Abstract
Dairy goats experience metabolic stress during the peripartal period, and their ability to navigate this stage of lactation is related to the occurrence and development of metabolic diseases. Unlike dairy cows, there is a lack of comprehensive analysis of changes in the plasma profiles of peripartal dairy goats, particularly using high-throughput techniques. A subset of 9 clinically-healthy dairy goats were used from a cohort of 96 primiparous Guanzhong dairy goats (BCS, 2.75 ± 0.15). Blood samples were collected at seven time points around parturition (d 21, 14, 7 before parturition, the day of kidding, and d 7, 14, 21 postpartum), were analyzed using untargeted metabolomics and targeted lipidomics. The orthogonal partial least squares discriminant analysis model revealed a total of 31 differential metabolites including p-cresol sulfate, pyruvic acid, cholic acid, and oxoglutaric acid. The pathway enrichment analysis identified phenylalanine metabolism, aminoacyl-tRNA biosynthesis, and citrate cycle as the top three significantly-altered pathways. The Limma package identified a total of 123 differentially expressed lipids. Phosphatidylserine (PS), free fatty acids (FFA), and acylcarnitines (ACs) were significantly increased on the day of kidding, while diacylglycerols (DAG) and triacylglycerols (TAG) decreased. Ceramides (Cer) and lyso-phosphatidylinositols (LPI) were significantly increased during postpartum period, while PS, FFA, and ACs decreased postpartum and gradually returned to antepartum levels. Individual species of FFA and phosphatidylcholines (PC) were segregated based on the differences in the saturation and length of the carbon chain. Overall, this work generated the largest repository of the plasma lipidome and metabolome in dairy goats across the peripartal period, which contributed to our understanding of the multifaceted adaptations of transition dairy goats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Milk production in the dairy goat industry has more than doubled in the last 50 years (Pulina et al. 2018). The metabolic status of dairy goats in the perinatal period is extremely important for milk production and quality (Stelletta et al. 2008; Matthews 2016). Similar to cows, dairy goats experience dramatic changes in energy demands during the transition into lactation and are highly-susceptible to negative energy balance (NEB) (Bell 1995; Simões and Gutiérrez 2017). The metabolic pressure can trigger common metabolic diseases such as hypocalcemia (milk fever), fatty liver syndrome, and ketosis in dairy cows (Adewuyi et al. 2005; McCarthy et al. 2015; Ringseis et al. 2015). Our previous study also detected a sustained state of oxidative stress in dairy goats during the around parturition (Huang et al. 2021). Metabolic diseases that occur in the peripartal period might affect milk production, quality, and animal welfare, all of which delay the resumption of estrous cyclicity, and even milk production in the subsequent lactation (McArt et al. 2012; Ribeiro et al. 2013; Sordillo and Raphael 2013; Raboisson et al. 2014). Thus, peripartal health management programs for dairy goats are critical for animal welfare and economic outcomes.
Although excessive lipolysis is presently recognized as an important factor for developing ketosis and fatty liver, the molecular basis of successful or impaired adaptations to the metabolic challenges in early lactation dairy goats remains incomplete (Ceciliani et al. 2018). In recent years, metabolomics has become a useful tool for understanding the disease pathophysiology and contributing to identify disease biomarkers for use in preventive protocols (Saleem et al. 2012; Hailemariam et al. 2014; Ceciliani et al. 2018; Wang et al. 2020). Untargeted metabolomics involves the qualitative determination of chemical signatures in a biological sample such as blood (Schrimpe-Rutledge et al. 2016). In contrast, targeted metabolomics analyzes specific metabolite clusters associated with certain metabolic pathways such as lipid species. Using untargeted metabolomics, a recent study identified variations in the metabolome, indicated enrichment in pathways such as lipid, glucose (GLU), and nucleotide metabolism after calving along with a decrease in amino acid metabolism (Luo et al. 2019). Some studies have also identified changes in acylcarnitines (ACs), glycerophospholipid, and sphingomyelin in the blood of peripartal dairy cows (Kenez et al. 2016). However, very little is known about metabolome profiles in peripartal dairy goats.
The present study used untargeted metabolomics and targeted lipidomics for analyzing plasma metabolite profiles in peripartal dairy goats at seven crucial time points (d 21, 14, 7 before parturition, on the kidding day, and d 7, 14, 21 postpartum). The objectives of this investigation involved (1) clarifying the metabolic changes in small molecule metabolites during the peripartal period; and (2) identifying potential biomarkers for characterizing new pathways that might be perturbed under metabolic stress.
Results
Plasma biochemical indices
An overview of the experimental design is shown in Fig. 1. Table 1 presents a summary of the biochemical data. Observations indicate significant changes in the plasma biochemical indices (non–esterified fatty acids [NEFA], β-Hydroxybutyrate [BHB], aminotransferase [AST], alanine aminotransferase [ALT], lactate dehydrogenase [LDH], total protein [TP], triglyceride [TG], total cholesterol [TC], urea, P < 0.05) during the peripartal period. Antepartum, plasma NEFA levels showed an increase, followed by a significant decrease postpartum. Plasma BHB levels displayed a significant increase at P-14 d and displayed an upward trend throughout the perinatal period. The goats exhibited low levels of plasma AST and LDH at P-7 d, subsequently reaching maximum levels at P + 7 d. Plasma ALT and TG levels presented a significant decrease at P-7 d with a sustained low level thereafter. Plasma TP levels indicated a decrease at P + 14 d and reached minimum levels at P + 21 d. Plasma TC levels showed a significant decrease at P 0 d and remained low over the observation period. Meanwhile, the plasma urea levels displayed a statistically significant increase at P + 7 d (P < 0.05) and persisted at high levels until the observation period's end.
Overview of the experimental design. Ninety-six multiparous healthy Guanzhong dairy goats (primiparous, Shaanxi, China) of similar age, BCS, and due date were selected. The subset of goats used was selected to include only healthy animals with a gap between expected and real kidding day of two days or less. Subsequently, A subset of 9 clinically-healthy dairy goats were used
Table 1 summarizes the biochemical data. The biochemical indices (NEFA, BHB, AST, ALT, LDH, TP, TG, TC, urea, P < 0.05) in plasma changed significantly during the peripartal period. The plasma NEFA levels was increased antepartum and then rapidly decreased postpartum. The plasma BHB levels increased significantly at P-14 d and maintained an upward trend over the perinatal period. The goats exhibited low plasma AST and LDH levels at P-7 d, and reached maximum levels at P + 7 d. The plasma ALT and TG levels decreased significantly at P-7 d and remained low thereafter. The plasma TP levels decreased at P + 14 d and reached the minimum levels at P + 21 d. The plasma TC levels were found to decrease significantly at P 0 d and remained low thereafter. The plasma urea levels increased significantly at P + 7 d and remained high until the end of the experiment (Fig. 1).
Untargeted metabolomics analysis
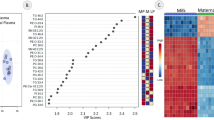
A total of 156 different metabolites were identified and quantified after quality control (QC). The principal component analysis (PCA) score plot (Fig. 2A) shows the distribution of samples from the 7-time points. Three groups before kidding and the other three groups after kidding were clustered together separately with no intersection and were separated from the P 0 d group. The orthogonal partial least squares discriminant analysis (OPLS-DA) analysis and the permutation tests (Fig. 2C and E) showed that based on the OPLS-DA models, the P-21 d and P 0 d groups were discriminated with R2Y = 0.963 and Q2 = 0.925. On the other hand, the P + 21d and P 0 d groups were discriminated with R2Y = 0.948 and Q2 = 0.88. There was a clear separation with no overlap for the OPLS-DA plots of the plasma metabolomics data. The stability and reliability of the OPLS-DA model were confirmed by the satisfactory explanatory and predictive values for the intercepts (R2, Q2) of the permutation testing (Fig. 2D and F).
Metabolite profiles of perinpartal dairy goats: A principal component analysis (PCA) score plot for the seven groups, B Venn diagram analyses for differential metabolites, C and D orthogonal partial least squares discriminant analysis (OPLS-DA) score plot and permutation test plots for P-21 d vs. P 0 d, E and F OPLS-DA score plot and permutation test plots for P 0 d vs. P + 21d. t[1], first principal component. to[2], second orthogonal component. The intercept limit of Q2, calculated by the regression line, is the plot of Q.2 from the permutation test in the OPLS-DA model. P-21 d, P-14 d, P-7 d (d 21,14 and 7 before the due date), P 0 d (the day of kidding), and P + 7 d, P + 14 d, P + 21 d (d 7, 14, and 21postpartum)
The high variable importance in projection (VIP) scores indicated the metabolites contributed greatly to the group separation. Based on the VIP value in the OPLS-DA model (VIP > 1) and P-value in the student’s t-test (P < 0.05), 23 and 20 differential metabolites were identified in P 0 d vs. P-21 d and P 0 d vs. P + 21 d groups, respectively (Table 2 and Fig. 2B). The VIP score plot highlighted the 15 top-scoring metabolites (Fig. 3). Most metabolites were more abundant in the P 0 d group, such as pyruvic acid, cholic acid, oxoglutaric acid, L-acctylcarnitine, indolelactic acid, stearoylcarnitine, oleic acid, compared to P + 21 d and P-21 d group, respectively. There were also changes in the differential metabolites during the peripartal period as demonstrated in Fig. S2 as box plots.
Functional annotation and pathway enrichment
Differentially altered metabolites were analyzed through the KEGG Metabolome Database and MetaboAnalyst. They were marked based on published articles and KEGG analysis (Fig. 4). The relative variation of differentially altered metabolites during the peripartal period was displayed in the form of heat maps in the metabolic network. To gain further insight into the changes in metabolic processes during the peripartal period, the pathway enrichment was analyzed using significantly-altered metabolites (Fig. 5). Here, phenylalanine metabolism, aminoacyl-tRNA biosynthesis, and citrate cycle (TCA cycle) were identified as significant pathways.
Differentially altered metabolites in lipid metabolism, energy metabolism, and amino metabolism pathways. The colors of the cells indicate the relative change during the peripartal period. P-21 d, P-14 d, P-7 d (d 21,14 and 7 before the due date), P 0 d (the day of kidding), and P + 7 d, P + 14 d, P + 21 d (d 7, 14, and 21postpartum)
Targeted lipidomics analysis
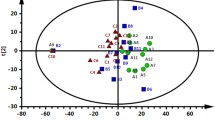
Numerous fatty acids and ACs related to beta-oxidation and mobilization of fat storage were identified as statistically significant. Thus, high-coverage lipidomics was performed for elucidating changes in lipid metabolism during parturition in dairy goats. The lipidomic analysis quantitated 466 lipids spanning 20 lipid classes (Fig. 6A). The TAG (n = 104) followed by PC (n = 89), PE (phosphatidylethanolamines, n = 55) and SM (Sphingomyelins, n = 29) had the highest diversity of individual lipid molecular species. Changes in each lipid subclass during the peripartal period are shown in Fig. S3. The differentially expressed lipids were screened by determining |log2FC|> 1 and FDR < 0.05 from Limma model (Fig. 6C and D). There were 101 and 54 differentially expressed lipids in P 0 d vs. P-21 d and P 0 d vs. P + 21 d groups, respectively (Table 3 and Fig. 6B). Compared to P-21 d, 12 TAG were significantly reduced (log2FC < -2) at P 0 d, while PS 40:5, PS 40:6 and PE 40:6 were significantly increased (log2FC > 2). Compared to P 0 d, there was significant reduction in 4 ACs and 7 PS (log2FC < -2) at P + 21 d, while LPI 18:0, LPI 20:3, Cer d(18:0/25:1), TAG 52:5(16:1), and TAG 56:4(18:1) were significantly increased (log2FC > 2) (Table 4).
Plasma target lipidomic analysis of transition dairy goats: A Percentage of lipid species, B Venn diagram analyses of differentially altered lipids, C Volcano plot showing differentially altered lipids between P-21 d (d 21 before the due date) and. P 0 d (the day of kidding), D Volcano plot showing differentially altered lipids between P 0 d and P + 21d (d 21postpartum). Differential altered lipids (|log2FC|> 2) are highlighted with lipid names. The two dashed vertical lines indicate log2FC of -1 (left) and 1 (right). The dashed horizontal line indicates a false discovery rate (FDR) of 0.05. ACs, acylcarnitine; CE, cholesteryl esters; Cer, ceramides; DAG, diacylglycerols; FFA, free fatty acids; GM3, monosialogangliosides; PA, phosphatidic acids; PC, phosphatidylcholines; LPC, lyso-PC; PE, phosphatidylethanolamines; PG, phosphatidylglycerols; PI, phosphatidylinositols; PS, phosphatidylserines; S1P, sphingosine-1-phosphate; SM, sphingomyelins; TAG, triacylglycerols
Hierarchical clustering heatmap plots for PC and FFA were generated using hierarchical clustering analysis (Fig. 7). PC38:6(18:0/22:6), PC40:6(18:0/22:6), PC40:5(20:1/20:4) were clustered together (Fig. 7A, group2) and did not change significantly in the peripartal period (Fig. 8A). However, the PC species with fatty acyl chains of 20:3 (Fig. 7A, group4) were clustered on the other side and decreased significantly at P 0 d (Fig. 8B). The polyunsaturated fatty acids (PUFA) with more than 20 carbons and other fatty acids were divided into two categories using hierarchical clustering (Fig. 7B, group1, and group2). The PUFA (C20:4, C22:4, C22:5, C22:6) increased at P-7 d, and then declined or remained unchanged at P 0 d (Fig. 8C). The SFA (saturated fatty acid, C16:0, C18:0) and MUFA (monounsaturated fatty acid, C16:1, C18:1) were significantly elevated at P 0 d (Fig. 8D). Lastly, there were interrelationships among the differential metabolites, screened by lipidomics and untargeted metabolomics, and biochemical markers (Fig. S4).
Peripartal changes in phosphatidylcholines (PC) and free fatty acids (FFA) with differences in carbon chain saturation and length: A PC with 22:6 and 20:4 carbon chains, B PC with 22:3 carbon chains, C PUFA, D SFA and MUFA. Mean values with different letters (a–c) show statistically significant differences (LSD, P < 0.05). P-21 d, P-14 d, P-7 d (d 21,14 and 7 before the due date), P 0 d (the day of kidding), and P + 7 d, P + 14 d, P + 21 d (d 7, 14, and 21postpartum)
Discussion
The untargeted metabolomics measurements revealed that metabolic profiles of goats in the antepartum and postpartal periods were different from those on the day of kidding. The resilience to metabolic stress is crucial for determining subsequent health, production, and reproductive performance (Matthews 2016). Thus, the present study aimed to investigate the changes and interactions of various small-molecular-weight metabolites in the plasma of dairy goats during the peripartal period.
Fatty acids derived from adipose tissue lipolysis are oxidized for providing energy primarily through mitochondrial β-oxidation. An increase in fatty acid oxidation induces excessive oxidative stress, and the observed increases in 2-hydroxybutyrate and uric acid reflected an imbalance between prooxidants and antioxidants (Ames et al. 1981; Gall et al. 2010; Fahrmann et al. 2015). Our previous study demonstrated a sustained state of oxidative stress in dairy goats during the peripartal period (Huang et al. 2021). Oxidative stress has the potential to restrict the functioning of α-ketoglutarate dehydrogenase in mitochondria, thus resulting in the accumulation of α-ketoglutarate (Tretter and Adam-Vizi 2005). Uric acid inhibits mitochondrial aconitase activity, but activates ATP citrate lyase (Lanaspa et al. 2012). A reduction in the activity of aconitase inhibits the formation of citric acid, leading to lower citric acid on the day of kidding. As important intermediates of the TCA cycle, changes in α-ketoglutarate and citric acid indicate TCA cycle flow interruption. A study of clinical ketosis in cows demonstrated that defects in TCA cycle flux can aggravate the severity of ketosis (Zhang et al. 2013). Therefore, the increase in the capacity of the TCA cycle is important to prevent energy metabolism disorders in transition dairy goats. Pyruvate not only enters the TCA cycle through the action of pyruvate dehydrogenase, but also generates glucose through the action of pyruvate carboxylase and phosphoenolpyruvate carboxylase (PEPCK). Alterations in the TCA cycle could account for the accumulation of pyruvate, channeling it to the gluconeogenesis pathway (Yoshimi et al. 2016). Greenfield et al. (Greenfield et al. 2000) speculated that an increase in abundance of pyruvate carboxylase and PEPCK mRNA during the early transition period was indicative of an increase in gluconeogenesis. The plasma glucose concentration during lactation was greater compared with late-pregnancy in Saanen goats (Sadjadian et al. 2013). Thus, alterations in the TCA cycle support gluconeogenesis during lactation in dairy goats.
To date, there is very little published information on amino acid metabolism in dairy goats during the transition period. In present sudy, multiple amino acid levels decreased after calving. The decrease in some amino acids (Trp, Tyr, and Val) can profoundly affect the overall rate of protein synthesis and contribute to lower plasma TP especially in the postpartum. Furthermore, some studies reported that fluctuations in the plasma amino acid levels are associated with ketosis in dairy cows (Zhang et al. 2013; Li et al. 2014). Changes in phenylacetylglycine and hippuric acid levels on the phenylalanine metabolic pathway were observed. Both the aromatic amino acids Trp and Tyr were also reduced on the day of kidding. All these observations point to the perturbation of phenylalanine metabolism. Phenylalanine is metabolized to tyrosine by phenylalanine hydroxylase and is involved in synthesizing various hormones (thyroid hormone, melanin, catecholamine, epinephrine, norepinephrine, and dopamine) and glycolipid metabolism (Lemmon and Schlessinger 2010). Deficiencies of Phe and Tyr reportedly impaired immune responses in chickens; Dietary supplementation of amino acids could enhance the immune response (Konashi et al. 2000). Changes in the phenylacetylglycine levels are associated with disorders of phospholipid metabolism (Delaney et al. 2004). The ratio of phenylacetylglycine to hippuric acid can serve as a biomarker for phospholipid disease (Kamiguchi et al. 2017; Malek et al. 2020). The increase in phenylacetylglycine and the decrease in hippuric after parturition in the present study suggested the possibility of altered phospholipid metabolism in dairy goats. Because phospholipids are important lipids involved in the cellular inflammatory response and immune regulation, the observed changes could be important to understand the occurrence and development of inflammation and disease in transition goats.
Indole sulfate (IS) and p-cresol sulfate (PCS) are metabolites of aromatic amino acids originating from protein fermentation in the intestine (Evenepoel et al. 2009; Opdebeeck et al. 2020). In clinical investigations, IS and PCS have been found to induce vascular toxicity followed by upregulation of inflammatory, coagulation, and oxidative stress pathways (Opdebeeck et al. 2020). There is a report that PCS in urine can predict nitrogen intake and efficiency of use (Bertram et al. 2011). Thus, the increase in the IS and PCS in early lactation might indicate a similar involvement for these compounds.
Phosphatidic acids, a central intermediate in synthesizing PS, PC, PE, and PI (Coleman and Mashek 2011), was reduced by nearly 40% on the day of kidding. The quantity of glycerophospholipids particularly PS and PE, which are important components of biofilm, were elevated postpartum (Cole et al. 2012). Multiple studies have shown that lysophospholipid (LPL), one of the phospholipid metabolites, is associated with fatty liver, steatohepatitis, diabetes, obesity, and even cancer (Grzelczyk and Gendaszewska-Darmach 2013). Thus, elevated levels of LPL in this study suggested a similar involvement in the peripartal period. Phosphatidic acid is hydrolyzed by phosphatidic acid phosphatase to DAG, the penultimate step in TAG biosynthesis (Coleman and Mashek 2011). An increased use of PA for synthesis of DAG and TAG might account for the decrease in PA and LPA at the day of kidding.
It is known that the changes of FFA were significant during the peripartal period. However, it was noteworthy that the distinctions have been identified with regards to alterations in SFA and PUFA. Whereas SFA are considered to negatively affect human health (Hu et al. 2001), PUFA are linked to anti-inflammatory events (Calder 2006) as well as improved immune system function (Srednicka-Tober et al. 2016). Supplemental PUFA in goat diets increases the number of follicles and ovulation rate, shortens the estrus cycles, and improves the immune response (Agazzi et al. 2004; Mahla et al. 2017; Stergiadis et al. 2019) Goat milk contains higher levels of PUFA compared to cow milk (Stergiadis et al. 2019). Hence, initiating lactation may be responsible for part of the lowered PUFA at the day of kidding. We observed that oleic acid and linoleic acid levels in the plasma increased significantly at the day of kidding. Consistently, in a study with dairy cows, the oleic acid levels were found to increase at the day of calving and in individuals with subclinical mastitis (Dervishi et al. 2016; Luo et al. 2019). Rukkwamsuk et al. (1999) found that cows on high-energy diet experienced a deeper negative energy balance compared to controls, and also showed a higher proportion of oleic acid in the plasma NEFA after parturition. Thus, dietary supplements rich in PUFA may be a better choice for dairy goats after parturition.
Carnitine is converted to ACs when fatty acids are shuttled into the mitochondria via carnitine palmitoyltransferase-1 (McGarry and Brown 1997). Increased fatty acid oxidation in liver and skeletal muscle is one of the reasons for carnitine's continued decline during the perinatal period (Krajcovicova-Kudlackova et al. 2000; Yang et al. 2019). Correspondingly, the level of ACs will increase (Ismaeel et al. 2019; Yang et al. 2019). We have focused on the long-chain ACs (C16, C17, C18) as it is associated with the increased fatty acid load (Schooneman et al. 2013; McFadden 2020; Schren et al. 2021). Rapidly changing fatty acid and ACs levels reflect the high energy demands during parturition. Neutral lipid depots are mobilized to provide energy for parturition, which perhaps saturates the mitochondrial capacity to cope with the fatty acid surplus and results in the temporal elevation of circulating ACs, dropping to the prenatal level soon after parturition.
Peripartal changes in the circulating NEFA and BHB have been reported in Surti goats and Saanen goats (Sadjadian et al. 2013; Manat et al. 2016; Huang et al. 2021). A rapid drop in NEFA levels at P + 7 d was also observed in dairy goats with a high energy diet (Celi et al. 2008). Circulating ACs and NEFA levels are rapidly reduced postpartum, suggesting that both the production and oxidation of NEFA were reduced postpartum in present study. Thus, increasing the dry matter intake of dairy goats during the peripartal period can effectively reduce the impact of negative energy balance.
Excess fatty acids promote the increase in TAG synthesis in the liver, known as re-esterification. The export of this part of the TAG from the liver depends on very-low-density lipoproteins (VLDL). The ability of the liver to synthesize cholesterol is also significantly increased in early lactation to ensure the VLDL synthesis (Schlegel et al. 2012; Kessler et al. 2014). Cholesterol and TAG synthesized by the liver are secreted into the circulation as VLDL. In ruminants, however, the ability of the liver to assemble and secrete VLDL is inherently lower than that in other mammals, and the accumulation of free fatty acids further impairs VLDL secretion (Bobe et al. 2004). Thus, the reduction in postpartum plasma TAG and CE might be due to restricted hepatic VLDL export and an increase in the clearance of circulating TAG by lipoprotein lipase in the mammary gland (Zang et al. 2019). The low levels of TAG and CE were maintained until the end of the experiment, which reflected the rate of VLDL secretion from liver maintained at lower levels, similar to a study in dairy cows (Van den Top et al. 2005; Kessler et al. 2014). Multiple studies in dairy cows have demonstrated that reduced hepatic TAG output leads to TAG accumulation, especially in cows with ketosis (Gross et al. 2013; Zang et al. 2019; Vogel et al. 2020).
Phosphatidylcholines is a major structural component of the cellular membranes, and is essential for synthesizing VLDL (Yao and Vance 1988; McFadden et al. 2020). Betaine and methionine drive the production of the universal methyl donor S-adenosylmethionine, supporting the production of PC through the phosphatidylethanolamine N-methyltransferase (PEMT) pathway (Pinotti et al. 2002). In addition, PC can be synthesized from choline through the cytidine-5′-diphosphate (CDP) choline pathway (McFadden et al. 2020). In nonruminants, Zeisel (Zeisel 1992) suggested choline deficiency (particularly PC) as the main reason for impaired secretion of VLDL. In some instances, supplementation with dietary methyl donors reduces lipid accumulation in the liver of dairy cows (Zang et al. 2019). Choline is an important methyl donor, existing in mammals in various forms such as acetylcholine, betaine, methionine, phosphatidylcholine, and sphingomyelin. Early lactating cows have a high demand for methyl compounds, but exogenous methyl donor supply is reduced due to lower DMI and extensive rumen degradation (Xue and Snoswell 1986). Hence, ruminants must adapt their metabolism of methyl groups during the transition into lactation. In this study, betaine (a product of choline oxidation) and methionine were observed to be at their highest levels around kidding. Feeding rumen-protected choline and methionine improves metabolic status, reduces oxidative stress, and enhances immune function (Shahsavari et al. 2016; Zhou et al. 2016; Batistel et al. 2018). Moreover, betaine-supplemented diets tend to improve production performance of dairy cows (Monteiro et al. 2017).
Mouse studies show that the CDP-choline pathway is biased toward the use of DAG-rich saturated or monounsaturated fatty acids (i.e., palmitic and oleic acids, respectively) as substrates; In contrast, the PEMT pathway prefers the use of long-chain and very-long-chain rich phosphatidylethanolamines of the PUFA as substrates, including eicosatetraenoic acid and docosahexaenoic acid (DHA) (DeLong et al. 1999). The circulating PC containing DHA has been identified as a biomarker of PEMT pathway activation in humans (da Costa et al. 2011). Our results suggest that inhibition of the CDP-choline pathway might be caused by choline deficiency and a compensatory increase of the PEMT pathway in early lactation. Quantification of VLDL secretion from primary bovine hepatocytes by ELISA showed that the VLDL output increased with choline supplementation (Chandler and White 2017). Cows with severe hepatic steatosis have lower serum PC levels compared to the clinically healthy cows (Imhasly et al. 2014). Thus, considering that the changes in choline metabolism might reflect the state of hepatic lipid metabolism in dairy goats, it can be suggested that dietary choline supplementation might reduce the pressure of hepatic lipid accumulation.
Circulating Cer levels were elevated postpartum, especially Cer(d18:0/25:1). Similar results of increased Cer levels in the transition from gestation to lactation were observed in dairy cows (Rico et al. 2015). Elevated Cer might be associated with hepatic fat accumulation, inflammation, and hydrolysis of SM (Peraldi et al. 1996; Rico et al. 2018). More interestingly, the increase in Cer often coincides with the decrease in insulin sensitivity (Rico et al. 2015). During early lactation, insulin resistance was found to enhance adipose tissue mobilization and promote the preferential allocation of glucose to the mammary gland, thereby increasing the synthesis of milk fat and lactose and milk production (Bell 1995; Zachut et al. 2013; Rico et al. 2015). However, there are no previous studies that have clarified the role of Cer(d18:0/25:1) in peripartal ruminants, limiting our understanding of the bioactivity of Cer(d18:0/25:1).
Materials and methods
Animals and study design
The experiments were conducted in accordance with the university’s guidelines for animal research at the experimental farm of Northwest A&F University (Shaanxi Province, China) in Western China (106°55′57″E, 34°48′41″N) in January–March 2019.
Ninety-six primiparous Guanzhong dairy goats (ranging in age from 1 to 2 years; body weight: 60 ± 5.2 kg; DMI: 1.45 ± 0.05 kg/d; mean ± standard deviation) were used as initial experimental animals. Goats were housed in a shaded open barn under natural lighting conditions and had free access to freshwater. Goats were fed the same diet offered twice daily at 0730 and 1530 ad libitum as a TMR. Diets were formulated to meet nutrient requirements of dairy goats according to the Nutrient Requirements of Small Ruminants (National Research Council, 2007). Ingredients of the diets are listed in Table 5.
Notably, in September, the farm goats included in this study were estrous synchronized such that kidding occurred in February. Blood samples collection was performed every other week from 21 d before kidding (22 to 20 d before the expected kidding date0) to 21 d postpartum. The subset of goats used was selected to include only healthy animals with a gap between expected and real kidding day of two days or less. Additionally, 7 goats were excluded for their litter size being 2 or 3. Finally, the final experimental sample size was 9. Over the period of the trial, the average milk yield per dairy goat was 1.8 ± 0.3 kg/d (mean ± standard deviation). The samples were divided into 7 groups according to sampling time: d 21,14 and 7 before due date (P-21 d, P-14 d, P-7 d), the day of kidding (P 0 d), and d 7, 14, and 21 postpartum (P + 7 d, P + 14 d, P + 21 d).
Blood samples collection
Blood was collected (10 mL) at 0600 h over seven time points (d 21, 14, 7 before and d 7, 14, 21 postpartum). In addition, Sample at P 0 d was collected within 1 h after kidding. Plasma was collected using EDTA as an anticoagulant, then centrifuged at 1,500 × g for 10 min at 4℃, and subsequently stored at − 80 °C until analysis.
Biochemical analyses
The concentrations of GLU, aminotransferase (AST), alanine aminotransferase (ALT), cholinesterase (CHE), γ-glutamyl transpeptidase (GGT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), total protein (TP), albumin (ALB), high-density cholesterol (HDL), low-density cholesterol (LDL), triglyceride (TG), total cholesterol (TC), urea in plasma were determined with a chemical autoanalyzer (Hitachi 7060, Hitachi, Tokyo).
The levels of non–esterified fatty acids (NEFA, kit no. FA115, enzymatic method) and β-hydroxybutyrate (BHB, kit no. RB1007, colorimetric method) were determined with the commercial kits (Randox Laboratories, Crumlin, UK).
Metabolome extraction
Samples were prepared according to a previous method (Song et al. 2020). Briefly, a 50 µL sample was mixed with 200 µL ice-cold 80% methanol in water, and incubated for 30 min at 1500 rpm and 4℃ followed by centrifugation for 10 min at 16,260 × g and 4℃. The supernatant was then removed into a clean 1.5 mL centrifuge tube, and dried using a SpeedVac. The dried extracts were redissolved with 1% acetonitrile in water, and the liquid in the upper layer was collected for liquid chromatography triple quadrupole mass spectrometry (LC–MS) analysis.
Untargeted metabolomics analysis
Untargeted metabolomics was conducted by LipidALL Technologies as previously described (Song et al. 2020). Metabolites were separated on an ACQUITY UPLC HSS T3 1.8 μm, 2.1 × 100 mm column (Waters, Dublin, Ireland) using ultra-performance liquid chromatography (Agilent 1290 II, Agilent Technologies, Germany) and analyzed on a Quadrupole-TOF MS (5600 Triple TOF Plus, AB SCIEX, Singapore). The MS parameters for detection were: ESI source voltage − 4.5 kV; vaporizer temperature, 500℃; drying gas (N2) pressure, 50 psi; nebulizer gas (N2) pressure, 50 psi; curtain gas (N2) pressure, 35 psi; the scan range was m/z 60–800. The information-dependent acquisition mode was used for MS/MS analyses of the metabolites. The collision energy was set at 35 ± 15 eV and the data acquisition and processing were performed using the Analyst® TF 1.7.1 Software (AB Sciex, Concord, ON, Canada). All detected ions were extracted using MarkerView 1.3 (AB Sciex, Concord, ON, Canada) into Excel in the format of a two-dimensional matrix, including mass to charge ratio (m/z), retention time, and peak areas. Then, the isotopic peaks were filtered. The MS/MS data were extracted, and a comparison was performed with the Metabolites database (AB Sciex, Concord, ON, Canada), HMDB, METLIN using the PeakView 2.2 (AB Sciex, Concord, ON, Canada), and the standard references were used for annotating the ion ID.
Targeted lipidomics analysis
Lipids were extracted from the plasma (20 µL) using a modified Bligh and Dyer’s extraction procedure (double rounds of extraction) and dried in a SpeedVac under OH mode (Song et al. 2020). Before analysis, the lipid extracts were resuspended in chloroform: methanol 1:1 (v/v) spiked with appropriate internal standards. The lipidomic analyses was carried out on an Exion UPLC system coupled with a QTRAP 6500 PLUS system (Sciex) under an electrospray ionization mode as described previously unless otherwise stated (Lam et al. 2021). All the quantification experiments were conducted using an internal standard calibration. The lipid types measured include, acylcarnitines (ACs), cholesteryl esters (CE); ceramides (Cer); diacylglycerols (DAG); free fatty acids (FFA); monosialogangliosides (GM3); phosphatidic acids (PA); phosphatidylcholines (PC); lyso-PC (LPC); phosphatidylethanolamines (PE); phosphatidylglycerols (PG); phosphatidylinositols (PI); phosphatidylserines (PS); sphingosine-1-phosphate (S1P); sphingomyelins (SM); triacylglycerols (TAG).
In brief, the polar lipids were separated on a Phenomenex Luna Silica 3 µm column (i.d. 150 × 2.0 mm) using the mobile phase A (chloroform: methanol: ammonium hydroxide, 89.5:10:0.5) and mobile phase B (chloroform: methanol: ammonium hydroxide: water, 55:39:0.5:5.5) at a flow rate of 270 µL/min and column oven temperature at 25℃. The individual polar lipid species were quantified by referencing to spiked internal standards including PC-14:0/14:0,PE14:0/14:0, d31-PS-16:0/18:1, PS-17:0/20:4, PA-17:0/17:0, PG-14:0/14:0,GluCer-d18:1/8:0, Cer-d18:1/17:0, C14:0-BMP, S1P-d17:1, Sph-d17:1,SM-d18:1/12:0, LPC-17:0, LPE-17:1, LPI-17:1, LPA-17:0, LPS-17:1 obtained from Avanti Polar Lipids and PI-8:0/8:0 from Echelon Biosciences, Inc. The monosialogangliosides (GM3) species were quantified using GM3d18:1/18:0-d3 from Matreya LLC.
The glycerol lipids including DAG and TAG were quantified using a modified version of reverse phase HPLC/MRM (Song et al. 2020). Neutral lipids were separated on a Phenomenex Kinetex-C18 2.6 µm column (i.d. 4.6 × 100 mm) using an isocratic mobile phase containing chloroform: methanol:0.1 M ammonium acetate 100:100:4 (v/v/v). Levels of short-, medium-, and long-chain TAGs were calculated with reference to the spiked internal standards of TAG(14:0)3-d5, TAG(16:0)3-d5, and TAG(18:0)3-d5 obtained from the CDN isotopes, respectively. The DAG were quantified using the d5-DAG16:0/16:0 and d5-DAG18:1/18:1as internal standards (Avanti Polar Lipids). The free cholesterols and CE were quantitated with d6-cholesterol and d6-C18:0 cholesteryl ester (CDN isotopes) as the internal standards under atmospheric pressure chemical ionization. Plasma lipid levels are expressed in nanomoles per L (nmol/L).
Data processing and statistical analyses
Data obtained from the biochemical analyses were statistically analyzed using GraphPad Prism 8.0 (GraphPad Software Inc., USA). Changes in the data from the biochemical analysis were analyzed using repeated measures ANOVA followed by Tukey’s multiple comparisons test. The repeated measures on each goat were considered (repeated factor: time during the peripartal period) and the results were expressed as means ± SEM.
Untargeted metabolomics data were pareto-scaled and pattern recognition analysis was performed using the SIMCA-P software (version 14.1, Umetrics, Umea, Sweden), comprising the unsupervised principal component analysis (PCA) and the supervised orthogonal partial least squares discriminant analysis (OPLS-DA). The intra-group aggregation and inter-group separation tendencies were determined using PCA and the inter-group differences were further determined using OPLS-DA. The OPLS-DA models were validated based on the interpretation of variation in Y (R2Y) and the forecast ability based on the model (Q2) in cross-validation and permutation tests by applying 200 iterations. The differential metabolites were screened using variable importance in projection (VIP) scores (VIP > 1) obtained from the OPLS-DA model and P-values (P < 0.05) from the paired t-test. Pathway analyses were performed using MetaboAnalyst 4.0 (http://www.metaboanalyst.ca) and the clustering was further performed using the R heatmap package (version 4.1.3, http://www.R-project.org).
Lipidomics data were analyzed in R using the Limma statistical package. The limma model compared the variation in lipidome within-subjects over time by treating the goats as random effect and estimated the correlation between measurements made on the same goat. The differentially-expressed lipids based on false discovery rate (FDR) and fold-change (FC) were considered statistically significant (FDR < 0.05 and |log2FC|> 1). A volcano plot was used for visualizing the differentially-expressed lipids. The boxplot was generated via the boxplot function in R and the differences was analyzed using R (LSD.test function in the “agricolae” package). A correlation matrix was produced using the corrplot function with the R package. To avoid false positives, correlations with adjusted P < 0.01 were selected. For visual simplicity, only significant correlations were shown.
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author upon request.
Abbreviations
- NEB:
-
Negative energy balance
- GLU:
-
Glucose
- ACs:
-
Acylcarnitines
- AST:
-
Aminotransferase
- ALT:
-
Alanine aminotransferase
- CHE:
-
Cholinesterase
- GGT:
-
γ-Glutamyl transpeptidase
- LDH:
-
Lactate dehydrogenase
- ALP:
-
Alkaline phosphatase
- TP:
-
Total protein
- ALB:
-
Albumin
- HDL:
-
High-density cholesterol
- LDL:
-
Low-density cholesterol
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- NEFA:
-
Non–esterified fatty acids
- BHB:
-
β-Hydroxybutyrate
- CE:
-
Cholesteryl esters
- Cer:
-
Ceramides
- DAG:
-
Diacylglycerols
- FFA:
-
Free fatty acids
- GM3:
-
Monosialogangliosides
- PA:
-
Phosphatidic acids
- PC:
-
Phosphatidylcholines
- LPC:
-
Lyso-PC
- PE:
-
Phosphatidylethanolamines
- PG:
-
Phosphatidylglycerols
- PI:
-
Phosphatidylinositols
- PS:
-
Phosphatidylserines
- S1P:
-
Sphingosine-1-phosphate
- SM:
-
Sphingomyelins
- TAG:
-
Triacylglycerols
- PCA:
-
Principal component analysis
- OPLS-DA:
-
Orthogonal partial least squares discriminant analysis
- VIP:
-
Variable importance in projection
- FDR:
-
False discovery rate
- FC:
-
Fold-change
- PEPCK:
-
Phosphoenolpyruvate carboxylase
- IS:
-
Indole sulfate
- PCS:
-
P-cresol sulfate
- LPL:
-
Lysophospholipid
- VLDL:
-
Very-low-density lipoproteins
- PEMT:
-
Phosphatidylethanolamine N-methyltransferase
- CDP:
-
Cytidine-5′-diphosphate
- DHA:
-
Docosahexaenoic acid
- SFA:
-
Saturated fatty acid
- MUFA:
-
Monounsaturated fatty acid
- PUFA:
-
Polyunsaturated fatty acids
References
Adewuyi AA, Gruys E, van Eerdenburg FJ (2005) Non esterified fatty acids (NEFA) in dairy cattle. A Review Vet Q 27(3):117–126. https://doi.org/10.1080/01652176.2005.9695192
Agazzi A, Cattaneo D, Dell’Orto V, Moroni P, Bonizzi L, Pasotto D, Bronzo V, Savoini G (2004) Effect of administration of fish oil on aspects of cell-mediated immune response in periparturient dairy goats. Small Ruminant Res 55(1–3):77–83. https://doi.org/10.1016/j.smallrumres.2004.02.007
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci U S A 78(11):6858–6862. https://doi.org/10.1073/pans.78.11.6858
Batistel F, Arroyo JM, Garces CIM, Trevisi E, Parys C, Ballou MA, Cardoso FC, Loor JJ (2018) Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J Dairy Sci 101(1):480–490. https://doi.org/10.3168/jds.2017-13185
Bell AW (1995) Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci 73(9):2804–2819. https://doi.org/10.2527/1995.7392804x
Bertram HC, Yde CC, Zhang X, Kristensen NB (2011) Effect of dietary nitrogen content on the urine metabolite profile of dairy cows assessed by nuclear magnetic resonance (NMR)-based metabolomics. J Agric Food Chem 59(23):12499–12505. https://doi.org/10.1021/jf204201f
Bobe G, Young JW, Beitz DC (2004) Invited review: pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J Dairy Sci 87(10):3105–3124. https://doi.org/10.3168/jds.S0022-0302(04)73446-3
Calder PC (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83(6 Suppl):1505S-1519S. https://doi.org/10.1093/ajcn/83.6.1505S
Ceciliani F, Lecchi C, Urh C, Sauerwein H (2018) Proteomics and metabolomics characterizing the pathophysiology of adaptive reactions to the metabolic challenges during the transition from late pregnancy to early lactation in dairy cows. J Proteomics 178:92–106. https://doi.org/10.1016/j.jprot.2017.10.010
Celi P, Di Trana A, Claps S (2008) Effects of perinatal nutrition on lactational performance, metabolic and hormonal profiles of dairy goats and respective kids. Small Ruminant Res 79(2–3):129–136. https://doi.org/10.1016/j.smallrumres.2008.07.010
Chandler TL, White HM (2017) Choline and methionine differentially alter methyl carbon metabolism in bovine neonatal hepatocytes. PLoS One 12(2):e0171080. https://doi.org/10.1371/journal.pone.0171080
Cole LK, Vance JE, Vance DE (2012) Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta 1821(5):754–761. https://doi.org/10.1016/j.bbalip.2011.09.009
Coleman RA, Mashek DG (2011) Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem Rev 111(10):6359–6386. https://doi.org/10.1021/cr100404w
da Costa KA, Sanders LM, Fischer LM, Zeisel SH (2011) Docosahexaenoic acid in plasma phosphatidylcholine may be a potential marker for in vivo phosphatidylethanolamine N-methyltransferase activity in humans. Am J Clin Nutr 93(5):968–974. https://doi.org/10.3945/ajcn.110.011064
Delaney J, Neville WA, Swain A, Miles A, Leonard MS, Waterfield CJ (2004) Phenylacetylglycine, a putative biomarker of phospholipidosis: its origins and relevance to phospholipid accumulation using amiodarone treated rats as a model. Biomarkers 9(3):271–290. https://doi.org/10.1080/13547500400018570
DeLong CJ, Shen YJ, Thomas MJ, Cui Z (1999) Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 274(42):29683–29688. https://doi.org/10.1074/jbc.274.42.29683
Dervishi E, Zhang G, Dunn SM, Mandal R, Wishart DS, Ametaj BN (2016) GC-MS metabolomics identifies metabolite alterations that precede subclinical mastitis in the blood of transition dairy cows. J Proteome Res 16(2):433–446. https://doi.org/10.1021/acs.jproteome.6b00538
Evenepoel P, Meijers BK, Bammens BR, Verbeke K (2009) Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 114:S12-19. https://doi.org/10.1038/ki.2009.402
Fahrmann J, Grapov D, Yang J, Hammock B, Fiehn O, Bell GI, Hara M (2015) Systemic alterations in the metabolome of diabetic NOD mice delineate increased oxidative stress accompanied by reduced inflammation and hypertriglyceremia. Am J Physiol Endocrinol Metab 308(11):E978-989. https://doi.org/10.1152/ajpendo.00019.2015
Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E, Group RS (2010) Alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5(5):e10883. https://doi.org/10.1371/journal.pone.0010883
Greenfield RB, Cecava MJ, Donkin SS (2000) Changes in mRNA expression for gluconeogenic enzymes in liver of dairy cattle during the transition to lactation. J Dairy Sci 83(6):1228–1236. https://doi.org/10.3168/jds.S0022-0302(00)74989-7
Gross JJ, Schwarz FJ, Eder K, van Dorland HA, Bruckmaier RM (2013) Liver fat content and lipid metabolism in dairy cows during early lactation and during a mid-lactation feed restriction. J Dairy Sci 96(8):5008–5017. https://doi.org/10.3168/jds.2012-6245
Grzelczyk A, Gendaszewska-Darmach E (2013) Novel bioactive glycerol-based lysophospholipids: New data - New insight into their function. Biochimie 95(4):667–679. https://doi.org/10.1016/j.biochi.2012.10.009
Hailemariam D, Mandal R, Saleem F, Dunn SM, Wishart DS, Ametaj BN (2014) Identification of predictive biomarkers of disease state in transition dairy cows. J Dairy Sci 97(5):2680–2693. https://doi.org/10.3168/jds.2013-6803
Hu FB, Manson JE, Willett WC (2001) Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 20(1):5–19. https://doi.org/10.1080/07315724.2001.10719008
Huang Y, Wen J, Kong Y, Zhao C, Liu S, Liu Y, Li L, Yang J, Zhu X, Zhao B, Cao B, Wang J (2021) Oxidative status in dairy goats: periparturient variation and changes in subclinical hyperketonemia and hypocalcemia. BMC Vet Res 17(1):238. https://doi.org/10.1186/s12917-021-02947-1
Imhasly S, Naegeli H, Baumann S, von Bergen M, Luch A, Jungnickel H, Potratz S, Gerspach C (2014) Metabolomic biomarkers correlating with hepatic lipidosis in dairy cows. BMC Vet Res 10:122. https://doi.org/10.1186/1746-6148-10-122
Ismaeel A, Franco ME, Lavado R, Papoutsi E, Casale GP, Fuglestad M, Mietus CJ, Haynatzki GR, Smith RS, Bohannon WT, Sawicki I, Pipinos II, Koutakis P (2019) Altered metabolomic profile in patients with peripheral artery disease. J Clin Med 8(9):1463. https://doi.org/10.3390/jcm8091463
Kamiguchi H, Murabayashi M, Mori I, Horinouchi A, Higaki K (2017) Biomarker discovery for drug-induced phospholipidosis: phenylacetylglycine to hippuric acid ratio in urine and plasma as potential markers. Biomarkers 22(2):178–188. https://doi.org/10.1080/1354750x.2016.1252958
Kenez A, Danicke S, Rolle-Kampczyk U, von Bergen M, Huber K (2016) A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics 12(11):165. https://doi.org/10.1007/s11306-016-1112-8
Kessler EC, Gross JJ, Bruckmaier RM, Albrecht C (2014) Cholesterol metabolism, transport, and hepatic regulation in dairy cows during transition and early lactation. J Dairy Sci 97(9):5481–5490. https://doi.org/10.3168/jds.2014-7926
Konashi S, Takahashi K, Akiba Y (2000) Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Brit J Nutr 83(4):449–456. https://doi.org/10.1017/S0007114500000556
Krajcovicova-Kudlackova M, Simoncic R, Bederova A, Babinska K, Beder I (2000) Correlation of carnitine levels to methionine and lysine intake. Physiol Res 49(3):399–402
Lam SM, Zhang C, Wang Z, Ni Z, Zhang S, Yang S, Huang X, Mo L, Li J, Lee B, Mei M, Huang L, Shi M, Xu Z, Meng FP, Cao WJ, Zhou MJ, Shi L, Chua GH, Li B, Cao J, Wang J, Bao S, Wang Y, Song JW, Zhang F, Wang FS, Shui G (2021) A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat Metab 3(7):909–922. https://doi.org/10.1038/s42255-021-00425-4
Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ (2012) Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem 287(48):40732–40744. https://doi.org/10.1074/jbc.M112.399899
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141(7):1117–1134. https://doi.org/10.1016/j.cell.2010.06.011
Li Y, Xu C, Xia C, Zhang H, Sun L, Gao Y (2014) Plasma metabolic profiling of dairy cows affected with clinical ketosis using LC/MS technology. Vet Q 34(3):152–158. https://doi.org/10.1080/01652176.2014.962116
Luo ZZ, Shen LH, Jiang J, Huang YX, Bai LP, Yu SM, Yao XP, Ren ZH, Yang YX, Cao SZ (2019) Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J Dairy Sci 102(5):4639–4650. https://doi.org/10.3168/jds.2018-15601
Mahla AS, Chaudhari RK, Verma AK, Singh AK, Singh SK, Singh G, Sarkar M, Dutta N, Kumar H, Krishnaswamy N (2017) Effect of dietary supplementation of omega-3 polyunsaturated fatty acid (PUFA) rich fish oil on reproductive performance of the goat (Capra hircus). Theriogenology 99:79–89. https://doi.org/10.1016/j.theriogenology.2017.05.023
Malek MR, Ahmadian S, Dehpour AR, Ebrahim-Habibi A, Shafizadeh M, Kashani-Amin E (2020) Investigating the role of endogenous opioid system in chloroquine-induced phospholipidosis in rat liver by morphological, biochemical and molecular modelling studies. Clin Exp Pharmacol Physiol 47(9):1575–1583. https://doi.org/10.1111/1440-1681.13332
Manat TD, Chaudhary SS, Singh VK, Patel SB, Puri G (2016) Hematobiochemical profile in Surti goats during post-partum period. Vet World 9(1):19–24. https://doi.org/10.14202/vetworld.2016.19-24
Matthews JG (2016) The periparturient goat. In: John W, Sons L (eds) Diseases of the Goat, Chichester, UK. pp 41–60
McArt JAA, Nydam DV, Oetzel GR (2012) Epidemiology of subclinical ketosis in early lactation dairy cattle. J Dairy Sci 95(9):5056–5066. https://doi.org/10.3168/jds.2012-5443
McCarthy MM, Mann S, Nydam DV, Overton TR, McArt JA (2015) Short communication: Concentrations of nonesterified fatty acids and beta-hydroxybutyrate in dairy cows are not well correlated during the transition period. J Dairy Sci 98(9):6284–6290. https://doi.org/10.3168/jds.2015-9446
McFadden JW (2020) Review: Lipid biology in the periparturient dairy cow: contemporary perspectives. Animal 14(S1):s165–s175. https://doi.org/10.1017/S1751731119003185
McFadden JW, Girard CL, Tao S, Zhou Z, Bernard JK, Duplessis M, White HM (2020) Symposium review: One-carbon metabolism and methyl donor nutrition in the dairy cow. J Dairy Sci 103(6):5668–5683. https://doi.org/10.3168/jds.2019-17319
McGarry JD, Brown NF (1997) The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244(1):1–14. https://doi.org/10.1111/j.1432-1033.1997.00001.x
Monteiro APA, Bernard JK, Guo JR, Weng XS, Emanuele S, Davis R, Dahl GE, Tao S (2017) Effects of feeding betaine-containing liquid supplement to transition dairy cows. J Dairy Sci 100(2):1063–1071. https://doi.org/10.3168/jds.2016-11452
NRC (2007) National Research Council (NRC) Nutrient Requirements of Small Ruminants National Academic Press, Washington, DC
Opdebeeck B, D’Haese PC, Verhulst AJT (2020) Molecular and Cellular Mechanisms That Induce Arterial Calcification by Indoxyl Sulfate and P-Cresyl Sulfate. Toxins (Basel) 12(1):58. https://doi.org/10.3390/toxins12010058
Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM (1996) Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 271(22):13018–13022. https://doi.org/10.1074/jbc.271.22.13018
Pinotti L, Baldi A, Dell’Orto V (2002) Comparative mammalian choline metabolism with emphasis on the high-yielding dairy cow. Nutr Res Rev 15(2):315–332. https://doi.org/10.1079/NRR200247
Pulina G, Milan MJ, Lavin MP, Theodoridis A, Morin E, Capote J, Thomas DL, Francesconi AHD, Caja G (2018) Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J Dairy Sci 101(8):6715–6729. https://doi.org/10.3168/jds.2017-14015
Raboisson D, Mounie M, Maigne E (2014) Diseases, reproductive performance, and changes in milk production associated with subclinical ketosis in dairy cows: a meta-analysis and review. J Dairy Sci 97(12):7547–7563. https://doi.org/10.3168/jds.2014-8237
Ribeiro ES, Lima FS, Greco LF, Bisinotto RS, Monteiro AP, Favoreto M, Ayres H, Marsola RS, Martinez N, Thatcher WW, Santos JE (2013) Prevalence of periparturient diseases and effects on fertility of seasonally calving grazing dairy cows supplemented with concentrates. J Dairy Sci 96(9):5682–5697. https://doi.org/10.3168/jds.2012-6335
Rico JE, Bandaru VV, Dorskind JM, Haughey NJ, McFadden JW (2015) Plasma ceramides are elevated in overweight Holstein dairy cows experiencing greater lipolysis and insulin resistance during the transition from late pregnancy to early lactation. J Dairy Sci 98(11):7757–7770. https://doi.org/10.3168/jds.2015-9519
Rico JE, Zang Y, Haughey NJ, Rius AG, McFadden JW (2018) Short communication: Circulating fatty acylcarnitines are elevated in overweight periparturient dairy cows in association with sphingolipid biomarkers of insulin resistance. J Dairy Sci 101(1):812–819. https://doi.org/10.3168/jds.2017-13171
Ringseis R, Gessner DK, Eder K (2015) Molecular insights into the mechanisms of liver-associated diseases in early-lactating dairy cows: hypothetical role of endoplasmic reticulum stress. J Anim Physiol an N 99(4):626–645. https://doi.org/10.1111/jpn.12263
Rukkwamsuk T, Kruip TA, Meijer GA, Wensing T (1999) Hepatic fatty acid composition in periparturient dairy cows with fatty liver induced by intake of a high energy diet in the dry period. J Dairy Sci 82(2):280–287. https://doi.org/10.3168/jds.S0022-0302(99)75234-3
Sadjadian R, Seifi HA, Mohri M (2013) Variations of energy biochemical metabolites in periparturient dairy Saanen goats. Comp Clin Pathol 22(3):449–456. https://doi.org/10.1007/s00580-012-1431-8
Saleem F, Ametaj BN, Bouatra S, Mandal R, Zebeli Q, Dunn SM, Wishart DS (2012) A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J Dairy Sci 95(11):6606–6623. https://doi.org/10.3168/jds.2012-5403
Schlegel G, Ringseis R, Keller J, Schwarz FJ, Eder K (2012) Changes in the expression of hepatic genes involved in cholesterol homeostasis in dairy cows in the transition period and at different stages of lactation. J Dairy Sci 95(7):3826–3836. https://doi.org/10.3168/jds.2011-5221
Schooneman MG, Vaz FM, Houten SM, Soeters MR (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62(1):1–8. https://doi.org/10.2337/db12-0466
Schren M, Snedec T, Riefke B, Slopianka M, Keck M, Gruendemann S, Wichard J, Brunner N, Klein S, Theinert KB (2021) Aspects of transition cow metabolomics—Part I: Effects of a metaphylactic butaphosphan and cyanocobalamin treatment on the metabolome in liver, blood, and urine in cows with different liver metabotypes. J Dairy Sci 104(8):9205–9226. https://doi.org/10.3168/jds.2020-19055
Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA (2016) Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom 27(12):1897–1905. https://doi.org/10.1007/s13361-016-1469-y
Shahsavari A, D’Occhio MJ, Al Jassim R (2016) The role of rumen-protected choline in hepatic function and performance of transition dairy cows. Brit J Nutr 116(1):35–44. https://doi.org/10.1017/S0007114516001641
Simões J, Gutiérrez C (2017) Nutritional and Metabolic Disorders in Dairy Goats. In: Simões J, Gutiérrez C (eds) Sustainable Goat Production in Adverse Environments. Springer International Publishing, Chan, pp 177–194
Song JW, Lam SM, Fan X, Cao WJ, Wang SY, Tian H, Chua GH, Zhang C, Meng FP, Xu Z, Fu JL, Huang L, Xia P, Yang T, Zhang S, Li B, Jiang TJ, Wang R, Wang Z, Shi M, Zhang JY, Wang FS, Shui G (2020) Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab 32(2):188-202 e185. https://doi.org/10.1016/j.cmet.2020.06.016
Sordillo LM, Raphael W (2013) Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet Clin North Am Food Anim Pract 29(2):267–278. https://doi.org/10.1016/j.cvfa.2013.03.002
Srednicka-Tober D, Baranski M, Seal CJ, Sanderson R, Benbrook C, Steinshamn H, Gromadzka-Ostrowska J, Rembialkowska E, Skwarlo-Sonta K, Eyre M, Cozzi G, Larsen MK, Jordon T, Niggli U, Sakowski T, Calder PC, Burdge GC, Sotiraki S, Stefanakis A, Stergiadis S, Yolcu H, Chatzidimitriou E, Butler G, Stewart G, Leifert C (2016) Higher PUFA and n-3 PUFA, conjugated linoleic acid, alpha-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: a systematic literature review and meta- and redundancy analyses. Brit J Nutr 115(6):1043–1060. https://doi.org/10.1017/S0007114516000349
Stelletta C, Gianesella M, Morgante M (2008) Metabolic and nutritional diseases. In: Cannas A, Pulina G (eds) Dairy goats feeding and nutrition. Bologna, Italy. pp 263–288
Stergiadis S, Norskov NP, Purup S, Givens I, Lee MRF (2019) Comparative nutrient profiling of retail goat and cow milk. Nutrients 11(10):2282. https://doi.org/10.3390/nu11102282
Tretter L, Adam-Vizi V (2005) Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci 360(1464):2335–2345. https://doi.org/10.1098/rstb.2005.1764
Van den Top AM, Van Tol A, Jansen H, Geelen MJ, Beynen AC (2005) Fatty liver in dairy cows post partum is associated with decreased concentration of plasma triacylglycerols and decreased activity of lipoprotein lipase in adipocytes. J Dairy Res 72(2):129–137. https://doi.org/10.1017/S0022029905000774
Vogel L, Gnott M, Kroger-Koch C, Dannenberger D, Tuchscherer A, Troscher A, Kienberger H, Rychlik M, Starke A, Bachmann L, Hammon HM (2020) Effects of abomasal infusion of essential fatty acids together with conjugated linoleic acid in late and early lactation on performance, milk and body composition, and plasma metabolites in dairy cows. J Dairy Sci 103(8):7431–7450. https://doi.org/10.3168/jds.2019-18065
Wang R, Li B, Lam SM, Shui G (2020) Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J Genet Genomics 47(2):69–83. https://doi.org/10.1016/j.jgg.2019.11.009
Xue GP, Snoswell AM (1986) Developmental changes in the activities of enzymes related to methyl group metabolism in sheep tissues. Comp Biochem Physiol B 83(1):115–120. https://doi.org/10.1016/0305-0491(86)90340-8
Yang Y, Sadri H, Prehn C, Adamski J, Rehage J, Danicke S, Saremi B, Sauerwein H (2019) Acylcarnitine profiles in serum and muscle of dairy cows receiving conjugated linoleic acids or a control fat supplement during early lactation. J Dairy Sci 102(1):754–767. https://doi.org/10.3168/jds.2018-14685
Yao ZM, Vance DE (1988) The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem 263(6):2998–3004. https://doi.org/10.1016/S0021-9258(18)69166-5
Yoshimi N, Futamura T, Kakumoto K, Salehi AM, Sellgren CM, Holmen-Larsson J, Jakobsson J, Palsson E, Landen M, Hashimoto K (2016) Blood metabolomics analysis identifies abnormalities in the citric acid cycle, urea cycle, and amino acid metabolism in bipolar disorder. BBA Clinical 5:151–158. https://doi.org/10.1016/j.bbacli.2016.03.008
Zachut M, Honig H, Striem S, Zick Y, Boura-Halfon S, Moallem U (2013) Periparturient dairy cows do not exhibit hepatic insulin resistance, yet adipose-specific insulin resistance occurs in cows prone to high weight loss. J Dairy Sci 96(9):5656–5669. https://doi.org/10.3168/jds.2012-6142
Zang Y, Samii SS, Myers WA, Bailey HR, Davis AN, Grilli E, McFadden JW (2019) Methyl donor supplementation suppresses the progression of liver lipid accumulation while modifying the plasma triacylglycerol lipidome in periparturient Holstein dairy cows. J Dairy Sci 102(2):1224–1236. https://doi.org/10.3168/jds.2018-14727
Zeisel SH (1992) Choline: an important nutrient in brain development, liver function and carcinogenesis. J Am Coll Nutr 11(5):473–481. https://doi.org/10.1080/07315724.1992.10718251
Zhang HY, Wu L, Xu C, Xia C, Sun LW, Shu S (2013) Plasma metabolomic profiling of dairy cows affected with ketosis using gas chromatography/mass spectrometry. BMC Vet Res 26(9):186. https://doi.org/10.1186/1746-6148-9-186
Zhou Z, Bulgari O, Vailati-Riboni M, Trevisi E, Ballou MA, Cardoso FC, Luchini DN, Loor JJ (2016) Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J Dairy Sci 99(11):8956–8969. https://doi.org/10.3168/jds.2016-10986
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 32273085, 32102742).
Author information
Authors and Affiliations
Contributions
Yan Huang: Software, Validation, Formal analysis, Writing-original draft. Yezi Kong: Software, Formal analysis, Investigation. Bowen Li: Software, Investigation. Chenxu Zhao: Writing-review & editing. Juan J. Loor: Writing-review & editing. Panpan Tan: Conceptualization, Validation. Yang Yuan: Software. Fangyuan Zeng: Software. Xiaoyan Zhu: Conceptualization. Simeng Qi: Software. Baoyu Zhao: Conceptualization. Jianguo Wang: Resources, Writing-review & editing, Supervision, Project administration, Funding acquisition. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures described were approved by the Animal Ethics and Welfare Committee of the Northwest A&F University (Yangling, Shaanxi, Chin).
Competing interests
The authors declare no conflict of interest.
Additional information
Handling editor: Meng-Hua Li.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Metabolite profiles of peripartal dairy goats: (A and B) orthogonal partial least squares discriminant analysis (OPLS-DA) score plot and permutation test plots for P-14 d vs. P 0 d, (C and D) OPLS-DA score plot and permutation test plots for P-7 d vs. P 0 d. P 0 d, (E and F) OPLS-DA score plot and permutation test plots for P 0 d vs. P+7d. P 0 d, (G and H) OPLS-DA score plot and permutation test plots for P 0 d vs. P+14d. t[1] = first principal component. to[2] = second orthogonal component. The intercept limit of Q2, calculated by the regression line, is the plot of Q2 from the permutation test in the OPLS-DA model. P-14 d, P-7 d (d 14 and 7 before the due date), P 0 d (the day of kidding), and P+7 d, P+14 d (d 7 and 14 postpartum). Fig. S2. Box-plot (middle bar = median, box limit = upper and lower quartile, extremes = Min and Max values) depicting the peripartal changes in differentially altered metabolites detected via non-targeted metabolomics. P-21 d, P-14 d, P-7 d (d 21,14 and 7 before the due date), P 0 d (the day of kidding), and P+7 d, P+14 d, P+21 d (d 7, 14, and 21postpartum). Mean values with different letters (a–d) show statistically significant differences based on least significant difference (LSD) (P < 0.05). Fig. S3. Line graph depicting the peripartal changes for all lipid species levels in dairy goats. P-21 d, P-14 d, P-7 d (d 21,14 and 7 before the due date), P 0 d (the day of kidding), and P+7 d, P+14 d, P+21 d (d 7, 14, and 21postpartum). Results are expressed as means ± SEM. ACs = acylcarnitine; CE = cholesteryl esters; Cer = ceramides; DAG = diacylglycerols; FFA = free fatty acids; GM3 = monosialogangliosides; PA = phosphatidic acids; PC = phosphatidylcholines; LPC = lyso-PC; PE = phosphatidylethanolamines; PG = phosphatidylglycerols; PI = phosphatidylinositols; PS = phosphatidylserines; S1P = sphingosine-1-phosphate; SM = sphingomyelins; TAG = triacylglycerols. Fig. S4. Correlations between plasma biochemical indices, differentially altered metabolites, and lipid levels at all time points combined. The left margin shows a dendrogram from hierarchical cluster analysis by which rows and columns are ordered.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Y., Kong, Y., Li, B. et al. Effects of perinatal stress on the metabolites and lipids in plasma of dairy goats. Stress Biology 3, 11 (2023). https://doi.org/10.1007/s44154-023-00088-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-023-00088-z