull Rev 4:285–299. https://doi.org/10.1134/S2079086414040070
Perelomov LV, Sizova OI, Rahman MM, Perelomova I, Minkina TM, Sokolov SV, Atroshchenko YM (2022) Metal-tolerant bacteria of wastewater treatment plant in a large city. Sustainability 14:11335. https://doi.org/10.3390/su141811335
Presentato A, Piacenza E, Cappelletti M, Turner RJ (2019) Interaction of Rhodococcus with metals and biotechnological applications. In: Biology of Rhodococcus. Microbiology Monographs, Alvarez, H., Eds. Springer, Cham. 16:333–357. https://doi.org/10.1007/978-3-030-11461-9_12
Priya AK, Gnanasekaran L, Dutta K, Rajendran S, Balakrishnan D, Soto-Moscoso M (2022) Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 307(4):135957. https://doi.org/10.1016/j.chemosphere.2022.135957
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnology Advances 30(6):1562–1574. https://doi.org/10.1016/j.biotechadv.2012.04.011
Roychoudhury A, Das N (2022) Sewage Sludge Treatment and Involvement of Microbes. In: Rajput VD, Yadav AN, Jatav HS, Singh SK, Minkina T (eds) Sustainable Management and Utilization of Sewage Sludge, Springer, Cham. https://doi.org/10.1007/978-3-030-85226-9_8
Shaddel S, Bakhtiary-Davijany H, Kabbe C, Dadgar F, Østerhus S (2019) Sustainable sewage sludge management: from current practices to emerging nutrient recovery technologies. Sustainability 11:3435. https://doi.org/10.3390/su11123435
Singh RK, Tirth V, Singh M (2022). Wastewater treatment processes with special reference to activated sludge process in indian conditions for water use sustainability. In: Yadav S, Negm AM, Yadava RN (eds) Wastewater Assessment, Treatment, Reuse and Development in India. Earth and Environmental Sciences Library. Springer, Cham, pp. 227–238. https://doi.org/10.1007/978-3-030-95786-5_12
Standard Methods for Examination of Water and Wastewater (2022) American Public Health Association, American Water Works Association, and Water Environment Federation, 24th edn. p 1516
State Standard of the Russian Federation (ГОСТ) R 17.4.3.07-2001 Nature protection. SOILS. Requirements for the properties of sewage sludge when used as fertilizers. https://docs.cntd.ru/document/1200017708. Accessed 25 Nov 2023 (in Rus)
Stiborova H, Strejcek M, Musilova L, Demnerova K, Uhlik O (2020) Diversity and phylogenetic composition of bacterial communities and their association with anthropogenic pollutants in sewage sludge. Chemosphere 238:124629. https://doi.org/10.1016/j.chemosphere.2019.124629
Stillman JA, Irwim RJ (1995) Extracting more information from the triangle task. Sensory Studies 10(1):105–112. https://doi.org/10.1111/j.1745-459X.1995.tb00007.x
Tirry N, Kouchou A, Laghmari G, Lemjereb M, Hnadi H, Amrani K, Bahafid W, El Ghachtouli N (2021) Improved salinity tolerance of Medicago sativa and soil enzyme activities by PGPR. Biocatalysis and Agricultural Biotechnology 31:101914
Uddin MJ, Haque F, Jabeen I, Shuvo SR (2022) Characterization and whole-genome sequencing of an extreme arsenic-tolerant Citrobacter freundii SRS1 strain isolated from Savar area in Bangladesh. Can J Microbiol 69(1):44–52. https://doi.org/10.1139/cjm-2022-0149
United Nations (2018) Department of Economic and Social Affairs. Population Dynamics. World Urbanization Prospects 2018. Available online: https://population.un.org/wup/ Accessed on 25.07.2023.
United Nations (2022) Population Division. World Population Prospects 2022: Summary of Results. Available online: https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022. Accessed on 25.06.2023.
Van der Geize R, Dijkhuizen L (2004) Harnessing the catabolic diversity of Rhodococci for environmental and biotechnological applications. Microbiology 7(3):255–261. https://doi.org/10.1016/j.mib.2004.04.001
Vargas-Ordóñez A, Aguilar-Romero I, Villaverde J, Madrid F, Morillo E (2023) Isolation of novel bacterial strains Pseudomonas extremaustralis CSW01 and Stutzerimonas stutzeri CSW02 from sewage sludge for paracetamol biodegradation. Microorganisms 11:96. https://doi.org/10.3390/microorganisms11010196
Wang X, Huang N, Shao J, Hu M, Zhao Y, Huo M (2018) Coupling heavy metal resistance and oxygen flexibility for bioremoval of copper ions by newly isolated Citrobacter freundii JPG1. J Environ Manag 226:194–200. https://doi.org/10.1016/j.jenvman.2018.08.042
Wang Y, Narayanan M, Shi X, Chen X, Li Z, Natarajan D, Ma Y (2022) Plant growthpromoting bacteria in metal-contaminated soil: Current perspectives on remediation mechanisms. Front Microbiol 13:966226. https://doi.org/10.3389/fmicb.2022.966226
Wierzba S (2015) Biosorption of lead(II), zinc(II) and nickel(II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis. Polish Journal of Chemical Technology 17(1):79–87. https://doi.org/10.1515/pjct-2015-0012
Wu L, Ning D, Zhang B, Li Y, Zhang P, Shan X, Zhang Q, Brown MR, Li Z, Van Nostrand JD et al (2019) Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat Microbiol 7:1183–1195. https://doi.org/10.1038/s41564-019-0426-5
Xie N, Zhong L, Ouyang L, Xu W, Zeng Q, Wang K, Zaynab M, Chen H, Xu F, Li S (2021) Community Composition and Function of Bacteria in Activated Sludge of Municipal Wastewater Treatment Plants. Water 13:852. https://doi.org/10.3390/w13060852
Ye L, Zhang T (2013) Bacterial communities in different sections of a municipal wastewater treatment plant revealed by 16S rDNA 454 pyrosequencing. Appl Microbiol Biotechnol 97:2681–2690. https://doi.org/10.1007/s00253-012-4082-4
Zhang Q, Hu J, Lee DJ, Chang Y, Lee YJ (2017) Sludge treatment: current research trends. Bioresour Technol 243:1159–1172. https://doi.org/10.1016/j.biortech.2017.07.070
Acknowledgements
The authors thank Joint Stock Company "Tulagorvodokanal" and personally Pavel Kulakov for providing samples of municipal sewage sludge.
Funding
This research was funded by the Russian Science Foundation Grant No. 22-24-20074 (regional competition), held jointly with the authorities of the subject of the Russian Federation: Tula region.
Author information
Authors and Affiliations
Contributions
L. Perelomov, Y. Atroshchenko and T. Minkina - study conception and design. O. Sizova, M. Gertsen, S. Kozmenko, I. Perelomova - experiment and analysis. L. Perelomov, O. Sizova and V. Rajput - the first draft of the manuscript. I. Perelomova - graphic design. V. Rajput, L. Perelomov, T. Minkina - manuscript editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Tula State Lev Tolstoy Pedagogical University Research Ethics Committee has confirmed that no ethical approval for research is required.
All authors agree with the principles of the Committee on Publication Ethics (COPE). The submitted work is original and is not have been published elsewhere in any form or language (partially or in full).
Consent for publication
All authors have read the contents of the manuscript and agreed to its publication in latest version.
Competing interests
The authors have no relevant financial or non-financial interests that are directly or indirectly related to the work submitted for publication, to disclose.
Additional information
Handling editor: Dr. Sudhakar Srivastava.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perelomov, L., Rajput, V.D., Gertsen, M. et al. Ecological features of trace elements tolerant microbes isolated from sewage sludge of urban wastewater treatment plant. Stress Biology 4, 8 (2024). https://doi.org/10.1007/s44154-023-00144-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-023-00144-8