Abstract
Viral RNAs have been known to contain N6-methyladenosine (m6A) modifications since the 1970s. The function of these modifications remained unknown until the development of genome-wide methods to map m6A residues. Increasing evidence has recently revealed a strong association between m6A modifications and plant viral infection. This highlight introduces advances in the roles of RNA m6A modifications in plant-virus interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Main text
N6-methyladenosine (m6A) is the most pivotal internal modification and is widely present in mRNA, rRNAs, and long non-coding RNA (lncRNA) in eukaryotes (Boccaletto et al. 2022). The modification has shown to be reversible and is catalyzed by methyltransferases (writers), removed by demethylases (erasers), and recognized by m6A binding proteins (readers) (Fu et al. 2014). m6A has been demonstrated to play a vital role in viral infection in mammals. In some cases, m6A is shown to serve as a negative regulator in viral infection (Gokhale et al. 2016; Lichinchi et al. 2016b). Nevertheless, some viruses can also take advantage of this modification for viral enhancement (Kennedy et al. 2016; Lichinchi et al. 2016a), indicating the pivotal role of m6A modification in host-virus interactions. In the meantime, mounting evidence shows that m6A modification also occurs in plant viruses, and its roles in the arms race of plants and viruses have been uncovered in recent work (Fig. 1).
Roles of m6A modifications in plant virus infection. m6A mRNA modification is catalyzed by a conserved m6A methyltransferase complex in plants containing MTA, MTB, FIP37, VIR, HAKAI, and HIZ2. The interactions among m6A components are supported by recent studies. m6A is removed by m6A demethylase, which belongs to the AlkB family and is recognized by ECT (evolutionarily conserved C-terminal regions) proteins in plants. Plant viral RNA undergoes m6A modification during viral infection. The addition of m6A in plant viral mRNAs has different functions in distinct viral life cycles. In some cases, m6A is shown to serve as a negative regulator in viral infection. For example, the m6A demethylase AtALKBH9B in Arabidopsis was found to interact with the envelope protein of alfalfa mosaic virus (AMV) and promote systemic viral invasion. Moreover, the ECT2/ECT3/ECT4/ECT5 module in Arabidopsis reduces AMV resistance, and the increased AMV resistance of alkbh9b mutants can be reverted by mutation of ECT2/ECT3/ECT5. The m6A modifications on PepMV genomic RNA were also found in infected Nicotiana benthamiana and Solanum lycopersium. The m6A writers MTA, HAKAI, and m6A readers NbECT2A/B/C negatively regulate pepino mosaic virus (PepMV) infection. NbECT2A/2B/2C can further mediate the PepMV RNA degradation in the processing body by recruiting RNA-decay-related host factors. However, some viruses acquire m6A modifications in viral RNA to promote viral genomic RNA stability and infection. For example, Triticum aestivum m6A methyltransferase B (TaMTB), a positive regulator for WYMV infection, interacts with wheat yellow mosaic virus (WYMV) NIb to stabilize the viral RNA. MTA, mRNA adenosine methylase A; MTB, mRNA adenosine methylase A; FIP37, FKBP12 Interacting Protein 37; VIR, VIRILIZER; HIZ2, HAKAI-interacting zinc finger protein 2; P-body, processing body; UPF3, up-frameshift protein 3; SMG7, suppressor with morphogenetic effects on genitalia 7
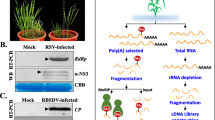
As early as the 1970s, m6A modification was identified in viral RNAs, such as influenza virus (Krug et al. 1976). In the last ten years, with the development of multiple detection technologies, the presence of m6A was reported in the genomic RNA of several plant viruses. Two members of the Bromoviridae family, alfalfa mosaic virus (AMV) and cucumber mosaic virus (CMV) have been reported to contain m6A modifications in the genomic RNAs by the methylated RNA immunoprecipitation sequencing (MeRIP-Seq) (Martínez-Pérez et al. 2017). Furthermore, two of these putative m6A-sites in the 3’-UTR of AMV RNA3 were reported to be involved in viral replication/accumulation and in vivo plus-strand accumulation (Alvarado-Marchena et al. 2022). The m6A distribution patterns on viral genomic RNA of rice black streaked dwarf virus (RBSDV) and rice stripe virus (RSV) were also revealed by Zhang and his colleagues. Clustered m6A peaks in the 5′ terminal of RBSDV genomic S1, S2, S3, S4, S5, S6, S9, and S10 and some discrete peaks in RSV RNA1 to RNA4 were observed (Zhang et al. 2021a). Two and four m6A peaks were significantly enriched in plum pox virus (PPV) and potato virus Y (PVY) genomes by MeRIP-seq (Yue et al. 2022). Four obvious m6A peaks in the coding region of RNA1 and one m6A peak in the 3’ terminal of RNA2 were found in the genomic RNAs of wheat yellow mosaic virus (WYMV). m6A modification occurring on the 6800th A in the WYMV RNA1 was further identified to be involved in the stability of viral CP transcripts (Zhang et al. 2022). The m6A modifications of pepino mosaic virus (PepMV) genomic RNA in infected Nicotiana benthamiana and Solanum lycopersium leaves were also mapped in the viral 3’-terminal in the latest study (He et al. 2023a).
Viral infection has been known to affect host m6A dynamics in mammals (Gokhale et al. 2016; Lichinchi et al. 2016a). Studies of the m6A dynamics in plant-virus interactions have also been revealed in the last three years. With an ultra-high performance liquid chromatography coupled with high-resolution tandem mass spectrometry (UHPLC − HR − MS/MS) method, Li et al. found that levels of m6A in Nicotiana tabacum appear to be decreased by tobacco mosaic virus (TMV) infection, which was in correspondence with the increased mRNA expression of the putative demethylase and decreased putative methyltransferase level after TMV infection (Li et al. 2018). In agreement with this finding, N. benthamiana m6A levels were reduced by infection of PPV and PVY (Yue et al. 2022). On the contrary, Zhang et al. analyzed the high-quality m6A methylomes in rice plants infected with RSV and RBSDV. They found that the m6A modification levels of rice mRNAs were enriched under infection of these two viruses (Zhang et al. 2021a). Interestingly, the m6A levels significantly increased to 1.397-fold in susceptible watermelon plants 24 h after cucumber green mottle mosaic virus (CGMMV) infection but significantly decreased to 0.757-fold at 48 h in resistant watermelons (He et al. 2021). These studies indicate that host m6A levels can be altered by viral infection, which might further affect the gene expression of hosts. CGMMV infection regulated the expression of 59 host cell genes by affecting the deposition of m6A, which involved multiple roles and signaling pathways such as resistance response, secondary biosynthesis and metabolism, and RNA processes. The high-quality m6A methylomes in rice plants infected with RSV and RBSDV were also analyzed, and several antiviral pathway-related genes, such as RNA silencing-, resistance-, and fundamental antiviral phytohormone metabolic-related genes, were m6A methylated upon RSV and RBSDV infection (Zhang et al. 2021a). In addition, transcriptome-wide m6A profiling in WYMV-infected resistant wheat variety and WYMV-infected sensitive wheat variety revealed significant changes in m6A and mRNA levels associated with plant defense responses (Zhang et al. 2021b). These studies deepen our understanding of the significant role of m6A in altering hosts’ physiological and pathological status in the context of viral infection.
In some cases, adding m6A in plant viral RNAs has antiviral function in distinct viral life cycles. Suppression of AtALKBH9B increased the relative abundance of m6A in the AMV genome, impairing the systemic invasion of the plant (Martínez-Pérez et al. 2017). Consistent with the above result, the downregulation of N. benthamiana AlkB homologs of the plant-specific ALKBH9 clade caused a significant decrease in PPV and PVY accumulation (Yue et al. 2022). Furthermore, overexpression of NbMETTL homologs (NbMETTL1 and NbMETTL2) promoted PPV resistance in N. benthamiana (Yue et al. 2023). Similarly, after LsMETTL3 and LsMETTL14, which encode m6A RNA methyltransferase in small brown planthopper (SBPHs), were knocked down, the titer of RBSDV in the midgut cells of SBPHs increased significantly (Tian et al. 2021).
Although m6A methylation plays an anti-viral role in plant viral infection, the underlying molecular mechanisms still need further study to reconcile these differing observations. Notably, the primary mechanism by which m6A exerts its effects is determined by which m6A-binding proteins (m6A readers) are recruited (Meyer and Jaffrey 2017). Recently, Martínez-Pérez et al. found that mutation of the ECT2/ECT3/ECT4/ECT5 module in Arabidopsis reduced AMV resistance and that the increased AMV resistance of alkbh9b mutants could be reverted by deficiencies of ECT2/ECT3/ECT5, indicating that the m6A-reader axis constituted a novel basal antiviral defense layer in plants (Martínez-Pérez et al. 2023). Supporting this conclusion, He et al. also found that the cytoplasmic YTH-domain family proteins NbECT2A/2B/2C could mediate the PepMV RNA degradation in the processing body by recruiting RNA-decay related host factors, including SMG7 and UPF3 proteins, thereby inhibiting virus infection through the RNA decay-related machinery (He et al. 2023a).
However, some viruses have also evolved anti-defense strategies to counterattack the plant defense responses mediated by m6A modification. For example, the PepMV-encoded RNA-dependent RNA polymerase (RdRP) exploits the autophagy pathway by interacting with an autophagy core protein, SlBeclin1, to promote the autophagic degradation of the SlHAKAI protein, thereby inhibiting the m6A modifications-mediated plant defense responses (He et al. 2023b). In addition, some viruses might acquire m6A modifications in viral RNA as a unique mechanism to promote viral genomic RNA stability and infection. A recently characterized susceptibility gene encoding Triticum aestivum m6A methyltransferase B (TaMTB) is identified as a positive regulator for WYMV infection. TaMTB is localized in the nucleus and is translocated into the cytoplasmic viral replication complexes by interacting with WYMV NIb to upregulate the m6A level of WYMV RNA1 and stabilize the viral RNA, thus promoting viral infection (Zhang et al. 2022). Interestingly, several plant viruses have been found to contain AlkB protein homologs or domains belonging to m6A demethylases, indicating that these viruses may exploit this as a novel counter-defense mechanism (Yue et al. 2022).
The work above demonstrates that plant RNA viruses undergo m6A modification during viral infection. Despite much progress, most studies to date focus on the qualitative and quantitative analyses of m6A using mass spectrometry (MS) or MeRIP-seq, which cannot enable absolute quantification of m6A at single-base resolution. Therefore, developing new techniques to map m6A modification with single-base resolution will help further dissect the roles of m6A modification in plant-virus interactions. Considering that the knockout of most m6A methyltransferases resulted in embryonic death, using small molecule inhibitors of m6A methyltransferases might help study the m6A modification in plant and virus interactions. In most cases, m6A modification plays an antiviral role in plant viral infection. However, the specific mechanisms still need further investigation. Of note, m6A is closely related to the alteration of hosts’ physiological and pathological status during plant viral infection. A comprehensive understanding of m6A methylation in plant-virus interactions and the crosstalk between m6A modification and other immunity-related pathways must be further explored. In addition, further studies will be necessary to answer whether m6A methylation occurs in the mRNA of plant DNA viruses.
Availability of data and materials
All the data supporting the claims contained in this manuscript are provided in the submission and can be shared publicly after acceptance of the manuscript for publication by Stress Biology.
Abbreviations
- m6A:
-
N6-methyladenosine
- lncRNA:
-
Long non-coding RNA
- AMV:
-
Alfalfa mosaic virus
- CMV:
-
Cucumber mosaic virus
- MeRIP-Seq:
-
Methylated RNA immunoprecipitation sequencing
- RBSDV:
-
Rice black streaked dwarf virus
- RSV:
-
Rice stripe virus
- PPV:
-
Plum pox virus
- PVY:
-
Potato virus Y
- WYMV:
-
Wheat yellow mosaic virus
- CP:
-
Coat protein
- PepMV:
-
Pepino mosaic virus
- UHPLC-HR-MS/MS:
-
Ultra-high performance liquid chromatography coupled with high-resolution tandem mass spectrometry
- TMV:
-
Tobacco mosaic virus
- CGMMV:
-
Cucumber green mottle mosaic virus
- SBPH:
-
Small brown planthopper
- RdRP:
-
RNA-dependent RNA polymerase
- TaMTB:
-
Triticum aestivum M6A methyltransferase B
- ECT:
-
Evolutionarily conserved C-terminal regions
- MS:
-
Mass spectrometry
References
Alvarado-Marchena L, Martínez-Pérez M, Úbeda JR, Pallas V, Aparicio F (2022) Impact of the potential m6A modification sites at the 3’UTR of alfalfa mosaic virus RNA3 in the viral infection. Viruses 14:1718. https://doi.org/10.3390/v14081718
Boccaletto P, Stefaniak F, Ray A, Cappannini A, Mukherjee S, Purta E, Kurkowska M, Shirvanizadeh N, Destefanis E, Groza P, Avşar G, Romitelli A, Pir P, Dassi E, Conticello SG, Aguilo F, Bujnicki JM (2022) MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res 50:D231–D235. https://doi.org/10.1093/nar/gkab1083
Fu Y, Dominissini D, Rechavi G, He C (2014) Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet 15:293–306. https://doi.org/10.1038/nrg3724
Gokhale NS, McIntyre ABR, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, Hopcraft SE, Quicke KM, Vazquez C, Willer J, Ilkayeva OR, Law BA, Holley CL, Garcia-Blanco MA, Evans MJ, Suthar MS, Bradrick SS, Mason CE, Horner SM (2016) N6-methyladenosine in Flaviviridae viral RNA genomes regulates infection. Cell Host Microbe 20:654–665. https://doi.org/10.1016/j.chom.2016.09.015
He H, Ge L, Chen Y, Zhao S, Li Z, Zhou X, Li F (2023) m6A modification of plant virus enables host recognition by NMD factors in plants. Sci China Life Sci. https://doi.org/10.1007/s11427-022-2377-1
He H, Ge L, Li Z, Zhou X, Li F (2023) Pepino mosaic virus antagonizes plant m6A modification by promoting the autophagic degradation of the m6A writer HAKAI. BaBIOTECH 4:83–96. https://doi.org/10.1007/s42994-023-00097-6
He Y, Li L, Yao Y, Li Y, Zhang H, Fan M (2021) Transcriptome-wide N6-methyladenosine (m6A) methylation in watermelon under CGMMV infection. BMC Plant Biol 21:516. https://doi.org/10.1186/s12870-021-03289-8
Kennedy EM, Bogerd HP, Kornepati AVR, Kang D, Ghoshal D, Marshall JB, Poling BC, Tsai K, Gokhale NS, Horner SM, CμLlen BR (2016) Posttranscriptional m6A editing of HIV-1 mRNAs enhances viral gene expression. Cell Host Microbe 22:830. https://doi.org/10.1016/j.chom.2016.04.002
Krug RM, Morgan MA, Shatkin AJ (1976) Influenza viral mRNA contains internal N6-methyladenosine and 5’-terminal methylguanosine in cap structures. J Virol 20:45–53. https://doi.org/10.1128/JVI.20.1.45-53.1976
Li Z, Shi J, Yu L, Zhao X, Ran L, Hu D, Song B (2018) N6-methyl-adenosine level in Nicotiana tabacum is associated with tobacco mosaic virus. J Virol 15:87. https://doi.org/10.1186/s12985-018-0997-4
Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Y, Mason CE, Rana TM (2016a) Dynamics of the human and viral m6A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol 1:16011. https://doi.org/10.1038/nmicrobiol.2016.11
Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C, Rana TM (2016b) Dynamics of human and viral RNA methylation during zika virus infection. Cell Host Microbe 20:666–673. https://doi.org/10.1016/j.chom.2016.10.002
Martínez-Pérez M, Aparicio F, Arribas-Hernández L, Tankmar MD, Rennie S, von Bülow S, Lindorff-Larsen K, Brodersen P, Pallas V (2023) Plant YTHDF proteins are direct effectors of antiviral immunity against an N6-methyladenosine-containing RNA virus. EMBO J 42:e113378. https://doi.org/10.15252/embj.2022113378
Martínez-Pérez M, Aparicio F, López-Gresa MP, Bellés JM, Sánchez-Navarro JA, Pallás V (2017) Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc Natl Acad Sci USA 114:10755–10760. https://doi.org/10.1073/pnas.1703139114
Meyer KD, Jaffrey SR (2017) Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol 33:319–342. https://doi.org/10.1146/annurev-cellbio-100616-060758
Tian S, Wu N, Zhang L, Wang X (2021) RNA N6-methyladenosine modification suppresses replication of rice black streaked dwarf virus and is associated with virus persistence in its insect vector. Mol Plant Pathol 22:1070–1081. https://doi.org/10.1111/mpp.13097
Yue J, Lu Y, Sun Z, Guo Y, San León D, Pasin F, Zhao M (2023) Methyltransferase-like (METTL) homologues participate in Nicotiana benthamiana antiviral responses. Plant Signal Behav 18:2214760. https://doi.org/10.1080/15592324.2023.2214760
Yue J, Wei Y, Sun Z, Chen Y, Wei X, Wang H, Pasin F, Zhao M (2022) AlkB RNA demethylase homologues and N6-methyladenosine are involved in Potyvirus infection. Mol Plant Pathol 23:1555–1564. https://doi.org/10.1111/mpp.13239
Zhang K, Zhuang X, Dong Z, Xu K, Chen X, Liu F, He Z (2021a) The dynamics of N6-methyladenine RNA modification in interactions between rice and plant viruses. Genome Biol 22:189. https://doi.org/10.1186/s13059-021-02410-2
Zhang T, Shi C, Hu H, Zhang Z, Wang Z, Chen Z, Feng H, Liu P, Guo J, Lu Q, Zhong K, Chen Z, Liu J, Yu J, Chen J, Chen F, Yang J (2022) N6-methyladenosine RNA modification promotes viral genomic RNA stability and infection. Nat Commun 13:6576. https://doi.org/10.1038/s41467-022-34362-x
Zhang T, Wang Z, Hu H, Chen Z, Liu P, Gao S, Zhang F, He L, Jin P, Xu M, Chen J, Yang J (2021b) Transcriptome-wide N6-methyladenosine (m6A) profiling of susceptible and resistant wheat varieties reveals the involvement of variety-specific m6A modification involved in virus-host interaction pathways. Front Microbiol 12:656302. https://doi.org/10.3389/fmicb.2021.656302
Acknowledgements
The authors acknowledge support from the National Key Research and Development Program of China (2021YFD1400400) and the National Natural Science Foundation of China (32320103010 and 31930089).
Funding
The study was funded by the National Key Research and Development Program of China (2021YFD1400400) to FL and the National Natural Science Foundation of China (32320103010 and 31930089) to FL and XZ.
Author information
Authors and Affiliations
Contributions
FL and XZ designed the project. HH, FL, MJ, and JL drafted the manuscript, and FL and XZ revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have given their consent for publication of this manuscript by Stress Biology if accepted.
Competing interests
XZ is an editorial board member but was not involved in the journal’s review of, or any decisions related to, this submission.
Additional information
Handling Editor: Aiming Wang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, H., Jia, M., Liu, J. et al. Roles of RNA m6A modifications in plant-virus interactions. Stress Biology 3, 57 (2023). https://doi.org/10.1007/s44154-023-00133-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-023-00133-x