Abstract
Helicobacter pylori is a widespread bacterium that has effectively colonized half of the global population, with Africa having over 70% of the total burden of H. pylori infections (HPI). Considering its acknowledged classification of as bacterial carcinogens and their significant contribution to the development of gastrointestinal disorders such as gastritis, peptic ulcers, and gastric neoplasia, together with their growing resistance to antibiotics. Gaining insight into the etiology of this organism is crucial in order to investigate and develop appropriate treatment strategies. Furthermore, the rise of bacteria that are resistant to antibiotics presents an extra danger in managing this detrimental bacterium. Our review focuses on investigating the presence of H. pylori in Africa and analyzing the various factors that contribute to its extensive prevalence. We simplified the complex mechanisms that H. pylori utilizes to flourish in the human body, with a specific emphasis on its virulence factors and antibiotic resistance. These variables pose significant challenges to conventional treatment strategies. In addition, we analyze both conventional and developing diagnostic methods, as well as the current treatment approaches implemented in various African nations. In addition, we tackle the distinct healthcare obstacles of the region and put-up practical remedies. The main goal of this review is to improve the formulation of more efficient methods for the management and treatment of HPI in Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Helicobacter pylori (H. pylori) is a bacterium with a flagellum, which is very invasive and has a coccoid form. It is primarily found in the stomach and is Gram-negative. This pathogenic microorganism is widely distributed, as it is the primary infectious agent accountable for gastrointestinal illnesses. Nearly half of the world populace, which amounts to over 3 billion humans, is documented to have H. pylori infection (HPI). Furthermore, Africa has the largest prevalence, constituting approximately 70.1% (nearly 2.1 billion) of the worldwide burden of HPI. Africa bears a significant burden of infectivity in comparison to other continents, with children in their early years being the most usually affected demographic [1]. In general, individuals, both children and adults, in undeveloped nations, particularly in Africa, have a greater occurrence of infection in comparison to individuals in developed nations. These issues, including inadequate hygiene practices, insufficient environmental sanitation, and limited availability of clean drinking water, are responsible for this situation in impoverished nations. Although it is known that transmission between people can occur through the oral-fecal routes, the specific mode of transmission is yet unknown [2].
Prior studies have confirmed a clear link between the incidence of H. pylori bacteria and the occurrence of several gastrointestinal conditions, such as gastric ulcers, mucosal lymphoma, and gastric adenocarcinoma. Recent research has revealed substantial evidence linking H. pylori to other disorders outside of the stomach, particularly in the neurological, cardiovascular, and immune systems. These disorders encompass coronary atherosclerosis, dementia, nonalcoholic steatosis, Alzheimer’s disease, iron deficiency anemia, Guillain–Barre syndrome and Parkinson’s disease. Long-lasting and protracted infection with H. pylori can lead to the formation of cancer of the stomach. Indeed, the World Health Organization (WHO) has categorized H. pylori as a Group 1 cancer-causing agent based on compelling evidence that establishes its involvement in the development of stomach cancer [3, 4]. Various factors define the persistence of HPI and its severe clinical effects. The components that are involved include the severity of the bacterial strain, the vulnerability of the infected host, additional ecological factors, the method utilized for diagnosis, and the treatment choices that are accessible.
Epidemiological studies have provided evidence indicating that this bacterium is prevalent among the majority of individuals residing in underdeveloped nations of Africa, with about half of the global population being affected. In less developed nations, the prevalence of this virus exceeds 80% of the population, with children being the most vulnerable. On the other hand, in industrialized countries, the illness is often less than 40% common, especially among children and teenagers compared to adults and older people [6]. On a continental level in Africa, this infection has caused substantial sickness and death, with stomach adenocarcinoma being the primary cause of mortality. According to multiple meta-analyses, people who have H. pylori infection are 2 to 3 times more expected to develop gastric cancer in comparison to individuals without the infection [6]. The correlation between lifestyle and HPI is not yet apparent, but the socio-economic conditions of each community significantly impact the severity of the infection. People in developing countries are at the greatest risk because of their fragile socioeconomic conditions, which include filthy settings, population growth, consumption of undercooked or inadequately cooked foods, and dependence on contaminated water supplies [7].
In Africa, it is widely known and commonly said that "prevention is preferable to treatment". This assertion is characterized by both logical and scientific reasoning. Hence, the elimination of H. pylori could potentially prevent the colonization and formation of lesions in the gastrointestinal tract, slow down the progression of stomach cancer, and lessen the chances of gastric ulcers and gastric malignancies recurring [8]. Despite significant efforts, there is a clear decrease in the rate of eradication and less than ideal outcomes associated with standard antibiotic therapy. Several studies have recorded a high rate of treatment failure, specifically 40% in children and 38% in adults, when using standard threefold therapy for HPI in China. Although the complete elimination of the problem may appear unattainable, it is crucial to continue investigating new and innovative methods of treatment. The global management of gastritis caused by H. pylori has become progressively challenging in recent years. This is a result of various reasons, including the appearance of novel strains of bacteria that are not sensitive to antibiotics [9]. In addition, the issue of poor patient adherence, particularly in Africa, exacerbates the situation during this period of antibiotic resistance [10]. Consensus standards for the validation and management of HPI have been created in Europe, America, and the Asia–Pacific [11]. These guidelines are periodically reviewed by their respective supervising groups, such as the Asia–Pacific Association of Gastroenterology, the American College of Gastroenterology and Helicobacter and Microbiota Study Group. Regrettably, there is a lack of unified agreement throughout Africa regarding the criteria and practices for diagnosing and treating HPI. Furthermore, regardless of the worldwide importance of HPI and the increasing worry about antibiotic resistance in HPI, there is a noticeable lack of extensive studies specifically examining the frequency, development, and antibiotic resistance patterns of this bacterium in African populations. This significantly hinders the progress of creating customized and efficient treatment approaches for HPI in Africa. This study aims to offer a thorough understanding of the development of H. pylori infection in the African continent. It specifically focuses on evaluating the prevalence of infection, factors that affect its prevalence in Africa, the mechanisms of infection, regional differences in antibiotic resistance, diagnostic methods, and treatment choices. Investigating these topics will aid in the creation of region-specific protocols for the control and prevention of HPI in Africa, specifically enhancing healthcare results and lessening the impact of this infection on the African continent.
2 Prevalence of H. pylori in Africa
The prevalence of H. pylori on a global scale is geographically varied with the greatest burden in developing countries. Among various continents, Africa exhibits a notably high prevalence of HPI, with a prevalence equal to or exceeding 70% [12]. The existing prevalence data in Africa does not encompass all African countries. Nevertheless, data from various African countries consistently demonstrates a higher incidence of the infection among patients whose major complaint upon admission to a healthcare center is chronic indigestion problem. Therefore, making it a prominent and frequently observed predisposing factor for HPI in the region [13]. For example, data from Ghana reported a prevalence of over 70% among dyspeptic patients [5]. In general, children are considered particularly susceptible to this infection as it is usually contracted during their early years.
Geography and socio-economic strata are other contingent factors responsible for fluctuations in the prevalence of H. pylori, even within a nation. These fluctuations can be further exacerbated by age, literacy level, dietary habits, and income [1]. The African situation could be explained by poor access to health care, environmental factors, host genetic characteristics, the sensitivity of diagnostic methods, immune response, type of H. pylori virulence factors, and underlying diseases like tumors. More so, factors like gender and ethnicity could also contribute to variations in prevalence [14]. The impact of each of these factors on H. pylori infection remains inadequately understood, which poses challenges in effectively addressing the escalating burden of this infection. Socioeconomic status stands out as a critical cause for the elevated prevalence of HPI in developing nations. The link is reinforced by suboptimal hygiene practices, which explain why HPI tends to be more prevalent during the early stages of life in the continent [15]. Also, the work of Awuku et al. sheds light on the levels of H. pylori prevalence in Sub-Saharan Africa. The study reported an unprecedented rate of infection in rural settings, and it also highlighted several contributing factors to this increase. These factors include the source of drinking water, open-air defecation practices, and the expanding size of households [16].
2.1 West Africa
A high incidence of HPI exists in West Africa with variations from country to country influenced by availability of and the type of diagnostic methods employed. The incidence rate in Nigeria is between 38.0% and 92%, and mostly in children [14]. The work of Oreh et al. showed a high incidence of HPI in patients with dyspepsia at the Garki clinic in Nigeria [17], indicating a a probable correlation between HPI and certain disease conditions. In the northern region of Nigeria, the infection prevalence is approximately 87.8% [12], while it is in the range of 28–51.4% in the southern region [18, 19]. Generally, the high incidence of H. pylori in Nigeria could be attributed to several identified risk factors, which include low socioeconomic status, absence of fresh water sources, high population and overcrowding, and bad lifestyles such as smoking [12, 20]. In other west African countries, H. pylori prevalence as reported by different studies include, 71.5% in Republic of Benin [21], about 73.2% in Cameroon[22], 93.1% in Togo [23] and 70.41% in Congo-Brazzaville [24], while their associated risk factors are mostly associated with family history, socioeconomic status, alcohol consumption, age, abuse of nonsteroidal anti-inflammatory drugs (NSAID), and disease conditions like chronic gastritis, anemia, and duodenal ulcer [25, 26]. Another example illustrating this trend is the elevated incidence of HPI observed during early life in Burkina-Faso. Furthermore, disease prevalence rates exceeding 50% have been reported in Ghana, Senegal, and Cote d’Ivoire [5].
2.2 East Africa
According to a review and analysis conducted by Melese et al. [27], Ethiopians exhibited high prevalence of HPI, with 50% or more of the participants infected. However, the study also reported a decline in infection rates when certain factors were implemented. These factors included adequate sanitation, behavioral changes, health education to raise awareness about transmission dynamics, the use of multiple tests for screening and diagnosis, and appropriate utilization of eradication therapy. This suggests that in many African countries experiencing increased infection rates and high prevalence, some or all of these factors may not have been adequately considered or implemented. Addressing these aspects could be a turning-point in combating the rising burden of HPI in the continent.
Furthermore, sickle cell anemia is now being recognized as a predisposing factor for HPI among children at Mulago Hospital in Uganda [26]. A prevalence rate of 70.8% was reported in Burundi. In another research conducted on individuals who sought medical care at the University Hospital Butare in Rwanda, over the course of a year, the researchers found a 75% positivity rate for HPI among the patients [1], suggesting this could be higher if a comprehensive testing is done.
2.3 North Africa
Bounder et al.[6] did a research to assess the incidence of H. pylori in Moroccan individuals, both with and without gastrointestinal symptoms. The researchers found that the prevalence of H. pylori seropositivity was 89.6% among individuals with gastrointestinal illnesses and 92.6% in asymptomatic Moroccans. In Algeria, approximately 71.4% prevalence was reported [28]. Furthermore, 64.6% prevalence was reported among children in Egypt with associated risk factors of illiteracy, overcrowding, and consumption of poorly cooked foods [29]. A study carried out in Tunisia, which included more than 1000 child participants, found that 51.4% of them had H. pylori infections. The study highlighted some predisposing factors common with H. pylori prevalence, especially overcrowded households, bed-sharing, lower socioeconomic status, and sharing cups. Additionally, a study conducted between 2006 and 2007, employing various diagnostic methods, revealed a 64% seroprevalence among blood donors and a 99% prevalence in patients who underwent gastroduodenal endoscopy. After this study, subsequent data has indicated a gradual reduction in prevalence in Tunis, as evidenced by culture results from biopsy specimens [30].
2.4 Southern Africa
Similar to several other African nations, the limited data available from Southern Africa regarding H. pylori prevalence indicate a significant and often exceeding 50% prevalence. In Harare, Zimbabwe, a prevalence rate of 67.7% was recorded among asymptomatic patients [12]. The prevalence is high in South Africa, about 77.6% [31]. In 2012, a prevalence of 75% was reported in the Rwandan population based on H. pylori endoscopy testing [32]. More so, among the 336 individuals assessed for H. pylori serology in Zambia, 6% of children and 93% of adults were seropositive [33] (Table 1).
3 Form of transmission of H. pylori
While the precise method through which H. pylori can be contracted has not been determined, we know that the fecal–oral and oral-oral routes [34] is the most common. HPI typically originate in childhood and can persist in the human stomach into maturity, serving as a reservoir for the pathogen and often going undetected [35]. While there are other means of transmission, the predominant mode is within the family. This phenomenon can be attributed to the extended lifespan of family members and their consistent interaction with one another [36]. An assessment of transmission between partners in couples and potential couples has revealed minimal or nonexistent association, as many couples with partners who tested positive have exhibited distinct strains of the bacterial infection [37]. This genomic alteration is linked to the adaptive traits of the bacteria that enable successful transmission [38]. These traits may arise from speciation and the absence of chromosomal integrity in the pathogenicity island of certain virulence proteins.
The bacterium’s remarkable capacity to extensively modify its genome in order to adapt to a new host is the primary factor for its rapid evolution and successful survival within the host’s internal environment [39]. The occurrence of infection within a family has been found to be higher with the duration of marriage and age grow, but it is relatively low among the upper class [36]. Various environmental factors, such as geographical location, lifestyle, age, food habits, socioeconomic situations, family size, and personal hygiene, have significantly contributed to the infection of H. pylori [40]. These factors are the primary determinants of the low and high rates of infection in most nations worldwide.
3.1 Pathogenesis of H. pylori
H. pylori is commonly implicated in numerous illnesses. Certain diseases originate in the stomach, whereas others are located in different organs. It induces gastritis, gastric ulceration, and gastric malignancy. HPI is associated with various conditions, including irritable bowel syndrome, ulcerative colitis, hepatobiliary diseases, neurological disorders like Guillian-Barre syndrome and Alzheimer’s disease, cardiovascular and cerebrovascular diseases like coronary artery disease and stroke, metabolic diseases like diabetes mellitus, bone diseases like osteoporosis, skin diseases like chronic urticarial, and non-gastric cancer such as colorectal cancer [41, 42]. The adaptability of this pathogen is enhanced through its outer surface membrane proteins (OMPs) and other virulence factors to be expressed, activated, or inactivated in response to the host’s reaction [43]. Urease, an outer membrane protein, is crucial for the bacterium’s pathogenesis and virulence due to its capacity to neutralize the acidity of the stomach. This allows other membrane proteins and cytokines to work together in initiating the disease [44]. CagA, a protein produced by H. pylori, induces an upregulation of inflammatory cytokines and a downregulation of autophagy in infected individuals. This process is a contributing factor to inflammation of the gastric tissues and serves as the catalyst for the formation of cancer [45].
3.1.1 H. pylori in carcinogenesis
H. pylori is linked with several forms of cancer both in the gastrointestinal region and beyond. These types of cancer encompass mucosa-associated lymphoid tissue (MALT), gastric cancer and duodenal cancer. Carcinogenesis, here, refers to the development of malignant cells in the host cells in response to evasive mechanisms triggered by virulence factors of H. pylori. A strong association exists between the gut microbiota and the occurrence of H. pylori in the cancer development of gastric region [47]. HPI increases gastrin levels, which in turn promotes bile reflux gastritis (BRG), another predisposing factor for the formation of cancer [48]. The development of gastric cancer is linked to the induction of Toll-like receptors, resulting in a release of inflammation-related chemokines and cytokines. This process ultimately raises the likelihood of developing the disease [49]. Mucosa associated lymphoid tissue (MALT), also known as low-grade B-cell lymphoma, forms in the stomach due to stimulation triggered by H. pylori [3].
Typically, both environmental and genetic conditions are necessary to initiate the formation of a malignant cell. The environmental factors encompass various elements such as dietary habits (including the consumption of tobacco, alcohol, and other harmful substances), exposure to cancer-causing chemicals (resulting from industrial and agricultural activities), geographical proximity to industrial areas with continuous emission of hazardous waste into the environment, and lifestyle choices (including inadequate personal health care and hygiene) [50]. All of these potentially exhibit characteristics that promote invasion and metastasis in cancer. Studies have demonstrated that every medical condition that can cause inflammation can also trigger the development of malignant cells. The growth of tumors is influenced by H. pylori virulence factors, which in turn are affected by oncogene-driven metabolic processes and microenvironment [51] (Fig. 1).
Various modes of transmission of H. pylori [46]
3.1.2 H. pylori in neurological disorders
H. pylori have been associated with some neurodegenerative conditions like Parkinson’s and Alzheimer’s diseasess, and Guillain–Barre syndrome in the brain [52]. Gastrointestinal abnormalities in autism spectrum disorder in children are also linked to H. pylori [53]. Recently, research has shown a link between H. pylori with dementia and decreased cognitive ability [54]. In the brain, nanosized-outer membrane vesicles (OMV) secreted by H. pylori play vital role in altering astrocyte function and thereby promote neuronal damage [52].
3.1.3 H. pylori in cardiovascular diseases
Dyslipidemia, a condition that expresses abnormal elevated cholesterol level in the blood and thereby enhances the likelihood of atherosclerosis and many heart diseases, is also associated with H. pylori [55]. Research efforts are ongoing to further establish the contribution and mechanisms of involvement of this bacterium in the etiology of heart diseases.
3.1.4 H. pylori in dermatological disorders
Some autoimmune inflammatory skin diseases like psoriasis, a chronic infection and urticarial clinical conditions were investigated and uncovered to be linked with HPI [52]. Similarly, research efforts are ongoing to further establish the contribution and mechanisms of involvement of this bacterium in the etiology of psoriasis and other dermatological diseases.
3.2 Mechanisms of adhesion and colonization
During colonization, the host epithelial cells receptors interact with H. pylori virulence factors, including OMPs and other proteins [56]. The bacterium may efficiently infiltrate and establish a presence in the stomach of acidic pH, aided by the ureases [34]. Urease is an essential outer membrane protein that the pathogen uses for the colonization and adherence to the stomach’s epithelial tissue. It achieves this by hydrolyzing urea into carbon dioxide and ammonia [56].
Outer membrane proteins (OMPs) exhibit geographical and regional variations and have significant correlations with each other, despite their varied functional roles. In addition to the OMPs, additional cytokines, such as Vacuole-forming cytotoxin A (VacA) and cytotoxin-associated-gene (CagA), are also implicated in the adherence of H. pylori to the gastric cells [43]. Inside the stomach wall, there is a slender organ that injects CagA and VacA at the point where two cells meet. This injection causes structural changes in the cells and triggers the activation of additional harmful factors [57]. There are other enzymatic activities that participate in neutralizing the acidity of the stomach. These activities can either be dependent or independent on the urease mechanism [56].
The pathogen’s ability to increase motility, manipulate its surroundings, and adhere to the microenvironment are mechanisms that also contribute to its colonization of the extremely acidic organ [58]. The enzymes’ capacity to disrupt cellular organization and interfere with the activities of signaling molecules within and around the cell has been a significant factor in the bacterium’s adaptation in the human stomach. This ability also poses a serious threat to human health, as bacterial infection is associated with various stomach and other organ diseases. H. pylori utilizes human cholesterol for adhesion by directly absorbing it from the stomach in the form of Cholesteryl α-D-glucoside 6-acyltransferase. This discovery is fascinating and requires more investigation due to the correlation between high cholesterol and cardiovascular illnesses [59]. The bacterium’s absorption of cholesterol is anticipated to decrease the likelihood of the condition instead.
3.3 Clinical features and manifestation
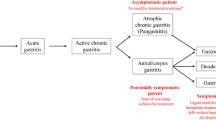
While some infected individuals may not show symptoms, those who do experience clinical characteristics and manifestations might exhibit a number of symptoms. Common clinical features of HPI include dyspepsia, typified by persistent or recurrent pain and discomfort in the upper abdomen, along with symptoms such as gas, bloating, and burning. Gastric ulcers, which can occur in the stomach (peptic ulcers) or the upper section of the small intestine (duodenal ulcers), are also common and may present with symptoms such as abdominal pain, sometimes accompanied by bleeding or vomiting of blood. Gastritis, characterized by abdominal discomfort, nausea, vomiting, and bloating, is another manifestation of HPI. Reflux esophagitis, which is characterized by heartburn, belching, and chest discomfort, is also associated with this infection. In addition to anemia, loss of appetite, and unexplained weight loss, individuals with HPI may experience persistent nausea and occasional vomiting [60].
If H. pylori infection is identified late or is severe, it can result in more severe consequences such as gastrointestinal bleeding, stomach cancer, and MALT lymphoma [61]. Nevertheless, these issues are infrequent. However, it is crucial to understand that not all infected individuals will experience symptoms or consequences. A significant number of individuals continue to be asymptomatic carriers of the bacteria, and the precise factors that determine why certain individuals develop symptoms while others do not are still not completely comprehended. Typically, due to the little or absent symptoms exhibited by H. pylori, early identification becomes challenging. Primarily, the underlying clinical state linked to H. pylori functions as a method for detecting H. pylori. This approach has demonstrated efficacy, although late detection of a disease condition can be deleterious if it has progressed to an advanced level. An established correlation with HPI is the presence of auto-immune diseases [62]. This is a contributing factor to the correlation of HPI with a number of other disorders in and outside the gastric tract.
3.4 Antibiotic resistance (AR) in H. pylori
The campaign to eliminate H. pylori has encountered an increasing challenge as a result of antibiotic resistance [63]. The ineffectiveness of antibiotics against H. pylori is mostly attributed to the emergence of novel strains and the existence of diverse virulence factors. The most effective approach is to implement frequent and precise antimicrobial susceptibility testing [64]. Excessive use of antibiotics in treating H. pylori results in the emergence of a drug-resistant strain of the bacterium. Low sensitivity to metronidazole, levofloxacin and clarithromycin has been observed in both first-line and second-line antibiotic interventions [65].
3.4.1 General concepts of antibiotic resistance; causes and public health
On a global scale, H. pylori has effectively become resistant to the several types of antibiotic options that are widely employed for its treatment [66]. The continuous utilization of antibiotics in medical therapy is a leading factor contributing to antibiotic resistance [67]. When metronidazole, clarithromycin, ciprofloxacin, doxycycline, amoxicillin, rifampicin, and Fosfomycin are administered together, there is a moderate level of resistance seen [68]. Although there have been multiple studies linking clarithromycin resistance to gene mutation, there is currently no evidence of mutation being connected with metronidazole resistance. Antibiotic resistance in China exhibits geographical variation and is believed to arise from genetic mutation [69]. A study [66] found that clarithromycin, levofloxacin, and metronidazole had a higher level of resistance.
The global impact of resistant H. pylori on human health is evident through three distinct profiles: single-resistance, multi-resistance, and hetero-resistance [70]. The repeated use of antibiotics such as tetracycline and metronidazole in previous treatments for common diseases like diarrhea is judged to be a significant cause contributing to resistance in H. pylori. This is because HPI is pronounced in children than adults, and the rate of resistance is linked to variances in age [71]. The degree of resistance to different drugs varies worldwide, and this variance is exacerbated by considerations such as human ecology, individual genetic composition, and lifestyle [72, 73].
Thorough testing of antibiotics is necessary to determine their effectiveness in specific locations, but this task has proven to be challenging. Vast studies have been conducted worldwide on the antibiotic resistance in H. pylori. However, some aspects have posed challenges in effectively addressing this issue. The challenging factors encompass a high level of genetic diversity, which arises from a rapid rate of mutation during cell division. Additionally, the organism has the capability to generate multiple virulent determinants that act both independently and in synergy, resulting in the development of various strains with differing degrees of virulence [72].
In the African context, the lack of sufficient facilities and resources for conducting regular and extensive antimicrobial testing worsened this problem. The emergence of counterfeit antibiotic products is becoming a growing concern. The rise in AR has resulted in a drop in the effectiveness of treatment, hence increasing the likelihood of developing gastritis [70]. Another contributing aspect to the phenomenon of antibiotic resistance is the capacity to generate biofilms that reduce bacteria’s susceptibility to antibiotics and facilitate long-term tolerance and persistence. Furthermore, cholesterol glycosylation plays multiple roles, including facilitating immune evasion, strengthening resistance to antibiotics, preserving the original helical shape, and supporting the activity of important virulence factors like CagA and VcaA. The escalating global resistance to antibiotics, resulting in treatment failure, along with the ongoing evolution of AR, is a matter of significant concern that requires uttermost attention [63].
4 Diagnostic methods of H. pylori
An accurate diagnosis is a crucial component of successful treatment for numerous gastroduodenal disorders [74]. Diagnosing of HPI requires both clinical examination and laboratory testing. In the diagnostic process, both invasive and non-invasive procedures may be utilized, each with its pros and cons depending on the specific clinical scenario [75]. Although none of these diagnostic approaches can be regarded as the definitive method, various ways have emerged offering more dependable outcomes.
4.1 Invasive diagnostic tests
In invasive diagnostic procedures, it is necessary to directly extract samples from the patient’s body. Specifically, samples obtained during endoscopy of the gastroduodenal region are subjected to invasive techniques. These biopsies can undergo cultivation or histopathological examination. In addition, PCR biopsies can be utilized to identify evolved strains of H. pylori and determine their resistance patterns [75].
4.1.1 Culture techniques
Despite its limited sensitivity and time-consuming nature, culture technique remains a dependable means of detecting H. pylori. The test is highly precise, with a 100% accuracy rate. It may be utilized to ascertain an organism’s susceptibility to antibiotics. This knowledge is crucial in order to select the most optimal treatment strategy, as it is important to stay informed about local antibiotic susceptibility and any changes that may occur. Nevertheless, the efficacy of isolating the bacteria can significantly differ among laboratories due to the challenging nature of working with this organism. Even the most proficient laboratories can only successfully retrieve the pathogen from 50 to 70% of infected biopsy samples [76]. The accuracy of this method is very high. However, its processing is labor-intensive and requires specific transportation conditions to maintain the bacterium’s viability. Additionally, the need for low oxygen of less than 5%, specialized tools, reagents, culture media, and high-skilled personnel are factors that collectively restrict its applicability [63, 78] (Fig. 2).
4.1.2 Histopathology examination
Histopathology is widely acknowledged as the most dependable method for directly identifying HPI. Nevertheless, the precision of histology can be affected by factors such as the location, quality and quantity of samples, the position and thickness of specimen, type of staining employed, the presence of proton pump inhibitors (PPIs) and antibiotics, as well as the proficiency of the pathologist performing the examination [79]. During a biopsy, specimens are extracted from the lower and middle sections of the stomach [75]. Various factors can impact the precision of examinations, and these may atrophy of the gastric region, metaplasia of the intestine, low bacterial number, uneven distribution of bacterial cell on the mucus, prolonged use of PPIs, and the administration of antibiotics or bismuth. Hence, a minimum of five stomach biopsy samples is required to precisely assess the phase and degree of Helicobacter-induced gastritis [80]. In addition to its ability to detect H. pylori and assess the degree of gastritis, histopathological examination is more efficient for detection of precancerous cells which may have been impacted by HPI [81].
4.1.3 Endoscopy
An endoscopic assessment is commonly executed to diagnose disorders related to H. pylori. It is a commonly utilized technique for obtaining samples, typically from stomach mucosa biopsies [74]. This approach has proven quite effective in individuals with no concerning signs of digestive issue. Gastritis is a condition that presents several symptoms, including inflammation, shrinking, and intestinal metaplasia. These symptoms might complicate the process of diagnosing the condition [81]. Modern endoscopic procedures utilize advanced imaging technologies such as blue laser imaging (BLI) and linked color imaging (LCI). Investigations have demonstrated that LCI is generally more efficient, while BLI is reliable for identifying metaplasia [82, 83].
A recent study proposed that artificial intelligence could mitigate the danger of inter-observer or intra-observer variability resulting from the absence of objective markers in endoscopy and endoscopic views [77]. Other research has supported this hypothesis, demonstrating that AI-assisted endoscopy can be advantageous by offering a second opinion and reducing the reliance on operators in diagnostic endoscopy. Endoscopic imaging was proven suitable for detection of both cancer and non-malignant growths in the intestines [84].
4.1.4 Rapid urease test (RUT)
RUT is an efficient and economical diagnostic tool for detecting HPI. It is well known for its quick results. This is an invasive procedure that may be conducted in a clinical environment and does not necessitate any specialist equipment. The test is a highly precise evaluation that may detect H. pylori within 12–24 h following a stomach biopsy taken during an endoscopy [85]. This test relies on the idea that the bacterium generates urease. This process results in the formation of ammonia, causing the pH to increase gradually, as sensed by the indicator—phenol red [76].
Occasionally, false-positive results may occur, but they are mostly caused by other bacteria (e.g., Enterobacter cloacae, Klebsiella pneumoniae, Proteus mirabilis) in the stomach that generate urease [86]. To enhance the accuracy rate, it is advised to get at least two biopsies done. Nevertheless, despite the challenges, research has shown that RUT has a significant level of precision, ranging from 95 to 100%, and is reasonably sensitive, with a rate of 85–95% [85].
4.2 Non-invasive diagnostic tests
Non-invasive examinations are medical procedures that do not involve the insertion of diagnostic devices into the patients’ bodies. In the case of H. pylori, the attending medical personnel can collect samples from saliva, breath, or stool.
4.2.1 Urea breath test (UBT)
UBT is a respiratory examination that utilizes the urease enzyme activity, secreted by H. pylori, to convert urea into ammonia. This process neutralizes the acid of the stomach, enabling the bacterium to attach to the wall of the gastric region. The UBT is predominant and very dependable non-invasive diagnostic procedure for about three decades. UBT usually consists of administering a dose of 75 mg of urea that is labeled with 13C/14C radioisotopes. The amount of urea in exhaled breath samples is then measured using a mass spectrometer before and after swallowing the urea [87]. Prior to administering the patient with 13C-labelled urea, it is necessary to collect two samples. In order to obtain the most precise outcomes, it is essential for the patient to refrain from consuming any food for minimum of 6 h, much better overnight. The initial twofold samples should be taken in the designated tubes or bags [88]. Once the patient has been weighed, they are administered a diluted solution of urea labeled with 13C. Subsequently, two further breath samples are collected within the following thirty minutes. Children should consume a recommended amount of 100 ml of orange juice. A positive test result is shown when the second pair of samples contains carbon dioxide containing C [77].
Although uncommon, false-positive results may occur in individuals who have recently undergone an endoscopy with a biopsy and gastrectomy, or in cases where the gastric pH is very alkaline. Additionally, false-positive results can be observed when bacteria in the mouth or stomach break down urea via urease [77]. Nevertheless, the UBT test offers a significant advantage over serology or stool antigen testing, particularly for those who have had gastrectomy or been on like antibiotics or PPIs in recent times [89] (Fig. 3).
4.2.2 Molecular tests
Molecular tests are procedures that utilize thermal cyclers for polymerase chain technology to detect the presence of H. pylori DNA in samples like feces, dental caries, saliva or stomach biopsies. Their accuracy rate is great, reaching up to 95%. However, they come with a significant cost and necessitate specialized laboratories and skilled personnel [90]. The invasiveness of molecular tests varies depending on the type of material being evaluated [91]. PCR offers the benefit to examine DNA obtained from test samples that can be conveniently transported through the mail without strict shipping rules [92]. However, the procedure of analyzing stomach biopsy samples using molecular testing is both time-consuming and demanding, since it requires the collection of several samples for the purpose of cultivating and sequencing H. pylori DNA. To realize the most precise identification of drug-resistant subpopulations, it is necessary to select numerous colonies of H. pylori for the purpose of DNA extraction [86].
4.2.3 Stool antigen test (SAT)
Based on a worldwide meta-analysis, the stool antigen test (SAT) has a 94% sensitivity and a 97% specificity for detecting HPI. It is a highly accurate non-invasive approach. This test finds out if stool samples contain the H. pylori antigen. There are of two kinds: enzyme immunoassay (EIA) and immunochromatography assay (ICA). Monoclonal or polyclonal antibodies may be used by any kind. With a precision rate of 90%, the SAT is a very useful diagnostic tool for precisely determining and validating bacterial persistence after treatment.
Physicians and patients frequently opt for the usage of SAT test, regardless of the patient’s condition, because of its cost-effectiveness compared to other treatments [76]. In order to achieve the best possible result, patients should refrain from using antimicrobials and bismuth medicines for a duration of 4 weeks, and PPIs for a duration of 2 weeks prior to the SAT evaluation. Although SATs offer numerous advantages, it is important to consider certain disadvantages. Differences in SAT scores can arise due to regional variations, as the antigens employed in the exam vary across different locations. SATs depend on an antigen–antibody interaction to give a result [92]. Because of the small number of bacteria in the stomach and the low level of H. pylori antigen in the sample, a negative SAT test may not always correctly indicate the absence of HPI [93]. Furthermore, SATs may lack precision in exceptional situations, such as cases involving persons experiencing gastrointestinal hemorrhage or using bismuth-based drugs. Despite being widely acknowledged, SATs are nevertheless regarded as inconvenient and unclean due to patients’ reluctance to provide feces samples [76].
4.2.4 Serology
Serological tests are minimally intrusive, economically efficient, and do not necessitate any specialized equipment, rendering them an excellent choice for screening purposes. The Western blotting, enzyme-linked immunosorbent test (ELISA), and latex agglutination assays are the three methods available for identifying antibodies. With a 95% utilization rate, ELISA is the most accurate and widely used of them. Within 3–4 weeks of H. pylori colonizing the digestive tract mucosa, the immune response of the infected individual is triggered, producing antibodies against the bacteria [77]. All three forms of antibodies (IgA, IgM, and IgG) may be detected, however only the long-term IgG test is thought to be dependable for detecting H. pylori. The accuracy of the test ranges from 80 to 95%. IgG antibodies are generated after an HPI and are high for about a year before decreasing to their baseline levels [75]. Their resistance to the effects of antibiotics, bismuth medications, PPIs, recent intestinal hemorrhage, and atrophic gastritis is a major benefit of serologic testing [95]. It is crucial to remember that a positive serology test does not always indicate that an infection is active. This is due to the possibility that non-specific cross-reacting antibodies from an earlier infection may have resulted in the existence of antibodies to specific antigens. These procedures are helpful for making an initial diagnosis or as a follow-up diagnostic procedure. Research has shown that even when the infection has been effectively eradicated, the levels of antibodies do not dramatically drop over an extended period of time [94].
4.3 Novel diagnostic approach
4.3.1 Matrix-assisted laser desorption/ionization- TIME-of-flight- mass spectrometry (MALDI-TOF MS): peptide mass fingerprinting technology
MALDI-TOF MS is an inventive, accurate, and reasonably priced Peptide Mass Fingerprinting Technology (PMF). It makes use of intricate fingerprints of certain biomarker molecules [96]. This method has several important benefits, including high sensitivity and accuracy, speedy process, and cheaper cost when compared to other traditional tests. Numerous species of bacteria may be reliably detected using MALDI-TOF MS. It can also reliably detect drug resistance and differentiate between many species of Helicobacter. Additionally, MALDI-TOF–MS analysis requires a minimum quantity of microbial biomass, usually between 104 and 106 CFU. Additionally, it has been shown that this technique may be used for analysis of both mix of microbes and homogeneous bacterial cultures [97].
4.3.2 Biosensors
A biosensor is a tool that measures and assesses biochemical interactions by generating outputs that correspond to the intensity or quantity of the material under examination. Biosensors are presently employed in several applications such as illness monitoring, identification of pathogenic microbes, detection of disease-associated biomarkers, identification of contaminants, and drug discovery [98]. Biosensors consist of three components: a bioreactor, a signal transducer, and an amplifier. The bio-receptor is tasked with the recognition of the analyte, while the signal transducer converts this interaction into a quantifiable signal [99].
4.3.2.1 Optoelectronic nano-biosensors for detection of H. pylori
Modified nanomaterials with remarkable fluorescence properties, such graphene, quantum dots, and gold nanoparticles, have found extensive applications because of their remarkable qualities. Optical techniques like surface-enhanced Raman scattering (SERs), colorimetry, and surface plasmon resonance (SPR) offer advantages in detecting and enhancing the original signal. These techniques are non-destructive and very sensitive [100]. Research has shown that the integration of PCR and FRET phenomena yielded a significant degree of precision in identifying H. pylori, while also being highly effective and rapid. In addition, urea-enzyme antibody-test strips were developed using CdSe core/ZnS shell quantum dots (QDs). These strips, when integrated with a Wi-Fi module, demonstrated a specificity of 97% and a sensitivity of 95% in identifying H. pylori [101].
In contrast, the method involved merging matching DNA with gold nanoparticles modified with oligonucleotide onto a probe and affix on a glass surface [75, 100]. Alternatively, a novel strategy for labeling involves the utilization of aptamers that have been labeled with fluorescent markers. Aptamers have garnered significant attention due to their utility in diagnosing infections, predicting disease outcomes, evaluating therapy efficacy, and forecasting metastasis. Aptamers having specific binding capabilities to a particular cell line are generated via a technique called SELEX, which relies on specific surface proteins on the target cell. Moreover, aptamers exhibit greater durability and a longer period of viability compared to antibodies [102].
4.3.2.2 Electrochemical biosensors for detecting H. pylori
Electrochemical biosensors are a cutting-edge tool employed in the medical field for diagnosis and monitoring purposes. In electrochemical systems, a common arrangement consists of a three-electrode system with a working, a counter, and a reference electrode. To enhance the performance of the sensors, various materials (e.g., metal nanoparticles) may be used to optimize the working electrode [75]. The primary electrochemical methods used are amperometry, and voltammetry-based techniques. Electrochemical methods have many benefits compared to conventional techniques for detecting H. pylori. These features include simplicity of use, rapidity, affordability, portability, compactness of devices, minimal sample needs, high precision, and specificity, among others [103]. Electrochemical biosensors may be classified into three categories: immunosensors, which rely on interactions between antibodies and antigens; genosensors, which use nucleotides; and aptasensors, which use DNA or RNA aptamers [104].
4.3.2.3 Piezoelectric sensors for detecting H. pylori
Quantifying vibration frequency and mass changes on the probe surface using a quartz crystal microbalance (QCM) is a useful technique used by piezoelectric devices to get information on the kinetics of the adsorbed layer. With the use of particular protein and QCM, a unique enzymatic immunoassay sensor was developed. The sensitivity may be increased by adding an adherent solution, which causes precipitate to develop on the surface [75]. A thorough description of an enzymatically amplified QCM Sensor that can measure the IgG levels in H. pylori was given by Singh et al. [105]. This method creates a sandwich-type immunocomplex by using anti-human IgG antibodies. However, Kuchmenko et al. developed a different technique to detect and quantify amines, aliphatic acids, and ammonia in exhaled air [106]. The quantity of urea present is strongly correlated with the amounts of ammonia and carbon dioxide in exhaled air, which makes them important indicators for the noninvasive detection of H. pylori. The approach was based on signal analysis from many piezoelectric sensing devices that were equipped with absorbent films such as Tween-40 and propolis. According to the research, the method showed a noteworthy 96% specificity and 90% sensitivity for ammonia [75] (Fig. 4; Table 2).
4.4 Availability and accessibility of H. pylori diagnostic tools in Africa
Due to the substantial prevalence of H. pylori in Africa and its associated negative health effects, it is essential to accurately and promptly diagnose the infection in order to effectively manage, treat, and eliminate HPI [107]. However, the availability and accessibility of the diagnostic tools used in diagnosing H. pylori in Africa may vary depending on the country and region. Diagnostic tools for H. pylori may have limited availability and accessibility in many African countries especially, in rural areas with insufficient healthcare infrastructure and resources. Nevertheless, large cities and urban areas in Africa typically have access to laboratories and medical facilities where H. pylori diagnostic testing is accessible.
A number of the previously mentioned techniques are being used in Africa to diagnose H. pylori infection. Nonetheless, there seems to be no agreed method adopted by the countries in the continent for diagnosing HPI, and inadequate health facilities, the inadequate number of healthcare specialists, and poor living conditions hinders the regular application of certain methods in some areas, additionally, the high cost to use some of these methods exacerbates the situation [107]. Local and regional studies have been carried out to compare various diagnostic techniques used in Africa. In West Africa, a blend of diagnostic techniques is predominately employed. During a retrospective analysis of patient records at a government health center in Accra, Ghana from 1999 to 2012, endoscopy and RUT were mostly employed for diagnosis of HPI [108]. Additional studies conducted in Ghana has similarly documented the predominant utilization of endoscopy and RUT for diagnosing H. pylori [5].
A variety of techniques, including SAT, histology, UBT, serology, endoscopy and culture are used in Nigeria to diagnose HPI. Histology and serology were used in a research by Olokoba et al. to identify H. pylori in dyspeptic patients in in the northern area [109]. Another research by Ray-Offor and Obiorah [14] in the south-south area of Nigeria used histology and endoscopy to diagnose H. pylori. Smith et al.’s research [107] compared traditional PCR to other diagnostic methods for the detection of H. pylori in patients from Nigeria.
In research carried out in Algeria, Moubri et al. [110] used both invasive and non-invasive methods to identify HPI in young people. They made use of endoscopy, RUT, Histology, 13C UBT, monoclonal SAT (HPStAR), and culture. Another epidemiological investigation was carried out by Kasmi et al. [28] on symptomatic patients in an Algerian tertiary hospital. Their only methods for diagnosing HPI were endoscopy and histological analysis of biopsies. Experts in Egypt, another nation in North Africa, came to an agreement in 2017 about the diagnosis of HPI there. The widely accessible SAT test in Egypt was one of the suggested diagnostic techniques, along with histological evaluation and detection of atrophy, RUT, and UBT [111]. The bulk of research studies conducted in Libya used serological approaches for HPI diagnosis [112,113,114].
A variety of techniques, comprising RUT, seology, endoscopy, polymerase chain reaction (PCR) and culture are used in Tunisia as diagnostic strategies HPI [30, 114]. Several publications state that SAT and serology are the main methods used in Ethiopia, an East African nation. Comparably, SAT [115] has been used in Cameroon to diagnose HPI, while SAT [116], endoscopy, histology, RUT [84, 117], serology [118] have all been well reported in Uganda. Different techniques are used in South Africa to diagnose HPI. These include endoscopy, RUT, and histology [119]. Additionally, the combination of endoscopy, culture and PCR [120] as well as endoscopy and histology [121] are commonly used.
4.5 Management practices for H. pylori infection (HPI)
Successful management of HPI often involves monitoring, identification, and therapy. Each of these necessitates sufficient healthcare resources. The insufficient data on HPI in Africa, as previously mentioned, can be linked to the underdeveloped healthcare systems in some African nations [107]. This is mostly due to the absence of diagnostic facilities and technical manpower. On the other hand, in industrialized nations, the effectiveness of the diagnosis is determined on the quality of available diagnostic choices [122]. Europe has a physician-to-population ratio of 1,286,645 doctors per million people, while Africa has only 141,192 medical professionals to cover the entire healthcare system [1, 123]. This situation has deteriorated further, since our medical personnel are now being discreetly recruited by the UK and other developed nations.
Furthermore, a mere 50% of African nations has educational and training establishments dedicated to basic or advanced endoscopy [123]. National endoscopic societies have been documented in countries such as Burkina Faso, Morocco, Nigeria, Ivory Coast, and South Africa.
Multiple guidelines suggest using non-invasive techniques, such as the SAT, in dyspeptic patients who do not show alarm signs and have received eradication treatment [95]. Despite other methods available, UBT remains the most efficient technique for identifying H. pylori infection because to its exceptional precision and user-friendly nature. Recently, the SAT has undergone changes in its format, incorporating monoclonal antibodies instead of polyclonal antibodies. This modification has led to a constant level of quality in the reagents. Unfortunately, there is currently a scarcity of laboratories equipped to perform HPI serology and Antigen–Antibody testing (SAT) in numerous undeveloped regions of Africa.
The load of H. pylori in the GIT can be reduced by administering antimicrobial medications, using antisecretory drugs, and treating ulcer bleeding. In premalignant and malignant lesions, such as intestinal metaplasia (IM) or MALT lymphoma, the amount of microorganisms present tends to be continuously low [124]. Moreover, in order to tackle the problem of eradication failure due to rising rates of resistance, it is advisable to conduct antibiotic susceptibility testing following the second unsuccessful effort at eradication. It is advisable to perform tests in order to ascertain the local prevalence of resistance.
Proton pump inhibitors (PPI) are now readily available, including as generic pharmaceuticals in many African nations, due to their effectiveness in alleviating discomfort, indigestion and stomach ache. Therefore, PPIs are commonly prescribed to provide relief from symptoms in the treatment of Dyspepsia. Thus, it is quite probable that a patient who seeks medical guidance for dyspeptic symptoms is already receiving proton pump inhibitor (PPI) medication. Multiple investigations have established that the use of PPIs leads to localized alterations in the stomach as a result of an elevation in gastric pH. This can cause a minimal presence of germs, especially in the antrum, leading to a negative result in diagnostic testing, save for serology [95].
Due to the presence of H. pylori antibodies long after the bacteria has been inhibited or destroyed, serology is the only test that remains unaffected by this factor. As a result, stopping the use of PPIs for 2 weeks prior to testing enables the pathogen to reestablish themselves in the GIT, leading to formerly negative tests being positive. Moreover, no studies have been carried out to ascertain the necessary length of time for washout after extended proton pump inhibitor (PPI) therapy. A study on UBT proposed that using a test meal with higher acidity could overcome the problem of false-negative tests [125]. The utilization of anti H2 medications may likewise contribute to a few inaccurate negative results, but to a considerably smaller extent. The panel determined that it was unnecessary to cease the use of anti H2 medications before testing, as long as citric acid is used instead [95].
By employing an endoscopy-based approach, one can perform a range of examinations, like the urease test, culturing, and histology. Culture is highly advantageous as it enables the evaluation of antimicrobial susceptibility. This is important since the presence of clarithromycin-resistant H. pylori significantly reduces the efficiency of threefold treatment that includes clarithromycin, resulting in a success rate of just about 10–30%. Several research investigations have shown that tailored treatments that specifically target H. pylori based on its susceptibility to antibiotics, rather than using the typical empirical triple therapy, result in a better rate of eradication [126]. Moreover, these customized therapies have the capacity to be economically efficient. As previously advised at the Maastricht meeting, it is recommended to conduct endoscopy in all instances after a second unsuccessful attempt. If it is not possible to do culture and routine susceptibility testing, molecular assays are advised [95, 127]. However, their accessibility in rural areas of the African continent is limited.
4.6 Treatment options for H. pylori and challenges in Africa
Due to the considerable influence of HPI and its correlation with GIT disorders, which comprises cancer, multiple medical institutes have put out treatment guidelines. The most current guidelines for the treatment of HPI can be found in the Maastricht V/Florence consensus, the Toronto consensus conference, and the plans provided by the American College of Gastroenterology (ACG) [133]. The guidelines recommend utilizing a combination of antisecretory medications that decrease the production of stomach acid (to augment the efficacy of antibiotics) and broad-spectrum antibiotics that are effective both in the gastrointestinal tract and systemically.
When choosing antibiotics, it is fundamental to consider specific resistance trends in the local area, any documented allergies to drugs (such as penicillin allergy), past therapy combinations used for eradication, and any other pertinent considerations. Laboratory investigations have shown a high level of antibiotics resistance, up to 100%, in routinely used antibiotics in specific regions like Nigeria [12, 130]. The prevalence of antibiotic abuse is the root cause of this scenario, as individuals are able to purchase medications without a prescription in the majority of countries on the continent. Enforcing limits or regulations in places where they are in existence presents a considerable problem. Multiple reports regarding the eradication HPI in Africa are available. The studies indicate varying rates between 6 to 44%.
Moreover, certain investigations have discovered that there is no substantial variation between the one-week and two-weeks therapy duration. The observed discrepancy in responses can be ascribed to the diversity of bacterial species and the diagnostic technique utilized. Another obstacle arises from the fact that the recommended approach of using endoscopy with biopsy and culture to determine therapy failure is not readily available or cheap. In locations with a rate of clarithromycin resistance over 15%, it is advised to utilize quadruple therapy with bismuth or non-bismuth option or concurrent remedy (which consists of three antibiotics and a PPI) [95].
If an endoscopy is required, the choice of second-line therapy should be made accordingly. It is essential to conduct culture and standard antibiotic susceptibility testing when this technique is requested in order to ascertain the best course of action. The justification for selecting a second-line therapy in the absence of endoscopy or if it is not wanted is to avoid the empirical use of clarithromycin because of the possibility of the formation of clarithromycin-resistant H. pylori strains [107]. Combining a PPI, bismuth, metronidazole, and either tetracycline or a triple therapy including a PPI, amoxicillin, and levofloxacin are advised second-line therapies. An alternative is to use a quadruple treatment that includes bismuth, levofloxacin, amoxicillin, and a PPI. Levofloxacin-based triple treatment is a viable option if standard triple therapy is futile and the local rate of fluoroquinolone resistance is less than 10%. Antimicrobial susceptibility testing and culture should be used to determine the third-line treatment option if clarithromycin-based or triple regimens prove unsuccessful. In this case, instead of using a PPI, amoxicillin, and levofloxacin, it is advised to think about using a combination of bismuth and other antibiotics or a therapy comprising rifabutin if the fluoroquinolones have not been used previously or if there is strong resistance to them [128, 129]. However, in several African nations, these options are either inaccessible or prohibitively expensive.
4.7 Emerging treatment options and future prospects
4.7.1 Emerging wide- range antibacterial agents
The majority of antibiotics utilized in therapeutic settings are sourced from natural sources and function by either impeding specific cellular processes in bacteria, such as DNA or protein synthesis, or by impacting the integrity of their cell membranes and walls. The distribution of these processes is extensive, and antibiotics that target them often demonstrate a wide range of effectiveness [131]. Nevertheless, the sensitivity to these antibiotics can greatly change among various bacterial genera, based on factors like the degree of similarity in the targeted pathways or the cell wall compositions. Following the increasing understanding of bacterial function systems, scientists are investigating novel avenues for the creation of artificial medications. The chosen pathways must fulfill vital roles in bacteria while being either absent or genetically different in mammals, in order to avoid any potential cross reactivity and toxicity. The Shikimate pathway is a potential target.
Plants and their extracts have been extensively used as therapeutic agents throughout history. Herbal therapy has frequently contributed to the identification and advancement of modern molecular medications. Several herbs commonly utilized in impoverished countries, particularly in African nations, have shown the capacity to either eradicate or hinder the proliferation of H. pylori, a bacterium responsible for gastritis and other gastrointestinal symptoms [132]. Zaidi et al. [133] and Ayala et al. [134] published two comprehensive reviews in this area. These studies suggest that consuming different plants and plant extracts, such as cranberry juice [135], broccoli sprouts [136], xanthones [137], green tea [138], and red wine [139], have the potential to lower inflammatory reaction or inhibit the bacterial replication.
In certain instances, the relationship between laboratory experiments and the corresponding results in living organisms was not significant, or the outcomes were equivocal since there were no trustworthy results from experiments conducted in living organisms [134]. A probable explanation for the difficulties encountered in discovering and separating pharmaceutical molecules from plants of medicinal value could be because their molecular mechanism is complex. In addition, some extracts obtained from plants demonstrated indirect positive effects, such as having qualities that can treat ulcers, but lack ability to completely eliminate or reduce H. pylori [140]. Certain plant materials have shown anti-adhesive properties. For instance, green tea extracts have been found to inhibit H. pylori from binding to epithelial cell lines in-vitro. However, the most effective results were observed when the bacteria were pre-incubated rather than when they were already attached [134]. In addition, extracts obtained from the roots of Vernomia kotschyma are traditionally used in Mali to treat stomach ulcers, show a partial suppression of H. pylori binding to human gastric cancer cells. Furthermore, studies have shown that extracts derived from the roots of liquorice (Glycyrrhiza glabra) exhibit a limited degree of attachment to sections of human stomach tissue [141].
Due to its osmotic effect, pH level, and hydrogen peroxide concentration, honey is well recognized for its effectiveness against bacteria [142]. Honey has been investigated as a possible biological agent having inhibitory effect against H. pylori. Experiments conducted in laboratories have demonstrated that honey and the compounds derived from it may inhibit the development of H. pylori. It has been shown that some types of honey have minimum inhibitory concentrations (MIC50) that are comparable to those of the widely used antibiotics amoxicillin and clarithromycin [143]. Additionally, research has been done on the possibility of probiotics, such as various strains of Lactobacillus, and Bacillus, as an adjuvant therapy to lessen the side effects of the existing antibiotic-based therapies for H. pylori. Probiotics are thought to have their therapeutic effects via binding to Toll-like receptors and modulating the production of cytokines that inhibit inflammation. This system contributes to the reduction of inflammation while concurrently inducing IgA secretion and monocyte and dendritic mobility [144, 145].
4.7.2 Emerging anti-virulence therapeutics for H. pylori treatment
Alternative method to address HPI involves specifically targeting its virulency, which are the proteins and processes involved for the harmful colonization of the stomach and the resulting symptoms. While a few of these highly infectious elements are not necessary, suppressing their activity can greatly diminish the ability to cause infection. This inhibition can be utilized as an independent treatment or in conjunction with conventional antibiotics to improve the treatment’s specificity and efficacy in eliminating the infection [146,147,148,149, 158].
Directing efforts towards preventing bacterial adhesion can be a potent substitute for antibiotic therapy, as evidenced by instances of bacterial bladder infections [152]. The adhesin BabA is crucial in the advancement of the disease [150, 151]. Parsley, borage, and turmeric extracts have the potential to deter the attachment of bacteria to parts of the stomach that express Lewis A and B [153]. While BabA lacks the ability to attach to Lewis A, there is speculation that the extract may also hinder the function of other adhesins. Nevertheless, the precise mechanism of action is still unidentified. A recent study investigated the composition of BabA adhesin and found a notable variation in the Lewis B-binding forms across diverse strains. The discovery poses a difficult prospect for the creation of adhesion inhibitors that are extremely effective and have a wide range of reactivity [154]. Nevertheless, a specific conserved disulfide located within the receptor-binding site was found to be vulnerable to the reducing chemical N-acetylcysteine (NAC). N-acetylcysteine (NAC) efficiently impeded the ability of BabA to stick to gastric tissue sections. The IC50 value for this inhibition was determined to be 10 mg/ml. Furthermore, NAC exhibited the capacity to lower the level of gastric infection and the infiltration of neutrophils in animal models. Significantly, in 2 limited human models, NAC demonstrated a coactive impact on the eradication of H. pylori when administered alongside threefold treatment. The mucolytic activities of NAC were responsible for this action [155].
4.8 Prevention strategies, control and need for surveillance for HPI in Africa
It is significant to emphasize the prevalence of HPI in Africa, while and a substantial proportion of individuals may carry the germs without exhibiting any symptoms or consequences. However, if there are any doubts or worries about HPI, it is advisable to seek healthcare support for accurate diagnosis and suitable treatment.
The degree to which stomach cancer may be prevented by eradicating a preexisting HPI mostly relies on the cancer risk of the person before the eradication. Thus, every eradication program has to decide whether to treat patients who have a high risk of stomach cancer alone without further surveillance. As an alternative, the program may provide a number of options depending on risk stratification, such monitoring to detect gastric cancer early on (secondary prevention) [62].
Primary prevention of HPI is the complete absence of the bacterium, hence preventing its transmission and acquisition. Given that humans are the primary reservoir, eliminating the pathogen also aids in the prevention of its spread, which is frequently observed in youngsters. The goal in persons who are already infected is to eradicate the infection, thereby stopping the advancement and spread of the pathogen to others. After eradicating the problem, the next logical step would be to assess the level of risk in order to identify those who need or could benefit from further actions. This technique is likely to be viable and adopted in nations that have substantial resources to sustain such a program and where stomach cancer is a widespread concern. For instance, a nation that is developing quickly may put in place a program that lasts from the age of 18 to the age of 25, with the intention of eliminating the infection in young people when the illness is still in its early stages and the danger of cancer is lowest.. This is done before they have children, in order to prevent the transmission of their infections to future generations. Furthermore, these systems could integrate pre-marriage screening. Similarly, by implementing primary preventive measures such as frequent handwashing, glove usage, sanitization of utensils, safe food handling practices, staff training, general inspection, and routine screening and testing in restaurants or food service industries, the risk of transmission and HPI can be lowered, thereby protecting both workers and customers. As a result, over a period of time, the population that does not HPI would gradually replace those who are infected. This would eventually lead to a situation where both H. pylori and stomach cancer are completely eradicated within the country. In numerous Western countries, including the United States, there has been a notable shift over the twentieth century where stomach cancer, once the most common kind of cancer, has become uncommon [157].
For secondary prevention, it is necessary to assess the condition and seriousness of atrophic gastritis when eliminating H. pylori. This evaluation is done in a non-invasive manner by conducting tests to measure the concentrations of pepsinogen I vis-à-vis the ratios of pepsinogen I to pepsinogen II [158]. To enhance this approach, the "ABC Method" can be utilized. This method involves measuring serum anti-H. pylori antibodies and serum pepsinogen levels. By doing so, the risk of cancer can be graded [159]. An extensive analysis, encompassing 20 trials, found that the utilization of this approach yielded a combined sensitivity and specificity of 74.7% and 95.6%, respectively in detecting atrophic gastritis [160]. Furthermore, a longitudinal study conducted over a span of 16 years demonstrated that persons who had a positive pepsinogen test at the start of the study had a roughly 3.5-fold increased likelihood of dying from stomach cancer [161]. An alternative approach could involve offering endoscopy to those who may be at risk, particularly those aged 40 or 50 and above, in order to assess the existence of atrophic gastritis and pre-malignant lesions [156]. It is clear that these solutions call for varied allocations of funds and resources, which might add up, particularly when taking into account the need for prolonged monitoring.
4.9 Summary and recommendations arising from this study
In conclusion, our review study on the pathophysiology and resistance of HPI in Africa emphasizes the significant gaps in knowledge and infrastructure and the urgent need for customized remedies. HPI in the African environment has distinct features:
-
Africa has a diverse variety of HPI rates, which are impacted by geography, socioeconomics, and hygiene behaviors. Comprehending these distinctions is crucial for directing intervention tactics. There is a complete absence or inadequate amount of data on the prevalence rate in certain places.
-
In Africa, HPI continues to be a notable predisposing factor for cancer of the stomach. This highlights the significance of identifying and treating the issue at an early stage in order to minimize the risk of a possibly lethal outcome.
-
The polymerase chain reaction (PCR) technique is a highly reliable approach for detection. PCR could detect even minute quantities of H. pylori DNA in a sample, rendering it extremely sensitive. Moreover, PCR exhibits a high level of specificity, enabling it to effectively distinguish between H. pylori and other closely related bacteria, hence increasing its dependability.
-
There is a need to increase the intensity of awareness and public education regarding proper hygiene practices and environmental sanitation, particularly in rural areas.
-
The healthcare infrastructure and resources in numerous African nations encounter obstacles that impact the control of HPI. The lack of adequate access to diagnosis and treatment, especially in rural regions, highlights the necessity for enhanced health systems and heightened awareness.
-
The government should allocate additional funds to the healthcare industry in order to acquire state-of-the-art diagnostic facilities and provide comprehensive training and re-training programs for medical workers.
-
The increasing prevalence of resistant-H. pylori strains in Africa is a concerning issue. This highlights the necessity for responsible utilization of antibiotics, surveillance of resistance patterns, and advancement of alternative treatment approaches.
-
Future study in Africa should prioritize the investigation of local epidemiology, genetic variety of H. pylori strains, and the effect of ecology on the dynamics of infection. This task should be accomplished through coordinated joint research efforts including both corporate and public sectors. Efforts should also be focused on ensuring efficient data collection and thorough documentation. Each of these factors would contribute to the advancement of more preventative and control methods.
-
The need of implementing and strengthening hygiene practices in households and sanitary measures in restaurants throughout Africa cannot be overstated. These should encompass, but are not restricted to, practices such as safe food handling, routine cleaning, thorough handwashing, utilization of gloves, sanitization of utensils, staff training, inspections, sick leave policies, awareness campaigns, and appropriate waste disposal systems.
5 Conclusion
In conclusion, effectively dealing with H. pylori infection in Africa necessitates a comprehensive strategy that integrates enhanced healthcare facilities, awareness initiatives, and appropriate antibiotic administration. Effective collaboration among health professionals, researchers, and policymakers is essential in order to decrease the impact of this bacterium and its related problems on the African continent. Furthermore, it is imperative to sustain continuous endeavors aimed at addressing antibiotic resistance so as to guarantee the efficiency of treatment protocols for HPI in Africa and worldwide.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Smith SI, Ajayi A, Jolaiya T, Onyekwere C, Setshedi M, Schulz C, Otegbayo JA, Ndip R, Dieye Y, Alboraie M et al (2022) Helicobacter Pylori Infection in Africa: update of the current situation and challenges. Dig Dis 40:535–544. https://doi.org/10.1159/000518959
Emerenini F, Nwolisa E, Iregbu F, Eke C, Ikefuna A (2021) Prevalence and risk factors for Helicobacter Pylori infection among children in Owerri, Nigeria. Nigerian J Clin Pract 24:1188–1193. https://doi.org/10.4103/njcp.njcp
Mladenova I (2021) Clinical relevance of Helicobacter Pylori infection. J Clin Med. https://doi.org/10.3390/jcm10163473
Roszczenko-Jasińska P, Wojtyś MI, Jagusztyn-Krynicka EK (2020) Helicobacter Pylori treatment in the post-antibiotics era—searching for new drug targets. Appl Microbiol Biotechnol 104:9891–9905. https://doi.org/10.1007/s00253-020-10945-w
Archampong TN, Asmah RH, Aidoo EK, Wiredu EK, Gyasi RK, Adjei DN, Beleza S, Bayliss CD, Krogfelt K (2017) Helicobacter Pylori CagA and VacA genes in dyspeptic ghanaian patients. BMC Res Notes 10:1–5. https://doi.org/10.1186/s13104-017-2542-8
Bounder G, Boura H, Nadifiyine S, Jouimyi MR, Bensassi M, Kadi M, Eljihad M, Badre W, Benomar H, Kettani A et al (2017) Epidemiology of Helicobacter Pylori Infection and related gastric pathologies in moroccan population. J Life Sci 11:211–218, https://doi.org/10.17265/1934-7391/2017.05.001
Chukwuma OM, Chukwuma G, Manafa P, Ibeh N, Jeremiah Z (2021) Prevalence and possible risk factors for Helicobacter Pylori seropositivity among peptic ulcerative individuals in Nnewi Nigeria. J Curr Biomed Res 1:39–45
Ford A, Forman D, Hunt R, Yuan Y, Moayyedi P, Ac F, Forman D, Hunt R, Yuan Y, Moayyedi P (2015) Erradicación del Helicobacter Pylori Para La Prevención Del Cáncer Gástrico (Revisión). Biblioteca Cochrane https://doi.org/10.1002/14651858.CD005583.pub2.www.cochranelibrary.com/es
Lineages S, Sequencing W, Zhou Y, Zhong Z, Hu S, Wang J, Deng Y, Li X, Chen X, Li X (2022) A survey of Helicobacter Pylori antibiotic-resistant genotypes. 1–15
Sokwala A, Shah MV, Devani S, Yonga G (2012) Helicobacter Pylori eradication: a randomised comparative trial of 7-day versus 14-day triple therapy. S Afr Med J 102:368–371. https://doi.org/10.7196/samj.5302
Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY et al (2009) Second Asia-Pacific consensus guidelines for Helicobacter Pylori infection. J Gastroenterol Hepatol (Australia) 24:1587–1600. https://doi.org/10.1111/j.1440-1746.2009.05982.x
Smith S, Fowora M, Pellicano R (2019) Infections with Helicobacter Pylori and challenges encountered in Africa. World J Gastroenterol 25:3183–3195. https://doi.org/10.3748/wjg.v25.i25.3183
Sjomina O, Pavlova J, Niv Y, Leja M (2018) Epidemiology of Helicobacter Pylori infection. Helicobacter 23:6–11. https://doi.org/10.1111/hel.12514
Ofori EG, Adinortey CA, Bockarie AS, Kyei F, Tagoe EA, Adinortey MB (2019) Helicobacter Pylori infection, virulence genes’ distribution and accompanying clinical outcomes: the West Africa situation. BioMed Res Int. https://doi.org/10.1155/2019/7312908
Khoder G, Sualeh Muhammad J, Mahmoud I, Soliman SSM, Burucoa C (2019) Prevalence of Helicobacter Pylori and its associated factors among healthy asymptomatic residents in the United Arab Emirates. Pathogens. https://doi.org/10.3390/pathogens8020044
Awuku YA, Simpong DL, Alhassan IK, Tuoyire DA, Afaa T, Adu P (2017) Prevalence of Helicobacter Pylori Infection among children living in a rural setting in Sub-Saharan Africa. BMC Public Health 17:1–6. https://doi.org/10.1186/s12889-017-4274-z
Oreh AC, Onu A, Moses AL (2021) Prevalence and determinants of Helicobacter Pylori infection among adult dyspeptic patients in Abuja, Nigeria. West African J Med 38:775–784
Alemzero DA, Iqbal N, Iqbal S, Mohsin M, Chukwuma NJ, Shah BA (2021) Assessing the perceived impact of exploration and production of hydrocarbons on households perspective of environmental regulation in Ghana. Environ Sci Pollut Res 28:5359–5371. https://doi.org/10.1007/s11356-020-10880-3
Alfaray RI, Saruuljavkhlan B, Ansari S, Fauzia KA, Yamaoka Y (2022) Review: epidemiology of Helicobacter pylori infection. Microb Health Dis 4:1–10
Olapeju WD, Chidozie BI, Thecla CE, Onyinye UA, Obumneme BE, Kenneth NO (2020) Seroprevalence and risk factors of Helicobacter Pylori infection among children in South-East Nigeria. J Gastroenterol Hepatol Res https://doi.org/10.17554/j.issn.2224-3992.2020.09.869
Kpossou AR, Kouwakanou B, Séidou F, Saké Alassane K, Vignon RK, Martin Sokpon CN, Ahouada C, Zoundjiekpon V, Souroko F, Kodjoh N et al (2020) Prevalence of Helicobacter Pylori infection and gastric preneoplastic lesions in patients admitted for upper gastro-intestinal endoscopy in Cotonou (Benin Republic). Gastroenterol Hepatol Open Access 11:208–213. https://doi.org/10.15406/ghoa.2020.11.00441
Aminde JA, Dedino GA, Ngwasiri CA, Ombaku KS, Mahop Makon CA, Aminde LN (2019) Helicobacter Pylori infection among patients presenting with dyspepsia at a primary care setting in cameroon: seroprevalence, five-year trend and predictors. BMC Infect Dis 19:1–9. https://doi.org/10.1186/s12879-019-3677-0
Lawson-Ananissoh LM, Bouglouga O, Bagny A, El-Hadj Yakoubou R, Kaaga L, Redah D (2015) Epidemiological profile of gastro duodenal ulcers in the campus teaching hospital of Lomé (Togo). J Africain d’Hepato-Gastroenterologie 9:99–103. https://doi.org/10.1007/s12157-015-0597-5
Birato YC, Masimango BA, Katabana DM, Shindano TA (2023) Risk factors of Helicobacter Pylori infection in Bukavu, democratic republic of the congo: a case-control study. Annals Med Surg 85:727–731. https://doi.org/10.1097/MS9.0000000000000409
Sukri A, Hanafiah A, Mohamad Zin N, Kosai NR (2020) Epidemiology and role of Helicobacter Pylori virulence factors in gastric cancer carcinogenesis. APMIS 128:150–161. https://doi.org/10.1111/apm.13034
Mubiru IS, Kasirye PG, Hume H, Ndeezi G (2022) Prevalence and factors associated with Helicobacter Pylori infection among children with sickle cell anemia attending Mulago Hospital, in Uganda. Afr Health Sci 22:135–145. https://doi.org/10.4314/ahs.v22i2.16
Melese A, Genet C, Zeleke B, Andualem T (2019) Helicobacter Pylori infections in ethiopia; prevalence and associated factors: a systematic review and meta-analysis. BMC Gastroenterol 19:1–15. https://doi.org/10.1186/s12876-018-0927-3
Kasmi H, Doukani K, Ali A, Tabak S, Bouhenni H (2020) Epidemiological profile of Helicobacter Pylori infection in patients with digestive symptoms in Algeria. J Epidemiol Glob Health 10:293–297. https://doi.org/10.2991/jegh.k.200527.001
Galal YS, Ghobrial CM, Labib JR, Abou-Zekri ME (2019) Helicobacter Pylori among symptomatic egyptian children: prevalence, risk factors, and effect on growth. J Egyptian Public Health Assoc. https://doi.org/10.1186/s42506-019-0017-6
Ben Mansour K, Fendri C, Battikh H, Garnier M, Zribi M, Jlizi A, Burucoa C (2016) Multiple and mixed Helicobacter Pylori infections: comparison of two epidemiological situations in Tunisia and France. Infect Genet Evol 37:43–48. https://doi.org/10.1016/j.meegid.2015.10.028
Palamides P, Jolaiya T, Idowu A, Loell E, Onyekwere C, Ugiagbe R, Agbo I, Lesi O, Ndububa D, Adekanle O et al (2020) Helicobacter Pylori patient isolates from South Africa and Nigeria differ in virulence factor pathogenicity profile and associated gastric disease outcome. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-66128-0
Kabakambira JD, Hategeka C, Page C, Ntirenganya C, Dusabejambo V, Ndoli J, Ngabonziza F, Hale D, Bayingana C, Walker T (2018) Efficacy of Helicobacter pylori eradication regimens in Rwanda: a randomized controlled trial. BMC Gastroenterol 18:1–9. https://doi.org/10.1186/s12876-018-0863-2
Hodges P, Kelly P, Kayamba V (2021) Helicobacter Pylori infection and hypochlorhydria in Zambian adults and children: a secondary data analysis. PLoS One 16:1–11. https://doi.org/10.1371/journal.pone.0256487
Öztekin M, Yılmaz B, Ağagündüz D, Capasso R (2021) Overview of Helicobacter Pylori infection: clinical features, treatment, and nutritional aspects. Diseases 9:66. https://doi.org/10.3390/diseases9040066
Kayali S, Aloe R, Bonaguri C, Gaiani F, Manfredi M, Leandro G, Fornaroli F, Di Mario F, De’angelis GL (2018) Non-invasive tests for the diagnosis of Helicobacter Pylori State of the Art. Acta Biomedica 89:58–64, https://doi.org/10.23750/abm.v89i8-S.7910
Yu XC, Shao QQ, Ma J, Yu M, Zhang C, Lei L, Zhou Y, Chen WC, Zhang W, Fang XH et al (2022) Family-based Helicobacter pylori infection status and transmission pattern in Central China, and its clinical implications for related disease prevention. World J Gastroenterol 28:3706–3719. https://doi.org/10.3748/wjg.v28.i28.3706
Hadji M, Mortazavi M, Saberi S, Esmaieli M, Amini N, Akrami R, Daroudian R, Shakeri F, Khedmat H, Pukkala E et al (2022) Helicobacter Pylori acquisition rates and the associated risk factors amongst newlywed couples; a prospective cohort study in Tehran, Iran. Microb Infect 24:104974. https://doi.org/10.1016/j.micinf.2022.104974
Linz B, Windsor HM, Gajewski JP, Hake CM, Drautz DI, Schuster SC, Marshall BJ (2013) Helicobacter Pylori genomic microevolution during naturally occurring transmission between adults. PLoS One 8:1–9. https://doi.org/10.1371/journal.pone.0082187
Salama NR, Hartung ML, Müller A (2013) Life in the stomach: persistence strategies Helicobacter Pylori. Nation Institute Health 11:385–399. https://doi.org/10.1038/nrmicro3016.Life
Li L, Tan J, Liu L, Li J, Chen G, Chen M, Xie J, Song Q, Huang X, Xie S (2020) Association between H. pylori infection and health Outcomes: an umbrella review of systematic reviews and meta-analyses. BMJ Open. https://doi.org/10.1136/bmjopen-2019-031951
Zhang L, Chen X, Ren B, Zhou X, Cheng L (2022) Helicobacter pylori in the oral cavity: current evidence and potential survival strategies. Int J Mol Sci. https://doi.org/10.3390/ijms232113646
Molaoa SZ (2021) Prevalence of Helicobacter Pylori infection and the incidence of the associated malignant and Peptic Ulcer Disease (PUD) at Nelson Mandela Academic Hospital: a retrospective analysis. J Drug Assessm 10:57–61. https://doi.org/10.1080/21556660.2020.1854560
Xu C, Soyfoo DM, Wu Y, Xu S (2020) Virulence of Helicobacter Pylori outer membrane proteins: an updated review. Eur J Clin Microbiol Infect Dis 39:1821–1830. https://doi.org/10.1007/s10096-020-03948-y
Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R (2021) Helicobacter Pylori virulence factors—mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 10:1–37. https://doi.org/10.3390/cells10010027
Zuo W, Yang H, Li N, Ouyang Y, Xu X, Hong J (2022) Helicobacter Pylori infection activates Wnt/β-catenin pathway to promote the occurrence of gastritis by upregulating ASCL1 and AQP5. Cell Death Discovery 8:1–10. https://doi.org/10.1038/s41420-022-01026-0
Duan M, Li Y, Liu J, Zhang W, Dong Y, Han Z, Wan M, Lin M, Lin B, Kong Q et al (2023) Transmission routes and patterns of Helicobacter Pylori. Helicobacter 28:1–16. https://doi.org/10.1111/hel.12945
Bakhti SZ, Latifi-Navid S (2021) Interplay and cooperation of Helicobacter Pylori and gut microbiota in gastric carcinogenesis. BMC Microbiol 21:1–16. https://doi.org/10.1186/s12866-021-02315-x
Shi X, Chen Z, Yang Y, Yan S (2022) Bile reflux gastritis: insights into pathogenesis, relevant factors, carcinomatous risk, diagnosis, and management. Gastroenterol Res Pract. https://doi.org/10.1155/2022/2642551
Meliț LE, Mărginean CO, Mărginean CD, Mărginean MO (2019) The Relationship between toll-like receptors and Helicobacter Pylori-related gastropathies: still a controversial topic. J Immunol Res. https://doi.org/10.1155/2019/8197048
Miranda-Galvis M, Loveless R, Kowalski LP, Teng Y (2021) Impacts of environmental factors on head and neck cancer pathogenesis and progression. Cells 10:1–16
Deng Q, Xu Y, Zhong Y, Tang L, Du S, Yang J, Wu L, Guo S, Huang B, Cao H et al (2022) MiR-30c increases the intracellular survival of Helicobacter Pylori by inhibiting autophagy. Cell Microbiol 2022:1–12. https://doi.org/10.1155/2022/4536450
Santos ML, de Brito BB, da Silva FA, Sampaio MM, Marques HS, Oliveira N, de Magalhães Queiroz DM, de Melo FF (2020) Helicobacter Pylori infection: beyond gastric manifestations. World J Gastroenterol. 26(28):4076–4093. https://doi.org/10.3748/wjg.v26.i28.4076
Bougafa F (2023) Prevalence of H Pylori Infection and related blood biomarker among Autistic children in Tobruk City, Libya. Alqalam J Med Appl Sci 6:274–278. https://doi.org/10.5281/zenodo.8007966
Erickson LD, White DS, Bassett P, Gale SD, Brown BL, Hedges D (2023) Cognitive function in UK adults seropositive for Helicobacter Pylori. PLoS One 18:1–12. https://doi.org/10.1371/journal.pone.0286731
Abdu A, Cheneke W, Adem M, Belete R, Getachew A (2020) Dyslipidemia and associated factors among patients suspected to have Helicobacter Pylori infection at Jimma University Medical Center, Jimma. Ethiopia Int J General Med 13:311–321. https://doi.org/10.2147/IJGM.S243848
Ansari S, Yamaoka Y (2019) Helicobacter Pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins 11:1–26. https://doi.org/10.3390/toxins11110677
Guo X, Yin B, Wang C, Huo H, Aziziaram Z (2021) Risk assessment of gastric cancer in the presence of Helicobacter Pylori CagA and HopQII genes. Cell Mol Biol (Noisy-le-grand) 67:299–305. https://doi.org/10.14715/cmb/2021.67.4.33
de Brito BB, da Silva FA, Soares AS, Pereira VA, Santos ML, Sampaio MM, Neves PH, de Melo FF (2019) Pathogenesis and clinical management of Helicobacter Pylori gastric infection. World J Gastroenterol. 25(37):5578. https://doi.org/10.3748/wjg.v25.i37.5578
Franceschi F, Covino M, Roubaud Baudron C (2019) Review: Helicobacter Pylori and extragastric diseases. Helicobacter 24:1–8. https://doi.org/10.1111/hel.12636
Shatila M, Thomas AS (2022) Current and future perspectives in the diagnosis and management of Helicobacter Pylori infection. J Clin Med. https://doi.org/10.3390/jcm11175086
Ali A, AlHussaini KI (2024) Helicobacter Pylori: a contemporary perspective on pathogenesis, diagnosis and treatment strategies. Microorganisms. https://doi.org/10.3390/microorganisms12010222
Kiesewetter B, Christiane C-B, Michael L, Fangtian W, Dupuis J, Barau C, Arcaini L, Paulli M, Lucioni M, Bonometti A et al (2021) Genetic characterization and clinical features of helicobacter tissue lymphoma. Cancers 13:1–14. https://doi.org/10.3390/cancers13122993
Hu Y, Zhu Y, Lu NH (2017) Novel and effective therapeutic regimens for Helicobacter Pylori in an era of increasing antibiotic resistance. Front Cell Infect Microbiol 7:1–20. https://doi.org/10.3389/fcimb.2017.00168
Bordin DS, Voynovan IN, Andreev DN, Maev IV (2021) Current Helicobacter Pylori diagnostics. Diagnostics 11:1–11. https://doi.org/10.3390/diagnostics11081458
Boyanova L, Hadzhiyski P, Gergova R, Markovska R (2023) Evolution of Helicobacter Pylori resistance to antibiotics: a topic of increasing concern. Antibiotics. https://doi.org/10.3390/antibiotics12020332
Jiang Z, Qian X, Wang Z, Dong Y, Pan Y, Zhang Z, Wang S (2022) Antibiotic resistance of Helicobacter Pylori isolated from patients in Nanjing, China: a cross-section study from 2018 to 2021. Front Cell Infect Microbiol 12:1–8. https://doi.org/10.3389/fcimb.2022.970630
Camorlinga-Ponce M, Gómez-Delgado A, Aguilar-Zamora E, Torres RC, Giono-Cerezo S, Escobar-Ogaz A, Torres J (2021) Phenotypic and genotypic antibiotic resistance patterns in Helicobacter Pylori Strains from ethnically diverse population in México. Front Cell Infect Microbiol 10:1–11. https://doi.org/10.3389/fcimb.2020.539115
Abouwarda AM, Ismail TA, Abu El-Wafa WM, Faraag AH (2022) Synergistic activity and molecular modelling of fosfomycin combinations with some antibiotics against multidrug resistant Helicobacter Pylori. World J Microbiol Biotechnol. 38:1–17. https://doi.org/10.1007/s11274-022-03289-2
Zhong Z, Zhang Z, Wang J, Hu Y, Mi Y, He B, Zhang Y, Zhang X, Xia X, Huang H et al (2021) A retrospective study of the antibiotic-resistant phenotypes and genotypes of Helicobacter Pylori strains in China. Am J Cancer Res 11:5027–5037
Tshibangu-Kabamba E, Yamaoka Y (2021) Helicobacter Pylori infection and antibiotic resistance — from biology to clinical implications. Nat Rev Gastroenterol Hepatol 18:613–629. https://doi.org/10.1038/s41575-021-00449-x
Essaidi I, Bounder G, Jouimyi RM, Boura H, Elyounsi I, Kheir FZ, Benomar H, Badre W, Zerouali K, Maachi F (2022) Comparative study of Helicobacter Pylori resistance to clarithromycin and metronidazole and its association with epidemiological factors in a moroccan population. Asian Pac J Cancer Prev 23:2755–2761. https://doi.org/10.31557/APJCP.2022.23.8.2755
Tacconelli E, Pezzani MD (2019) Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis 19:4–6. https://doi.org/10.1016/S1473-3099(18)30648-0
Vital JS, Tanoeiro L, Lopes-Oliveira R, Vale FF (2022) Biomarker characterization and prediction of virulence and antibiotic resistance from Helicobacter Pylori next generation sequencing data. Biomolecules. 12(5):691. https://doi.org/10.3390/biom12050691
Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SSW, Wu JY, Kuo CH, Huang YK, Wu DC (2015) Diagnosis of Helicobacter Pylori infection: current options and developments. World J Gastroenterol 21:11221–11235. https://doi.org/10.3748/wjg.v21.i40.11221
Cardos AI, Maghiar A, Zaha DC, Pop O, Fritea L, Miere F, Cavalu S (2022) Evolution of diagnostic methods for Helicobacter Pylori infections: from traditional tests to high technology, advanced sensitivity and discrimination tools. Diagnostics. https://doi.org/10.3390/diagnostics12020508
Miftahussurur M, Yamaoka Y (2016) Diagnostic methods of Helicobacter Pylori infection for epidemiological studies: critical importance of indirect test validation. BioMed Res Int. https://doi.org/10.1155/2016/4819423
Mărginean CO, Meliț LE, Săsăran MO (2022) Traditional and modern diagnostic approaches in diagnosing pediatric Helicobacter Pylori infection. Children 9:1–16. https://doi.org/10.3390/children9070994
Dechant FX, Dechant R, Kandulski A, Selgrad M, Weber F, Reischl U, Wilczek W, Mueller M, Weigand K (2020) Accuracy of different rapid urease tests in comparison with histopathology in patients with endoscopic signs of gastritis. Digestion 101:184–190. https://doi.org/10.1159/000497810
Tongtawee T, Bartpho T, Kaewpitoon S, Kaewpitoon N, Dechsukhum C, Leeanansaksiri W, Loyd RA, Talabnin K, Matrakool L, Panpimanmas S (2018) Genetic polymorphisms in TLR1, TLR2, TLR4, and TLR10 of Helicobacter Pylori- associated gastritis: a prospective cross-sectional study in Thailand. Eur J Cancer Prev 27:118–123. https://doi.org/10.1097/CEJ.0000000000000347
Abut E, Yaşar B, Güveli H, Blkba C, Bölükbaş FF, Dalay AR, Kurdaş OO (2010) Effect of the mucolytic erdosteine on the success rate of PPI-based first-line triple therapy for Helicobacter Pylori eradication: a prospective, double-blind, randomized, placebo-controlled study. Scandinavian J Gastroenterol 45:677–683. https://doi.org/10.3109/00365521003702726
Lee JY, Kim N (2015) Diagnosis of Helicobacter Pylori by invasive test: histology. Annals Trans Med 3:1–8. https://doi.org/10.3978/j.issn.2305-5839.2014.11.03
Zhu Y, Wang F, Zhou Y, Xia G, Dong L, He W, Xiao B (2019) Blue laser magnifying endoscopy in the diagnosis of chronic gastritis. Experim Therapeut Med. https://doi.org/10.3892/etm.2019.7811
Alsulaimany FAS, Awan ZA, Almohamady AM, Koumu MI, Yaghmoor BE, Elhady SS, Elfaky MA (2020) Prevalence of Helicobacter Pylori infection and diagnostic methods in the Middle East and North Africa region. Medicina (Lithuania) 56:1–15. https://doi.org/10.3390/medicina56040169
Obayo S, Muzoora C, Ocama P, Cooney MM, Wilson T, Probert CS (2015) Upper gastrointestinal diseases in patients for endoscopy in South-Western Uganda. Afr Health Sci 15:959–966. https://doi.org/10.4314/ahs.v15i3.33
Pohl D, Keller PM, Bordier V, Wagner K (2019) Review of current diagnostic methods and advances in Helicobacter Pylori diagnostics in the era of next generation sequencing ISBN 0000000212298
Mărginean CO, Meliț LE, Săsăran MO (2021) Gastric microenvironment—a partnership between innate immunity and gastric microbiota tricks Helicobacter Pylori. J Clin Med. https://doi.org/10.3390/jcm10153258
Kosikowska P, Berlicki Ł (2011) Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin Ther Pat 21:945–957. https://doi.org/10.1517/13543776.2011.574615
Broekaert IJ, Borrelli O, Dolinsek J, Martin-De-Carpi J, Mas E, Miele E, Pienar C, Ribes-Koninckx C, Thomassen R, Thomson M et al (2022) An ESPGHAN position paper on the use of breath testing in paediatric gastroenterology. J Pediatr Gastroenterol Nutr 74:123–137. https://doi.org/10.1097/MPG.0000000000003245
Zhou Q, Li L, Ai Y, Pan Z, Guo M, Han J (2017) Diagnostic accuracy of the 14C-urea breath test in Helicobacter Pylori infections: a meta-analysis. Wien Klin Wochenschr 129:38–45. https://doi.org/10.1007/s00508-016-1117-3
Dore MP, Pes GM (2021) What is new in Helicobacter Pylori diagnosis. an overview. J Clin Med 10:1–15. https://doi.org/10.3390/jcm10102091
Bachir M, Allem R, Benejat L, Tifrit A, Medjekane M, Drici AEM, Megraud F, Douidi KT (2018) Molecular detection of mutations involved in Helicobacter Pylori antibiotic resistance in Algeria. J Antimicrob Chemother 73:2034–2038. https://doi.org/10.1093/jac/dky167
Qiu E, Li Z, Han S (2021) Methods for detection of Helicobacter Pylori from stool sample: current options and developments. Braz J Microbiol 52:2057–2062. https://doi.org/10.1007/s42770-021-00589-x
El-Shabrawi M, El-Aziz NA, Hassanin F, Eskander A, Abou-Zekri M, Mansour H, Meshaal S, El-Adly TZ (2018) Stool antigen detection versus13C-urea breath test for non-invasive diagnosis of pediatric Helicobacter Pylori infection in a limited resource setting. Arch Med Sci 14:69–73. https://doi.org/10.5114/aoms.2016.61031
Lmj B, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, Yaghoobi M, Ks G (2018) Non-invasive diagnostic tests for Helicobacter Pylori infection. Cochrane Database System Rev https://doi.org/10.1002/14651858.CD012080.pub2.www.cochranelibrary.com
Malfertheiner P, Megraud F, O’Morain C, Gisbert JP, Kuipers EJ, Axon A, Bazzoli F, Gasbarrini A, Atherton J, Graham DY et al (2017) Management of Helicobacter Pylori infection-the maastricht V/florence consensus report. Gut 66:6–30. https://doi.org/10.1136/gutjnl-2016-312288
Berlamont H, De Witte C, De Bruyckere S, Fox JG, Backert S, Smet A, Boyen F, Haesebrouck F (2021) Differentiation of gastric helicobacter species using maldi-tof mass spectrometry. Pathogens 10:1–15. https://doi.org/10.3390/pathogens10030366
Tanno D, Saito K, Ohashi K, Toyokawa M, Yamadera Y, Shimura H (2022) Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry with time-of-flight peak analysis for rapid and accurate detection of group b streptococcus in pregnant women. Microbiology Spectrum 10:1–10. https://doi.org/10.1128/spectrum.01732-21
Kumaran A, Jude Serpes N, Gupta T, James A, Sharma A, Kumar D, Nagraik R, Kumar V, Pandey S (2023) Advancements in CRISPR-based biosensing for next-gen point of care diagnostic application. Biosensors. https://doi.org/10.3390/bios13020202
Mobed A, Baradaran B, Guardia M, Agazadeh M, Hasanzadeh M, Rezaee MA, Mosafer J, Mokhtarzadeh A, Hamblin HR (2019) Advances in detection of fastidious bacteria: from microscopic observation to molecular biosensors. Trends Anal Chem 113:157–171. https://doi.org/10.1016/j.trac.2019.02.012
Shahrashoob M, Mohsenifar A, Tabatabaei M, Rahmani-Cherati T, Mobaraki M, Mota A, Shojaeic TR (2016) Detection of Helicobacter Pylori genome with an optical biosensor based on hybridization of urease gene with a gold nanoparticles-labeled probe. J Appl Spectrosc 83:322–329. https://doi.org/10.1007/s10812-016-0290-5
Zheng Y, Wang K, Zhang J, Qin W, Yan X, Shen G, Gao G, Pan F, Cui D (2016) Simultaneous quantitative detection of Helicobacter Pylori based on a rapid and sensitive testing platform using quantum dots-labeled immunochromatiographic test strips. Nanoscale Res Lett 11:1–11. https://doi.org/10.1186/s11671-016-1254-7
Zhao Y, Xu D, Tan W (2017) Aptamer-functionalized nano/micro-materials for clinical diagnosis: isolation, release and bioanalysis of circulating tumor cells. Integrat Biol (United Kingdom) 9:188–205. https://doi.org/10.1039/c6ib00239k
Vidic J, Manzano M (2021) electrochemical biosensors for rapid pathogen detection. Curr Opin Electrochem 29:100750. https://doi.org/10.1016/j.coelec.2021.100750
McConnell EM, Morrison D, Rey Rincon MA, Salena BJ, Li Y (2020) Selection and applications of synthetic functional DNAs for bacterial detection. Trends Anal Chem 124:115785. https://doi.org/10.1016/j.trac.2019.115785
Singh A, Sharma A, Ahmed A, Sundramoorthy AK, Furukawa H, Arya S, Khosla A (2021) Recent advances in electrochemical biosensors: applications, challenges, and future scope. Biosensors 11:1–31. https://doi.org/10.3390/bios11090336
Kuchmenko TA, Shuba AA, Kuchmenko DA, Umarkhanov RU (2020) Development of a method for assessing Helicobacter Pylori activity based on exhaled air composition with the use of an array of piezoelectric chemical sensors. J Anal Chem 75:553–562. https://doi.org/10.1134/S1061934820040085
Ajayi A, Jolaiya T, Smith SI (2021) Direct detection of Helicobacter Pylori from biopsies of patients in Lagos, Nigeria using real-time PCR—a Pilot Study. BMC Res Notes. https://doi.org/10.1186/s13104-021-05505-y
Darko R, Yawson AE, Osei V, Owusu-Ansah J, Aluze-Ele S (2015) Changing patterns of the prevalence of Helicobacter Pylori among patients at a Corporate Hospital in Ghana. Ghana Med J 49:147–153. https://doi.org/10.4314/gmj.v49i3.4
Olokoba AB, Gashau W, Bwala S, Adamu A, Salawu FK (2013) Helicobacter Pylori infection in Nigerians with dyspepsia. Ghana Med J 47:79–81
Moubri M, Kalach N, Larras R, Berrah H, Mouffok F, Guechi Z, Cadranel S (2019) Adapted first-line treatment of Helicobacter Pylori infection in Algerian Children. Ann Gastroenterol 32:60–66. https://doi.org/10.20524/aog.2018.0317
Alboraie M, Elhossary W, Aly OA, Abbas B, Abdelsalam L, Ghaith D, Shady Z, Gaber Y, Adel E, Peura D et al (2019) Egyptian recommendations for management of Helicobacter Pylori infection: 2018 Report. Arab J Gastroenterol 20:175–179. https://doi.org/10.1016/j.ajg.2019.09.001
Altyar IA, Abdalla AM, Abdalwahab SS, Abdalwahab SS (2015) The seroprevalence of Helicobacter Pylori infection in Tripoli (Libya). Nat Sci 13:81–86
Almehdawi KA, Ali RH (2016) The prevalence of Helicobacter Pylori infection in Benghazi, Libya. IOSR J Dental Med Sci 15:73–77. https://doi.org/10.9790/0853-150787377
Nami A, Grima N, Almadni M, Huwiage G, Shahlol AMA, Khalifallah HM (2019) Helicobacter Pylori infection in healthy and dyspeptic adult populations resident in Tripoli and Sabha of Libyan Cities: a seroepidemiologic study. J Pure Appl Sci 18:206–209
Kouitcheu Mabeku LB, Bello Epesse M, Fotsing S, Kamgang R, Tchidjo M (2021) Stool antigen testing, a reliable noninvasive method of assessment of Helicobacter Pylori infection among patients with gastro-duodenal disorders in Cameroon. Dig Dis Sci 66:511–520. https://doi.org/10.1007/s10620-020-06219-0
Segamwenge IL, Kagimu M, Ocama P, Opio K (2014) The utility of the Helicobacter Pylori stool antigen test in managing dyspepsia: an experience from a low resource setting. Afr Health Sci 14:829–834. https://doi.org/10.4314/ahs.v14i4.9
Oling M, Odongo J, Kituuka O, Galukande M (2015) Prevalence of Helicobacter Pylori in dyspeptic patients at a tertiary hospital in a low resource setting surgery. BMC Res Notes 8:4–9. https://doi.org/10.1186/s13104-015-1184-y
Aitila P, Mutyaba M, Okeny S, Ndawula Kasule M, Kasule R, Ssedyabane F, Okongo B, Onyuthi Apecu R, Muwanguzi E, Oyet C (2019) Prevalence and risk factors of Helicobacter Pylori infection among children aged 1 to 15 years at Holy Innocents Children’s Hospital, Mbarara, South Western Uganda. J Trop Med 2019:1–6. https://doi.org/10.1155/2019/9303072
Mk K, Ks M, Rheeder P (2016) The prevalence of Helicobacter pylori infection in bleeding and non-bleeding gastric ulcers: a cross-sectional case-control study. J Bioanal Biomed. https://doi.org/10.4172/1948-593x.1000153
Tanih NF, Okeleye BI, Ndip LM, Clarke AM, Naidoo N, Mkwetshana N, Green E, Ndip RN (2010) Helicobacter Pylori prevalence in dyspeptic patients in the Eastern Cape Province - race and disease status. S Afr Med J 100:734–737. https://doi.org/10.7196/samj.4041
Idowu A, Mzukwa A, Harrison U, Palamides P, Haas R, Mbao M, Mamdoo R, Bolon J, Jolaiya T, Smith S et al (2019) Detection of Helicobacter Pylori and its virulence genes (CagA, DupA, and VacA) among patients with gastroduodenal diseases in Chris Hani Baragwanath Academic Hospital, South Africa. BMC Gastroenterol 19:1–10. https://doi.org/10.1186/s12876-019-0986-0
Agha A, Graham DY (2005) Evidence-based examination of the African Enigma in relation to Helicobacter Pylori infection. Scand J Gastroenterol 40:523–529. https://doi.org/10.1080/00365520510012280
Steurs L (2019) European aid and health system strengthening: an analysis of donor approaches in the DRC, Ethiopia, Uganda, Mozambique and the Global Fund. Global Health Action. https://doi.org/10.1080/16549716.2019.1614371
Makristathis A, Hirschl AM, Mégraud F, Bessède E (2019) Review: diagnosis of Helicobacter Pylori infection. Helicobacter 24:1–7. https://doi.org/10.1111/hel.12641
Shirin H, Levine A, Shevah O, Shabat-Sehayek V, Aeed H, Wardi J, Birkenfeld S, Eliakim R, Avni Y (2005) Eradication of Helicobacter Pylori can be accurately confirmed 14 days after termination of triple therapy using a high-dose citric acid-based 13c urea breath test. Digestion 71:208–212. https://doi.org/10.1159/000087045
Wenzhen Y, Yumin L, Quanlin G, Kehu Y, Lei J, Donghai W, Lijuan Y (2010) Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter Pylori infection? - meta-analysis of randomized controlled trials. Intern Med 49:1103–1109. https://doi.org/10.2169/internalmedicine.49.3031
Vécsei A, Innerhofer A, Binder C, Gizci H, Hammer K, Bruckdorfer A, Riedl S, Gadner H, Hirschl AM, Makristathis A (2010) Stool polymerase chain reaction for Helicobacter Pylori detection and clarithromycin susceptibility testing in children. Clin Gastroenterol Hepatol 8:309–312. https://doi.org/10.1016/j.cgh.2009.12.002
Chey WD, Leontiadis GI, Howden CW, Moss SF (2017) ACG clinical guideline: treatment of Helicobacter Pylori infection. Am J Gastroenterol 112:212–238. https://doi.org/10.1038/ajg.2016.563
Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P et al (2016) The toronto consensus for the treatment of Helicobacter Pylori infection in adults. Gastroenterology 151:51-69.e14. https://doi.org/10.1053/j.gastro.2016.04.006
Harrison U, Fowora MA, Seriki AT, Loell E, Mueller S, Ugo-Ijeh M, Onyekwere CA, Lesi OA, Otegbayo JA, Akere A et al (2017) Helicobacter Pylori strains from a Nigerian cohort show divergent antibiotic resistance rates and a uniform pathogenicity profile. PLoS One 12:1–16. https://doi.org/10.1371/journal.pone.0176454
Debraekeleer A, Remaut H (2018) Future perspective for potential Helicobacter Pylori eradication therapies. Future Microbiol 13:671–687. https://doi.org/10.2217/fmb-2017-0115
Bakal SN, Bereswill S, Heimesaat MM (2017) Finding novel antibiotic substances from medicinal plants — antimicrobial properties of Nigella sativa directed against multidrug resistant bacteria. Eur J Microbiol Immunol 7:92–98. https://doi.org/10.1556/1886.2017.00001
Zaidi SF, Muhammad JS, Usmanghani K, Sugiyama T (2015) Pharmacological Ins and outs of medicinal plants against Helicobacter Pylori: a review. Pakistan J Pharmaceut Sci 28:1171–1176
Ayala G, Escobedo-Hinojosa WI, de La Cruz-Herrera CF, Romero I (2014) Exploring alternative treatments for Helicobacter Pylori infection. World J Gastroenterol 20:1450–1469. https://doi.org/10.3748/wjg.v20.i6.1450
Seyyedmajidi M, Ahmadi A, Hajiebrahimi S, Seyedmajidi S, Rajabikashani M, Firoozabadi M, Vafaeimanesh J (2016) Addition of cranberry to proton pump inhibitor-based triple therapy for Helicobacter Pylori eradication. J Res Pharm Pract 5:248. https://doi.org/10.4103/2279-042x.192462
Yanaka A (2011) Sulforaphane enhances protection and repair of gastric mucosa against oxidative stress in vitro, and demonstrates anti-inflammatory effects on Helicobacter Pylori Infected gastric mucosae in mice and human subjects. Curr Pharm Des 17:1532–1540. https://doi.org/10.2174/138161211796196945
Nontakham J, Charoenram N, Upamai W, Taweechotipatr M, Suksamrarn S (2014) Anti-Helicobacter pylori xanthones of garcinia fusca. Arch Pharmacal Res 37:972–977. https://doi.org/10.1007/s12272-013-0266-4
Stoicov C, Saffari R, Houghton JM (2009) Green tea inhibits helicobacter growth in vivo and in vitro. Int J Antimicrob Agents 33:473–478. https://doi.org/10.1016/j.ijantimicag.2008.10.032
Ruggiero P, Rossi G, Tombola F, Pancotto L, Lauretti L, Del Giudice G, Zoratti M (2007) Red wine and green tea reduce H. Pylori- or VacA-induced gastritis in a mouse model. World J Gastroenterol 13:349–354. https://doi.org/10.3748/wjg.v13.i3.349
da Silva Junior IF, Balogun SO, de Oliveira RG, Damazo AS, de Oliveira Martins DT (2016) Piper umbellatum L.: A medicinal plant with gastric-ulcer protective and ulcer healing effects in experimental rodent models. J Ethnopharmacol. 192:123–31. https://doi.org/10.1016/j.jep.2016.07.011
Inngjerdingen KT, Thöle C, Diallo D, Paulsen BS, Hensel A (2014) Inhibition of Helicobacter Pylori adhesion to human gastric adenocarcinoma epithelial cells by aqueous extracts and pectic polysaccharides from the roots of Cochlospermum Tinctorium A. Rich and Vernonia Kotschyana Sch. Bip. Ex Walp. Fitoterapia 95:127–132. https://doi.org/10.1016/j.fitote.2014.03.009
Boyanova L, Ilieva J, Gergova G, Vladimirov B, Nikolov R, Mitov I (2015) Honey and green/black tea consumption may reduce the risk of Helicobacter Pylori infection. Diagn Microbiol Infect Dis 82:85–86. https://doi.org/10.1016/j.diagmicrobio.2015.03.001
Manyi-Loh CE, Clarke AM, Munzhelele T, Green E, Mkwetshana NF, Ndip RN (2010) Selected South African honeys and their extracts possess in vitro anti-helicobacter pylori activity. Arch Med Res 41:324–331. https://doi.org/10.1016/j.arcmed.2010.08.002
Gill HS (2003) Probiotics to enhance anti-infective defences in the gastrointestinal tract. Bailliere’s Best Pract Res Clin Gastroenterol 17:755–773. https://doi.org/10.1016/S1521-6918(03)00074-X
Patel A, Shah N, Prajapati JB (2014) Clinical application of probiotics in the treatment of Helicobacter pylori infection—a brief review. J Microbiol Immunol Infect 47(5):429–37. https://doi.org/10.1016/j.jmii.2013.03.010
Ruer S, Pinotsis N, Steadman D, Waksman G, Remaut H (2015) Virulence-targeted antibacterials: concept, promise, and susceptibility to resistance mechanisms. Chem Biol Drug Des 86:379–399. https://doi.org/10.1111/cbdd.12517
Peek RM (2005) Pathogenesis of Helicobacter Pylori infection. Springer Semin Immunopathol 27:197–215. https://doi.org/10.1007/s00281-005-0204-8
Prinz C, Schöniger M, Rad R, Becker I, Keiditsch E, Wagenpfeil S, Classen M, Rösch T, Schepp W, Gerhard M (2001) Key importance of the Helicobacter Pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Can Res 61:1903–1909
Posselt G, Backert S, Wessler S (2013) The functional interplay of Helicobacter Pylori factors with gastric epithelial cells induces a multi-step process in pathogenesis. Cell Commun Signal 11:1. https://doi.org/10.1186/1478-811X-11-77
Javaheri A, Kruse T, Moonens K, Mejías-Luque R, Debraekeleer A, Asche CI, Tegtmeyer N, Kalali B, Bach NC, Sieber SA et al (2016) Helicobacter Pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat Microbiol 2:1–12. https://doi.org/10.1038/nmicrobiol.2016.189
Ishijima N, Suzuki M, Ashida H, Ichikawa Y, Kanegae Y, Saito I, Borén T, Haas R, Sasakawa C, Mimuro H (2011) BabA-mediated adherence is a potentiator of the Helicobacter Pylori Type IV secretion system activity. J Biol Chem 286:25256–25264. https://doi.org/10.1074/jbc.M111.233601
Spaulding CN, Klein RD, Ruer S, Kau AL, Schreiber HL, Cusumano ZT, Dodson KW, Pinkner JS, Fremont DH, Janetka JW, Remaut H (2017) Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature. 546(7659):528–32. https://doi.org/10.1038/nature22972
O’Mahony R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, Holton J, Basset C (2005) Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter Pylori. World J Gastroenterol 11:7499–7507. https://doi.org/10.3748/wjg.v11.i47.7499
Kristof M, Pär G, Suresh S, Jeanna B, Romaõ E, Melissa M, Nordén J, Fallah M, Rakhimova L, Shevtsova A et al (2017) Structural insight into polymorphic ABO glycan binding by Helicobacter Pylori. Cell Host Microbe 176:498–503. https://doi.org/10.1016/j.neuroimage.2016.03.072.Cortical
Cammarota G, Sanguinetti M, Gallo A, Posteraro B (2012) Review article: biofilm formation by Helicobacter Pylori as a target for eradication of resistant infection. Aliment Pharmacol Ther 36:222–230. https://doi.org/10.1111/j.1365-2036.2012.05165.x
Dore MP, Cipolli A, Ruggiu MW, Manca A, Bassotti G, Pes GM (2018) Helicobacter Pylori eradication may influence timing of endoscopic surveillance for gastric cancer in patients with gastric precancerous lesions. Medicine (United States) 97:1–5. https://doi.org/10.1097/MD.0000000000009734
Graham DY (2014) History of Helicobacter Pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol 20:5191–5204. https://doi.org/10.3748/wjg.v20.i18.5191
Kikuchi S, Kato M, Katsuyama T, Tominaga S, Asaka M (2006) Design and planned analyses of an ongoing randomized trial assessing the preventive effect of Helicobacter pylori eradication on occurrence of new gastric carcinomas after endoscopic resection. Helicobacter. 11(3):147–51
Yoshida T, Kato J, Inoue I, Yoshimura N, Deguchi H, Mukoubayashi C, Oka M, Watanabe M, Enomoto S, Niwa T et al (2014) Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter Pylori antibody titer. Int J Cancer 134:1445–1457. https://doi.org/10.1002/ijc.28470
Zagari RM, Rabitti S, Greenwood DC, Eusebi LH, Vestito A, Bazzoli F (2017) Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter Pylori Antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther 46:657–667. https://doi.org/10.1111/apt.14248
Chiang TH, Chiu SYH, Chen SLS, Yen AMF, Fann JCY, Liu CY, Chou CK, Chiu HM, Shun CT, Wu MS et al (2019) Serum pepsinogen as a predictor for gastric cancer death a 16-year community-based cohort study. J Clin Gastroenterol 53:E186–E193. https://doi.org/10.1097/MCG.0000000000000992
Acknowledgements
Not applicable.
Funding
Authors declare that no funding was received for this study.
Author information
Authors and Affiliations
Contributions
BE: Conceptualization, literature research, writing – original draft, writing – review and editing, graphical visualization; DP, MP, and IA: Literature research, writing – original draft, writing – review and editing; KO: Conceptualization, Formatting and Formal Analyses, writing – original draft, writing – review and editing, Supervision, and coordination. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethics approval and consent to participate
This is a review paper and not a research paper; hence, no participants were used for this study, and ethics approval was not required.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emmanuel, B.N., Peter, D.A., Peter, M.O. et al. Helicobacter pylori infection in Africa: comprehensive insight into its pathogenesis, management, and future perspectives. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00166-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00166-6