Abstract
Background
Adipose tissue has recently become one of the most promising and predominant sources of mesenchymal stem cells owing to its high accessibility, culturing properties, regenerative potential, and relatively fewer ethical considerations. From the time of the adipose-derived stem cells (ADSCs) discovery, many beneficial properties have been found, including their regenerative, anti-inflammatory, immunomodulatory, and antimicrobial effects. The number of publications and clinical trials using ADSCs has increased significantly worldwide, attesting to the promising nature of the therapeutic properties of ADSCs.
Main body of the abstract
In clinical studies, ADSCs are mainly used to treat wounds, multiple sclerosis, soft tissue trauma, aging, diabetes, Parkinson’s disease, bone and cartilage regeneration, strokes, and spinal cord injuries. Few and insignificant adverse effects after ADSC treatment have been documented, suggesting their relative safety for clinical use. Despite significant progress in ADSC-related studies, several issues are yet to be addressed, including a lack of standardization of ADSC-associated protocols and the methods used to obtain them, inconsistent dosages, small numbers of patients in each treatment group, and variable graft purity. This severely complicates our ability to compare these studies, making the results even of similar studies controversial.
Short conclusion
This review described the current stage of ADSCs-based treatment outcomes and their limitations, associated with standardization of ADSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Regenerative medicine is a relatively new branch of science that aims to replace aged, damaged, and disease- or trauma-affected tissues and organs, and to stimulate organismal regenerative potential.
Stem cell therapy involves several mechanisms of action. One is direct replacement of damaged cells and tissues [1]. Another is a paracrine mechanism that involves modulation of the microenvironment, activation of the native immunity, anti-inflammatory effect and prevention of fibrosis development, pain relief through the secretion of cytokines, regulation of cell death, and immunomodulatory effect [2, 3]. Stem cell therapy is considered one of the most promising and highly effective treatment methods for several inflammatory diseases, infectious diseases, non-communicable diseases, cancer, age-related pathologies, pediatric diseases and rejuvenation [4,5,6].
Despite this, stem cell therapy is still not widespread and is even forbidden in some countries. Based on available data, no more than 15 allogeneic mesenchymal stem cell (MSC) products have been approved worldwide [7]. Implementation rates of stem-cell-based therapeutic products remain low, but they have been gradually increasing; as of 17 August 2022, twenty-four cellular and gene therapy products have been licensed by the Office of Tissues and Advanced Therapies (USA) [8]. Medical tourism to seek stem-cell-based therapies has increased significantly despite the small number of clinical studies and poor evidence base for such therapies [7, 9].
Initially MSCs were first found in bone marrow in 1976. It has been shown that MSCs, which are multipotent, can differentiate into mesenchymal, endodermal, and ectodermal cell lines [10]. Bone marrow is the gold-standard source of MSCs [11]. The most common harvesting site for bone marrow is the iliac crest, followed by the proximal femur [12]. However, bone marrow aspiration has significant drawbacks due to its high invasiveness and low MSCs yield [13]. Even MSCs harvested from different bones of the same individual differ in terms of their regenerative potential and cell concentration, and their effects vary between in-vivo and in-vitro settings [14]. Bone marrow biopsy is poorly tolerated by patients because of post-procedure pain, and most patients experience anxiety before and during the procedure, even in the case of experienced bone marrow donors [15, 16]. Owing to these limitations, alternative MSC donor sites and new approaches are in high demand. Connective tissue and stromal components of inner organs are graft-rich sources for MSCs isolation. One of these is adipose tissue, which is in abundance in a human body. The high proliferation and differentiation capacity of adipose-derived stem cells (ADSCs) and their more accessible donor sites make them a more promising and less invasive alternative to bone-marrow MSCs (BM-MSCs) for stem-cell-based therapies [17]. ADSCs and BM-MSCs have similar characteristics in terms of their morphology, properties, and receptors[18]. There are also other sources of adult tissue-derived MSC such as peripheral blood, endometrium, tooth pulp, and breast milk [19]. Umbilical cord, cord blood, placenta and amniotic fluid are the neonatal sources of stem cells [20]. Adult and neonatal stem cells have various clinical applications and their own advantages and disadvantages. Neonatal stem cells have higher proliferative capacity, potential growth by multi-layering due to the absence of contact inhibition, no senescence over passaging and lower immunogenicity, and higher immunosuppressive capacity [20]. Despite possessing better immunological properties, neonatal stem cells have several disadvantages that limit their clinical application such as low cell amount in a single cord blood unit, single time collection, high storage cost etc. [21].
In most circumstances, only the allogeneic application of neonatal stem cells is possible, while BM-MSCs, ADSCs and peripheral blood stem cells can also be used in autologous settings, which significantly facilitates ethical issues, prevents infections from spreading, and provides a limitless source of cells [22]. Moreover, BM-MSCs are not immune-privileged and have immunogenic potential in allogeneic settings [23]. Two strategies exist for prolonging their persistence and improving the efficacy of stem cell therapy: modifying the host immune system response or modifying the antigen properties of MSCs [24].
The range of application of stem cells-based treatment in clinical medicine expands every year, especially adipose tissue as one of the favorable sources of stem cells found a broad application in tissues engineering [25, 26]. This review aims to summarize current understandings of ADSC biology, to discuss the latest ADSC-based experimental studies and clinical trials, and to highlight the current advantages and limitations of using ADSCs in medicine.
A systematic search in the PubMed and Scopus database was conducted on 12 October 2023 for all studies including ADSCs, BM-MSCs and MSCs. Original articles, review articles, meta-analysis, clinical cases and case series written in English were selected for review. The search strategy included usage of the following terms: “adipose-derived stem cells”, “fat-derived stem cells”, “bone marrow-derived stem cells”, “mesenchymal stem cells” and their synonyms. Retrieved articles, relative to the review topic, were stored in a database and duplicates were removed.
2 Main text
2.1 Status of research regarding regenerative medicine using ADSCs
ADSCs were first retrieved from lipoaspirates by Zuk et al. in 2001 [27]. While BM-MSCs were historically discovered earlier than ADSCs, their clinical application is sometimes limited. This is why an alternative source of MSCs is required. One of them is ADSCs that have been intensively studied worldwide owing to their relative ease of isolation, few ethical considerations, non-invasive harvesting procedure, good culturing properties, and promising results in in-vitro and in-vivo research.
Medical stem cell therapy is flourishing worldwide; however, patients sometimes have unsubstantiated expectations regarding stem cell therapy. Sometimes, stem cell treatment is provided without proper indications and has life-threatening consequences [28]. The high cost of treatment, low quality, long waiting times, jurisdictional legal restrictions, inability to participate in clinical trials, and lack of access to unapproved treatments lead patients to engage in stem cell tourism. The leaders in international MSC tourism are the USA, China, India, Thailand, and Mexico [29].
In Japan, in addition to laws governing clinical trials conducted under the International Conference on Harmonisation – Good Clinical Practice and the requirement for the approval of regenerative medical products (Pharmaceutical and Medical Device Act), the Act on the Safety of Regenerative Medicine governs the implementation of regenerative medicine in clinical trials or as a treatment. Due to the Regenerative Medicine Act all the procedures were classified into risk categories (high, intermediate and low risk, which are Class I, II and III respectively), among which treatment and research using ADSCs are being conducted in various clinical departments, including the orthopedic and dental fields [30].
Around the world, stem cell therapies, including those using ADSCs, are offered in clinical practice, with the main clinical indications being multiple sclerosis, cellular therapy of cornea injuries, chronic pulmonary disease, rejuvenation, Parkinson's disease, bone and soft tissues augmentation and regeneration which were destroyed due periodontitis, stroke therapy, severe spinal cord injury, cerebral palsy, chronic wound healing, autism, amyotrophic latent arteriosclerosis, Alzheimer's disease, and inflammatory joints disease [31].
A search of “adipose-derived stem cells” on www.clinicaltrials.gov found that more than 394 clinical trials using ADSCs have been conducted worldwide, 132 of which have already been completed. In clinical trials, ADSCs have been used for face rejuvenation, keloid treatment, reconstructive surgery, alopecia treatment, arthritis therapy, periodontal therapy, diabetic wound healing, and many other purposes.
2.2 Biology of adipose tissue
Adipose tissue is a connective tissue with special properties. Approximately 20–25% percent of a healthy individual’s weight is adipose tissue. Based on morphological differences, adipocytes were distributed into white, brown and bright (beige) adipocytes. Depending on its location, adipose tissue is classified into subcutaneous (located under the skin) or visceral (around inner organs) fat. White adipocytes are found in white adipose tissue (WAT), and cell shape varies from spherical to oval or polyhedral. Almost the entire cell volume is occupied by a unilocular lipid droplet which occupied the central part of an adipocyte and flattens to the periphery nucleus. The adipocyte`s lipid droplets are lost on a histological section during the traditional way of tissues preparation, which gives WAT a thin polygonal mesh appearance [32, 33]. Visceral adipose tissue (VAT) presented as abdominal viscera, mesenterium and omentum, has completely different qualities compared to WAT. Adipocytes type, their secretome, endocrine regulation, proliferation rate, lipolytic activity, sensitivity to insulin and other hormones differ between subcutaneous WAT and visceral fat. Macrophages are more prevalent in VAT compared with subcutaneous WAT [34].
Brown adipose tissue is common in newborns and located in the neck, back and shoulder areas. With maturation, brown fat scatters around the body. In adults it is located around the neck and inner organs such as the kidneys, adrenal glands, aorta and mediastinum. Brown adipocytes are much smaller compared to white and beige adipocytes, and their lipids are distributed into numerous lipid droplets, with their nucleus located at the cell center. These cells are abundant in mitochondria, with a brown appearance. The major function of these cells is to produce heat. There are two types of brown adipocytes: high- and low-thermogenic adipocytes [35].
Beige adipocytes are a recently discovered type of brown adipocyte located in subcutaneous fat depots, such as the inguinal and anterior subcutaneous WAT; however, a small number can also be found in VAT [36]. Beige adipocytes have a multilocular morphology. Properties, cultural and functional differences of white, brown and beige adipocytes summarized in Table 1.
Similar to every connective tissue, adipose tissue presented as cells surrounded by an extracellular matrix. Cells percentage in adipose tissue is significantly prevail under the extracellular matrix component. Adipocyte is a minimal structural and functional unit of adipose tissue. Besides adipocytes, adipose tissue also consists of preadipocytes, fibroblasts, capillary endothelial cells, macrophages, and stem cells, all of which form the stromal vascular fraction (SVF) that supports, supplies, and protect adipocytes [36]. Adipose tissue has a good blood supply and is innervated by unmyelinated nerves [37].
In mammals, adipose tissue has the following important functions: energy storage, hormone secretion, metabolism, protection, and thermogenesis. In recent years, adipose tissue has been considered as a powerful endocrine organ because it produces several hormones such as estrogen, leptin, adiponectin, resistin, and biologically active substances such as TNF, IL-6, IL-1, CCL2, MCP1, PAI-1, and complement factors [38, 39].
SVF is one of the adipose tissue components that is a mixture of cells contained within adipocytes that is traditionally isolated by enzymatic digestion. After adipocytes extraction, connective tissue and blood from lipoaspirate, come the SVF, a mix including MSC, endothelial precursor, T-reg, adipose tissue macrophages, smooth muscle cells, pericytes and preadipocytes [40, 41].
2.3 Adipose tissue as a source of MSC
WAT is a huge source of MSCs with superior culturing properties. In humans that WAT has an abundance of CD-34+ -cells, immunohistochemical analysis has confirmed that CD-34+ cells are evenly distributed among white adipocytes [10]. It has been shown that about 5 × 105 stem cells can be isolated from a few milligrams of adipose tissue with the possibility of continuously culturing in vitro for up to one month without cell passaging [42]. Adipose tissue is a prospective source of MSCs owing to variable donor sites, the large quantity of biological sources from deceased donors, and routine deceased-donor workups [43, 44]. Studies have shown that WAT harvested from the abdomen of deceased, research-consenting donors indicated that the total nucleated cell count was even higher than that in living donors, and the morphology and functional properties (growth potential, gene expression level, and differentiation ability) of the cell culture were similar [43, 44]. However, changes in the properties and biology of adipose tissue in obese individuals are a general health condition [39, 45,46,47]. Isolated ADSCs from VAT and subcutaneous WAT had no differences in morphology and had the same expression of CD antigens. However, the growth rate of subcutaneous WAT ADSCs is 1.75 faster than ADSCs isolated from VAT also ADSCs were different in terms of angiogenic and inflammatory cytokines level. ADSCs from subcutaneous WAT have significantly lower concentrations of chitinase 3-like 1, IL-1β, EGF, MCP-1, Cystatin C, IL-6, IL-8, Pentraxin 3, TGF-β, plasminogen activator urokinase receptor and TNF-α [48].
There are three main criteria for ADSCs. Firstly, MSCs must have adherent growth; trilineage mesenchymal differentiation (adipocytes, osteoblasts, and chondroblasts). Secondly, ADSCs must express surface specific antigens such as expressing MSCs markers like CD44, CD105, CD90, and CD73, which are progenitors in subcutaneous WAT, and their phenotype is similar to BM-MSCs. Thirdly, ADSCs do not express the HLA-DR protein or MHC Class I molecules, which enable the possibility of allogeneic transplantation [49].
Some scientists considered that ADSCs to be immune-privileged cells [41]. The concept of immune privilege means that some biological grafts can survive in the recipient’s body for a certain time without triggering a graft-versus-host response or large-scale destructive inflammation in the place of application [50]. However, other studies have shown that MSCs are not completely immune-privileged, due to the triggering of both humoral and cellular immune responses in vivo, which depends on the microenvironment [24]. For example, the second transplantation of allogeneic MSCs from the same donor in mice resulted in accelerated rejection of cells, which attests to the formation of T-cell memory [51]. It was reported that ADSCs have superior immunomodulatory action because of the less MHC class II expression that makes them a prospective graft material for allogenic treatment [52]. Allogeneic ADSCs have immunological potential and can trigger graft rejection and inflammation in the recipient’s body. Introducing the human cytomegalovirus US2/US3 gene into ADSCs reduced ADSC immunogenicity and graft rejection by decreasing MHC I protein expression [53]. This method is promising for obtaining the same effect after transplantation of allogeneic ADSCs as autogenetic ADSCs [53]. Was reported that immunomodulatory effect related to the regenerative capacity has been increasing [52]. Moreover, it was shown that MSCs are able to produce molecules which have antimicrobial and analgetic properties, making them a prospective therapeutic agent against cytokine storm- infections [54, 55].
Several studies also indicate promising clinical results with brown adipocyte transplantation for the treatment of diabetes and obesity [56]. In experimental research, brown adipocyte transplantation improved the regulation of adipose tissue and glucose homeostasis as well as insulin resistance [57]. However, the specific mechanisms behind these effects have not yet been discovered [35].
2.4 Mechanism of ADSCs action
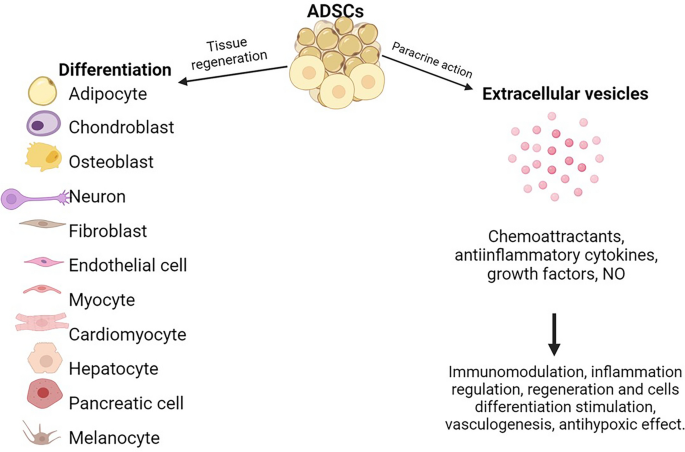
ADSCs therapy is based on direct replacement of damaged cells with differentiated ADSCs or modification of local paracrine signaling by extracellular vesicles (see Fig. 1). Studies report that under different conditions in vitro, ADSCs can differentiate into ectodermal, mesodermal, and endodermal progenitors [11, 17, 58, 59]. However, only several of these studies reported a successful result in in-vivo studies or clinical trials. Differentiation of ADSCs in vivo is challenging due to poor cell survival, mostly because of the transplantation of cells into organs with a hypoxic environment. However, compared with mature adipocytes, ADSCs have higher survival rates because of less sensitivity to ischemia and secretion of angiogenic factors that stimulate local angiogenesis [60].
Studies reported the successful usage of ADSCs in endometrial injury treatment. ADSCs underwent differentiation into mature endometrial epithelial cells, which resulted in endometrial structure and function regeneration [61]. However, most of studies are limited to the in vitro demonstration of ADSCs differentiation such as differentiation of ADSCs into insulin-producing cells, cells with hepatocytic function, osteocytes, adipocytes etc. [58, 60, 62, 63]. Nowadays, clinical translation of ADSC-based therapy for a direct cell’s replacement is difficult since most of the mechanisms for stem cells differentiation in the in vivo setting remains unclear. Such treatment might possibly result in the initial stages of cancer development and other adverse results [64]. ADSCs under inflammation regulate the inflammatory stimuli, triggering the synthesis of pro-angiogenic factors such as VEGF-A, hepatocyte growth factors, and IGF-1 as well as that of hematopoietic cytokines such as macrophage-colony stimulating factor, granulocyte-colony stimulating factor, IL-6, TNF-α [65].
Another more promising implication of ADSCs is via regulation of local tissue homeostasis. ADSCs possess unique paracrine characteristics. It is realized through extracellular vesicles (EVs) which contain products of cell secretion and transport it to the target cells to regulate cell function and change their phenotype via cell signaling. EVs are secreted by many different cell types, including ADSCs. They contain microRNA, mRNA, lipids, and proteins, and are classified as microvesicles (50–1000 nm in size) and exosomes (30–100 nm)[66, 67].
Recently, several promising results of treatment using isolated from ADSCs exosomes were shown. Exosomes of ADSCs contain numerous growths regulating cytokines that enhance recovery of damaged tissue and growth factors that mediate tissue regeneration. These growth factors are: basic fibroblast growth factor, VEGF-A, insulin-like growth factor 1, hepatocyte growth factors, and transforming growth factor, brain-derived neurotrophic factor, nerve growth factor, and glial-derived neurotrophic factor, matrix metalloproteinase- (MMP-) 3 and MMP-9 [68, 69].
ADSCs exosomes treatment showed promising results in therapy of neurological diseases, liver fibrosis, myocardial ischemic injuries, endocrine diseases, bone and skin regeneration. Isolated ADSCs exosomes were used for the treatment of ischemic brain injury. They reduced brain ischemia caused by the microglial polarization, which was caused by the delivery of microRNA to inhibit the expression of signal transducers and activators of transcription 1 and phosphatase and tensin homolog deleted on chromosome ten (PTEN) [70]. Metastasis-associated lung adenocarcinoma transcript 1 was identified as one of the ADSCs exosomes component that contributes to increased neuronal survival and proliferation in traumatic brain injury or other neurodegenerative diseases [71, 72]. Mouse ADSC EVs reduced apoptosis of motor neurons of in vitro amyotrophic lateral sclerosis model under the condition of oxidative stress alteration [73].
Further, exosomes of ADSCs decrease hepatic fibrosis development through the suppression of autophagy, PI3K/AKT/mTOR,,TGF-β/smad, Wnt/β-catenin, LPS/TLR4, EMT/ERK1, PPAR-γ, NF-κB signaling pathways and by the changing of lipid metabolism through regulation of choline metabolism [74, 75]. ADSCs exosomes also suppress the proliferation rate of stellate cells through stimulation of apoptosis and arrest of G1 phase of the cell cycle, and through the inhibition of profibrogenic proteins and epithelio-mesenchymal transition [76]. ADSCs exosome therapy reduced liver damage by downregulation of collagen I, vimentin, α-SMA and fibronectin in liver via selectively transfer of miR-181-5p to affected hepatocytes [77].
Exosomes isolated from ADSCs are used for the therapy of diabetes mellitus associated erectile dysfunction. They enhance the secretion of the endothelial markers and downregulate caspase-3 after the operation [78]. ADSCs exosomes activate functional recovery and activate endogenous repair mechanisms of corpus cavernosum via micro RNA 126, 130a and 132 that provides angiogenesis and restore erectile function, and inhibit fibrosis in corpus cavernosum by antifibrotic properties of micro RNA-let7b and c [79]. Zhao et al. showed that ADSCs exosomes-based treatment induces endometrial regeneration and fertility restoration by collagen remodeling and enhancement of integrin-β3, LIF, and VEGF expression [74]. EV isolated from human ADSCs increase wound healing and restore the function and prevent scar formation via activation of PI3K/AKT pathway in sebocytes on a murine model [80].
However, ADSCs and their exosomes have very variable biological properties and cytokine content, even if they were harvested from the SCAT of the same donor but from a different anatomical location. Thus, the thigh fat had a significantly higher cytokines profile except for IL-1β and IL-6, compared with abdominal and chin sites [81]. Nowadays, standardised issue of ADSC-based therapy, that determine their mechanism of action, is one of a several major limitations of its clinical translation.
2.5 Factors impacting the clinical effectiveness of ADSCs treatment
The result of stem cell treatment depends on the general health of the cell donor. Thus, in patients with diabetes mellitus, type II ADSCs exhibit impaired viability and proliferation rate, mitochondrial dysfunction, senescence phenotype, impaired glucose homeostasis, and insulin sensitivity. Significantly low secretion of VEGF, adiponectin, and CXCL-12, in the background of hypo concentration of leptin, were observed among type-II ADSC samples [82]. General systemic diseases lead to disturbances in the function and morphology of ADSCs and reduce their therapeutic properties.
Co-transplantation of ADSCs and platelet-rich plasma (PRP) resulted in significantly increased alveolar bone and gingiva regeneration [83, 84]. Moreover, PRP activates ADSCs by increasing cytokines and growth factors production, and a fibrin network can be used as a scaffold for the stem cells and to create a conducive microenvironment that increases stemness and prolongs cellular survival rate and duration [83, 85, 86]. Mechanical tension significantly enhances osteoblastic ADSCs differentiation; however, the mitotic activity of ADSCs is not affected by mechanical tension [85]. Li et al. showed that pretreatment of freshly isolated ADSCs with thymosin beta 4 (Tβ) upregulates the expression of genes associated with cell division, decrease cells doubling time and apoptosis [87].
In reconstructive surgery, transplantation of ADSCs alone for regenerative purposes is not as effective as co-transplantation with a composition of different cells to create a favorable environment for revascularization, preventing graft resorption and necrosis. In particular, transplantation of ADSCs, adipocytes, and endothelial cells implanted into the extracellular matrix has shown a higher cellular survival rate and volume maintenance when compared to non-prevascularized control grafts [86].
The injection of ADSCs along with intraoral administration of sildenafil citrate, which enhances blood supply and NO synthesis in animal models, significantly improves the healing rate after colon anastomosis and better reduces inflammation when compared with ADSCs alone [88]. For promoting hair growth ADSCs pretreated with bee venom is reported to increase the release of fibroblast -1 and -6, endothelial and platelet growth factors and enhancement of cells migration [89].
The actions of ADSCs are determined by their environment. Human ADSCs transferred to non-inflamed mouse lungs resulted in development of mild low-grade inflammation, which can be associated with apoptotic graft or heterotransplant clearance. T-cells that produce IFNγ can activate the immune response to efferocytosis, thus altering lung homeostasis [90]. The combination of Shilajit (a herbomineral natural substance) and an alginate hydrogel environment induced osteogenic differentiation of ADSCs into osteoblasts in a short period of time [91]. Thus, a proper microenvironment can significantly enhance the outcome of ADSCs clinical applications. There are still many concerns about safety of ADSCs therapy, thus, EV from ADSCs showed suppression of breast cancer tumor growth meanwhile the components of cell growth medium had an opposite effect of a tumor [92].
2.6 Standardization of ADSCs
The translation of novel findings in stem cell therapy to clinical practice has been discouragingly limited and ambiguous, with the effectiveness of some forms of stem cell therapies remaining poorly supported by evidence. The main problem that limits the clinical application of stem cells, in addition to many other biological medical products, is poor standardization and a lack of comprehensive guidelines [93]. Standardization of biological grafts is necessary because it offers an opportunity to compare research outcomes, which leads to the optimization of ADSC-based treatment.
It is impossible to effectively translate the results of basic research to clinical settings due to differences in cell origins, cultivation conditions, obtainment methods, and the number of cell passages. Tragoonlugkana et al. showed that cell culture plates coated with platelet lysate significantly increased properties of ADSCs such as adhesion, proliferation speed and growth as well as the cells’ viability [94]. Thus, the same method of adipose tissue harvesting, but used by different commercial systems, influences the cellular content and cytokine secretion of ADSCs [95]. Distinctive changes in gene expression have been observed after a 48-h ADSCs cultivation period. Regulatory genes are involved in cell morphogenesis and metabolism, cell-to-substrate adhesion, glycoprotein metabolic processes, and regulation of fiber molecular structure organization. Downregulated genes were those involved in cell proliferation, differentiation, and transformation [96].
Cultural, biological, and functional properties of ADSCs depend on the anatomical location of fat, age, gender, and BMI of patients [97,98,99]. It is not yet clear whether isolated cells are actually ADSCs or what types of cells they are able to generate. Researchers agree that not all MSCs have identical characteristics, which can depend on the patient’s age, donor site, isolation technique, and growth [100]. Close attention should also be paid to the origin of the allogeneic graft, since several studies have underlined that donor age, sex, tissue source, and method of isolation have an effect on cellular and molecular variability [101, 102]. Another problem is the safety of the graft and its possibility of being infected with diverse latent viruses that do not trigger a manifestation of the disease under normal conditions. ADSCs harvested from a dog`s omentum with canine distemper disease were found to be infected with canine morbillivirus [103]. In this study, before the clinical use of ADSCs, cells were checked for the presence of latent viruses.
Currently, the major dilemma with fat grafting, as well as with other biological grafts and substances, is inconsistent results of experimental and clinical findings attributable to poor standardization resulting from wide varieties of harvesting methods, donor sites, and patients’ initial state of health, as well as a lack of established, objective methods for assessment of clinical results and a lack of knowledge on the precise mechanism of stem cell action and regenerative mechanisms. There is also a lack of data and evidence from which to draw conclusions regarding the safety, effectiveness, and impact of ADSCs and other adipose tissue grafts on tissue regeneration [104].
The main issues that should undergo standardization are adipose tissue harvesting and processing, donor`s health condition, age, cryopreservation and storage procedure, freezing media that was used, quantification of ADSCs number and their phenotypical markers, storage duration, dosage used for the treatment of particular disease etc. Moreover, apart from three main widely accepted criteria for ADSCs – such as plastic-adherent during culturing, trilineage mesenchymal differentiation and expression of specific cell-surface antigens – a functional analysis of ADSCs properties (doubling time, specter and quantity of cytokines secretion, migration speed etc.) should be checked and compared to some standard in order to receive a predictable treatment result.
2.7 Latest clinical studies implementing ADSCs
The number of clinical trials using MSCs has recently significantly increased owing to notable successes and breakthroughs in basic research and experimental studies. New properties and clinical actions of MSCs have been discovered, and their clinical applications and indications have broadened.
Clinical studies have shown that infusion of MSCs leads to vigorous anti-inflammatory effects characterized by lymphocytosis and a decrease in levels of overactivated pro-inflammatory immune cells and TNF-α, in contrast to upregulation of IL-10 secretion. MSCs are known to auto-induce and address their microenvironment to promote cell proliferation and tissue regeneration. MSCs act via paracrine effects on cells and the organ environment, reducing cytokine storms and severe inflammation [105]. MSCs have been shown to demonstrate antimicrobial properties, increasing the immune response through the production of bactericide peptides and proteins, and the expression of indoleamine 2,3-dioxygenase (enzyme that decrease reproduction rate of viruses, some mammalian cells) and IL-17 [106]. ADSCs have proven efficient in the treatment of pulmonary diseases in vivo targeting a paracrine pathway, through the promotion of the epitheliocytes mitosis and apoptosis suppression [107]. The outcomes and limitations of clinical and randomized clinical trials with adipose tissue grafting products are shown in Table 2.
3 Conclusion
The last five years have witnessed a huge breakthrough in the translation of basic research and experimental studies of ADSCs into clinical practice. ADSCs are the most promising and easy-to-obtain cells when compared to other MSCs because of their satisfactory cultural, biological, and clinical properties. The future of ADSC-based therapies likely belongs to allogeneic ADSCs. ADSC-based treatment is a highly promising method that utilizes etiological treatment approaches for diseases that are accompanied by cell death or acute tissue loss such as diabetes mellitus type I, xerostomia, periodontitis, and wound treatment for them stem cell therapy. ADSCs act through their differentiation to the specific type of mature cells which are determined by the particular microenvironment or cell stimuli or via paracrine regulation, such as secretion of growth factors and cytokines.
The clinical translation of ADSCs requires proper validation in large controlled trials, discovery of the exact mechanism of action, research standardization, and the adoption of pre-determined therapeutic guidelines.
The vast majority of pre-clinical in vivo studies showed positive treatment outcomes, however there were only a few clinical trials performed, indicating that enough clinical evidence is not yet available to allow broad ADSCs implementation into clinical practice. Among the limiting factors are: small patient sample sizes, predominantly short-term observation time, a lack of adipose tissue graft standardization (procedure and cite of the graft harvesting, cell culturing protocols), deficit of clinical protocols and guidelines, and subjective scoring methods for clinical study results (such as visual assessment and patients’ response to pain) [22]. Currently, most preclinical studies and clinical trials reported that ADSCs are relatively safe and effective [104, 115]. The issue of dose, quality of the graft and indications remains unresolved and debatable [62]. According to the reviewed literature analysis, adipose tissue-derived biological products such as (ADSCs, SFV, ADSCs-Ev) showed promising results in clinical and in vivo setting. However, one of the main limitations of ADSCs therapy, as all other biologics-based drugs, is a process of the graft standardization. Such standardization should take into consideration the functional properties of the graft, such as doubling time, specter and quantity of cytokines secretion, migration speed etc., all of which varied based on the procedure of their isolation, localization, and pretreatment used in order to provide predictable and effective treatment outcomes.
Data availability
Not applicable for review paper.
Availability of data and materials
Not applicable.
Abbreviations
- ADSCs:
-
Adipose-derived stem cells
- MSC:
-
Mesenchymal stem cell
- BM-MSCs:
-
Bone marrow-derived stem cells
- RM:
-
Regenerative medicine
- VAT:
-
Visceral adipose tissue
- WAT:
-
White adipose tissue
- SVF:
-
Stromal vascular fraction
- EVs:
-
Extracellular vesicles
- VEGF:
-
Vascular endothelial growth factor
- TGF:
-
Transforming growth factor
- MMP:
-
Matrix metalloproteinase
- TNF:
-
Tumor necrosis factor
- PRP:
-
Platelet-rich plasma
- IFN-γ:
-
Interferon gamma
- PPAR-γ:
-
Peroxisome proliferator-activated receptor gamma
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
References
Yang L, Hu Z-M, Jiang F-X, Wang W (2022) Stem cell therapy for insulin-dependent diabetes: Are we still on the road? WJSC 14:503–512. https://doi.org/10.4252/wjsc.v14.i7.503
Cheng W, Zeng Y, Wang D (2022) Stem cell-based therapy for pulmonary fibrosis. Stem Cell Res Ther 13:492. https://doi.org/10.1186/s13287-022-03181-8
Ma J, Lei P, Chen H et al (2022) Advances in lncRNAs from stem cell-derived exosome for the treatment of cardiovascular diseases. Front Pharmacol 13:986683. https://doi.org/10.3389/fphar.2022.986683
Hoang DM, Pham PT, Bach TQ et al (2022) Stem cell-based therapy for human diseases. Signal Transduct Target Ther 7:272. https://doi.org/10.1038/s41392-022-01134-4
Yan S, Campos de Souza S, Xie Z, Bao Y (2023) Research progress in clinical trials of stem cell therapy for stroke and neurodegenerative diseases. Ibrain 9:214–230. https://doi.org/10.1002/ibra.12095
El Sayed R, Shankar KM, Mankame AR, Cox CS Jr (2023) Innovations in cell therapy in pediatric diseases: a narrative review. Transl Pediatr 12:1239–1257. https://doi.org/10.21037/tp-23-92
Orozco-Solares TE, León-Moreno LC, Rojas-Rizo A et al (2022) Allogeneic mesenchymal stem cell-based treatment legislation in Latin America: the need for standardization in a medical tourism context. Stem Cells Dev 31:143–162. https://doi.org/10.1089/scd.2022.0013
Glenn Cohen I, Simana S (2018) Regulation of stem cell therapy travel. Curr Stem Cell Rep 4:220–227. https://doi.org/10.1007/s40778-018-0134-8
Şovrea AS, Boşca AB, Constantin AM et al (2019) State of the art in human adipose stem cells and their role in therapy. Rom J Morphol Embryol 60:7–31
Frese L, Dijkman PE, Hoerstrup SP (2016) Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother 43:268–274. https://doi.org/10.1159/000448180
Youn GM, Woodall BM, Elena N et al (2018) Arthroscopic bone marrow aspirate concentrate harvesting from the intercondylar notch of the knee. Arthrosc Tech 7:e1173–e1176. https://doi.org/10.1016/j.eats.2018.07.016
Pittenger MF, Mackay AM, Beck SC et al (1979) (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. https://doi.org/10.1126/science.284.5411.143
Sivasubramaniyan K, Ilas DC, Harichandan A et al (2018) Bone marrow-harvesting technique influences functional heterogeneity of mesenchymal stem/stromal cells and cartilage regeneration. Am J Sports Med 46:3521–3531. https://doi.org/10.1177/0363546518804807
Tanasale B, Kits J, Kluin PM et al (2013) Pain and anxiety during bone marrow biopsy. Pain Manag Nurs 14:310–317. https://doi.org/10.1016/j.pmn.2011.06.007
Filbet M, Fawoubo A, Tricou C et al (2020) Management of the procedural pain induced by bone marrow biopsies: an observational study. Ann Clin Oncol. https://doi.org/10.31487/j.ACO.2020.01.06
Mazini L, Rochette L, Amine M, Malka G (2019) Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). IJMS 20:2523. https://doi.org/10.3390/ijms20102523
Bourin P, Bunnell BA, Casteilla L et al (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Macrin D, Joseph JP, Pillai AA, Devi A (2017) Eminent sources of adult mesenchymal stem cells and their therapeutic imminence. Stem Cell Rev and Rep 13:741–756. https://doi.org/10.1007/s12015-017-9759-8
Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. https://doi.org/10.1186/1478-811X-9-12
Sanchez-Petitto G, Rezvani K, Daher M et al (2023) Umbilical cord blood transplantation: connecting its origin to its future. Stem Cells Transl Med. https://doi.org/10.1093/stcltm/szac086
Malhotra A, Thebaud B, Paton MCB et al (2023) Advances in neonatal cell therapies: proceedings of the First Neonatal Cell Therapies Symposium (2022). Pediatr Res 94:1631–1638. https://doi.org/10.1038/s41390-023-02707-x
Zhang J, Huang X, Wang H et al (2015) The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther 6:234. https://doi.org/10.1186/s13287-015-0240-9
Ankrum JA, Ong JF, Karp JM (2014) Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32:252–260. https://doi.org/10.1038/nbt.2816
Biniazan F, Stoian A, Haykal S (2024) Adipose-derived stem cells: angiogenetic potential and utility in tissue engineering. Int J Mol Sci 25:2356. https://doi.org/10.3390/ijms25042356
Nasser RA, Arya SS, Alshehhi KH et al (2024) Conducting polymer scaffolds: a new frontier in bioelectronics and bioengineering. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2023.11.017
Zuk PA, Zhu M, Mizuno H et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228. https://doi.org/10.1089/107632701300062859
Madhavan AA, Summerfield D, Hunt CH et al (2020) Polyclonal lymphocytic infiltrate with arachnoiditis resulting from intrathecal stem cell transplantation. Neuroradiol J 33:174–178. https://doi.org/10.1177/1971400920902451
Lyons S, Salgaonkar S, Flaherty GT (2022) International stem cell tourism: a critical literature review and evidence-based recommendations. Int Health 14:132–141. https://doi.org/10.1093/inthealth/ihab050
Tobita M, Konomi K, Torashima Y et al (2016) Japan’s challenges of translational regenerative medicine: Act on the safety of regenerative medicine. Regen Ther 4:78–81. https://doi.org/10.1016/j.reth.2016.04.001
Connolly R, O’Brien T, Flaherty G (2014) Stem cell tourism–a web-based analysis of clinical services available to international travellers. Travel Med Infect Dis 12:695–701. https://doi.org/10.1016/j.tmaid.2014.09.008
Rehfeld A, Nylander M, Karnov K (2017) Adipose tissue. Compendium of histology. Springer International Publishing, Cham, pp 201–207
Mescher AL, Junqueira LCU (2013) Junqueira’s basic histology: text and atlas, 13th edn. McGraw-Hill Medical, New York, NY
Ibrahim MM (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11:11–18. https://doi.org/10.1111/j.1467-789X.2009.00623.x
Shinde AB, Song A, Wang QA (2021) Brown adipose tissue heterogeneity, energy metabolism, and beyond. Front Endocrinol 12:651763. https://doi.org/10.3389/fendo.2021.651763
Tordjman J (2013) Histology of adipose tissue. In: Bastard J-P, Fève B (eds) Physiology and physiopathology of adipose tissue. Springer Paris, Paris, pp 67–75
Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL (2019) The importance of peripheral nerves in adipose tissue for the regulation of energy balance. Biology (Basel) 8:10. https://doi.org/10.3390/biology8010010
Beregova TV, Neporada KS, Skrypnyk M et al (2017) Efficacy of nanoceria for periodontal tissues alteration in glutamate-induced obese rats-Multidisciplinary considerations for personalized dentistry and prevention. EPMA J. https://doi.org/10.1007/s13167-017-0085-7
González-Muniesa P, Mártinez-González M-A, Hu FB et al (2017) Obesity. Nat Rev Dis Primers 3:17034. https://doi.org/10.1038/nrdp.2017.34
Nguyen A, Guo J, Banyard DA et al (2016) Stromal vascular fraction: a regenerative reality? Part 1: current concepts and review of the literature. J Plast Reconstr Aesthet Surg 69:170–179. https://doi.org/10.1016/j.bjps.2015.10.015
Ramakrishnan VM, Boyd NL (2018) The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev 24:289–299. https://doi.org/10.1089/ten.teb.2017.0061
Zhu Y, Liu T, Song K et al (2008) Adipose-derived stem cell: a better stem cell than BMSC. Cell Res 18:S165–S165. https://doi.org/10.1038/cr.2008.255
Cieśla J, Tomsia M (2021) Cadaveric stem cells: their research potential and limitations. Front Genet 12:798161. https://doi.org/10.3389/fgene.2021.798161
Rao P, Deo D, Marchioni M et al (2019) 24: deceased donor adipose tissue: an untapped source of mesenchymal stem cells. Transplantation 103:S5–S6. https://doi.org/10.1097/01.tp.0000581296.60427.ad
Kim SM, Lun M, Wang M et al (2014) Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab 20:1049–1058. https://doi.org/10.1016/j.cmet.2014.10.010
Skrypnyk M, Petrushanko T, Neporada K et al (2022) Colonization resistanCe of oral muCosa in individuals with diverse body mass index. J Stomatol. https://doi.org/10.5114/jos.2022.119168
Skrypnyk M, Petrushanko T, Neporada K et al (2022) Dependence of the dental status of young individuals with different body weights on their eating behavior. Acta Facult Med Naissensis. https://doi.org/10.5937/afmnai39-35901
Wada Y, Ikemoto T, Morine Y et al (2019) The differences in the characteristics of insulin-producing cells using human adipose-tissue derived mesenchymal stem cells from subcutaneous and visceral tissues. Sci Rep 9:13204. https://doi.org/10.1038/s41598-019-49701-0
Yoon H-J, Jung Ho W (2019) Minimum criteria for adipose derived stem cells. Clin Med Rep. https://doi.org/10.15761/CMR.1000145
Hong S, van Kaer L (1999) Immune privilege: keeping an eye on natural killer T cells. J Exp Med 190:1197–1200. https://doi.org/10.1084/jem.190.9.1197
Zangi L, Margalit R, Reich-Zeliger S et al (2009) Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 27:2865–2874. https://doi.org/10.1002/stem.217
Mazini L, Rochette L, Admou B et al (2020) Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. IJMS 21:1306. https://doi.org/10.3390/ijms21041306
Ren M-L, Peng W, Yang Z-L et al (2012) Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transplant 21:2711–2721. https://doi.org/10.3727/096368912X654966
Gentile P, Sterodimas A (2020) Adipose stem cells (ASCs) and stromal vascular fraction (SVF) as a potential therapy in combating (COVID-19)-disease. Aging Dis 11:465. https://doi.org/10.14336/AD.2020.0422
Copcu HE (2020) Potential using of fat-derived stromal cells in the treatment of active disease, and also, in both pre- and post-periods in COVID-19. Aging Dis 11:730. https://doi.org/10.14336/AD.2020.0621
Cypess AM, Weiner LS, Roberts-Toler C et al (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21:33–38. https://doi.org/10.1016/j.cmet.2014.12.009
Liu X, Zheng Z, Zhu X et al (2013) Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res 23:851–854. https://doi.org/10.1038/cr.2013.64
Khazaei S, Keshavarz G, Bozorgi A et al (2022) Adipose tissue-derived stem cells: a comparative review on isolation, culture, and differentiation methods. Cell Tissue Bank 23:1–16. https://doi.org/10.1007/s10561-021-09905-z
Si Z, Wang X, Sun C et al (2019) Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother 114:108765. https://doi.org/10.1016/j.biopha.2019.108765
Tsuji W (2014) Adipose-derived stem cells: Implications in tissue regeneration. WJSC 6:312. https://doi.org/10.4252/wjsc.v6.i3.312
Shao X, Ai G, Wang L et al (2019) Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote 27:367–374. https://doi.org/10.1017/S096719941900042X
Mazini L, Ezzoubi M, Malka G (2021) Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: flow chart and regulation updates before and after COVID-19. Stem Cell Res Ther 12:1. https://doi.org/10.1186/s13287-020-02006-w
Raposio E, Simonacci F, Perrotta RE (2017) Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann Med Surg (Lond) 20:87–91. https://doi.org/10.1016/j.amsu.2017.07.018
Dalerba P, Diehn M, Weissman IL, Clarke MF (2020) Stem Cells, Cell Differentiation, and Cancer. In: Abeloff’s Clinical Oncology. Elsevier, pp 97–107.e5
Xin L, Lin X, Pan Y et al (2019) A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater 92:160–171. https://doi.org/10.1016/j.actbio.2019.05.012
Wong DE, Banyard DA, Santos PJF et al (2019) Adipose-derived stem cell extracellular vesicles: a systematic review✰. J Plast Reconstr Aesthet Surg 72:1207–1218. https://doi.org/10.1016/j.bjps.2019.03.008
Bazzan E, Tinè M, Casara A et al (2021) Critical review of the evolution of extracellular vesicles’ knowledge: from 1946 to today. Int J Mol Sci 22:6417. https://doi.org/10.3390/ijms22126417
Gokce A, Abd Elmageed ZY, Lasker GF et al (2014) Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie’s disease. Andrology 2:244–251. https://doi.org/10.1111/j.2047-2927.2013.00181.x
Braga Osorio Gomes Salgado JA, Goncalves Reis LR, Jorge Carvalho Sousa N, et al (2010) Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. CSCR 5:103–110. https://doi.org/10.2174/157488810791268564
Hu X, Pan J, Li Y et al (2022) Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res Ther 13:21. https://doi.org/10.1186/s13287-021-02668-0
el Bassit G, Patel RS, Carter G et al (2016) MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCδII in HT22 cells. Endocrinology. https://doi.org/10.1210/en.2016-1819
Patel NA, Moss LD, Lee J-Y et al (2018) Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflammation 15:204. https://doi.org/10.1186/s12974-018-1240-3
Bonafede R, Scambi I, Peroni D et al (2016) Exosome derived from murine adipose-derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res 340:150–158. https://doi.org/10.1016/j.yexcr.2015.12.009
Zhao S, Qi W, Zheng J et al (2020) Exosomes derived from adipose mesenchymal stem cells restore functional endometrium in a rat model of intrauterine adhesions. Reprod Sci 27:1266–1275. https://doi.org/10.1007/s43032-019-00112-6
Ma L, Wei J, Zeng Y et al (2022) Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv 29:440–453. https://doi.org/10.1080/10717544.2022.2030428
Zhang Z, Shang J, Yang Q et al (2023) Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnol 21:29. https://doi.org/10.1186/s12951-023-01788-4
Qu Y, Zhang Q, Cai X et al (2017) Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med 21:2491–2502. https://doi.org/10.1111/jcmm.13170
Chen F, Zhang H, Wang Z et al (2017) Adipose-derived stem cell-derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med 14:1084–1094. https://doi.org/10.1016/j.jsxm.2017.07.005
Zhu LL, Huang X, Yu W et al (2018) Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50:e12871. https://doi.org/10.1111/and.12871
Zhang Y, Zouboulis CC, Xiao Z (2024) Exosomes from adipose-derived stem cells activate sebocytes through the PI3K/AKT/SREBP-1 pathway to accelerate wound healing. Cell Tissue Res. https://doi.org/10.1007/s00441-024-03872-z
Santos J, Dalla P (2021) A molecular analysis of cytokine content across extracellular vesicles, secretions, and intracellular space from different site-specific adipose-derived stem cells. IJMS 23:397. https://doi.org/10.3390/ijms23010397
Alicka M, Major P, Wysocki M, Marycz K (2019) Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. JCM 8:765. https://doi.org/10.3390/jcm8060765
Tobita M, Uysal CA, Guo X et al (2013) Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy 15:1517–1526. https://doi.org/10.1016/j.jcyt.2013.05.007
Tobita M, Uysal AC, Ogawa R et al (2008) Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A 14:945–953. https://doi.org/10.1089/ten.tea.2007.0048
Wang L, Lu Y, Cai G et al (2022) Polycystin-2 mediates mechanical tension-induced osteogenic differentiation of human adipose-derived stem cells by activating transcriptional co-activator with PDZ-binding motif. Front Physiol 13:917510. https://doi.org/10.3389/fphys.2022.917510
Louis F, Sowa Y, Irie S, Higuchi Y, Kitano S, Mazda O, Matsusaki M (2022) Injectable prevascularized mature adipose tissues (iPAT) to achieve long-term sur-vival in soft tissue regeneration. Adv Healthcare Mater. https://doi.org/10.1002/adhm.202201440
Li W, Yang Y, Lin Y, Mu D (2024) In vitro study of thymosin beta 4 promoting transplanted fat survival by regulating adipose-derived stem cells. Aesthetic Plast Surg. https://doi.org/10.1007/s00266-024-03861-1
Demir M, Yılmaz EM, İpek E et al (2022) Effects of adipose tissue-derived mesenchymal stem cells and/or sildenafil citrate in experimental colon anastomosis model. Ulus Travma Acil Cerrahi Derg 28:1373–1381. https://doi.org/10.14744/tjtes.2021.57500
Kim JH, Kim TY, Goo B, Park Y (2024) Bee venom stimulates growth factor release from adipose-derived stem cells to promote hair growth. Toxins (Basel) 16:84. https://doi.org/10.3390/toxins16020084
Tynecka M, Janucik A, Niemira M et al (2022) The short-term and long-term effects of intranasal mesenchymal stem cell administration to noninflamed mice lung. Front Immunol 13:967487. https://doi.org/10.3389/fimmu.2022.967487
Kangari P, Roshangar L, Iraji A et al (2022) Accelerating effect of Shilajit on osteogenic property of adipose-derived mesenchymal stem cells (ASCs). J Orthop Surg Res 17:424. https://doi.org/10.1186/s13018-022-03305-z
Felthaus O, Vedlin S, Eigenberger A et al (2024) Exosomes from adipose-tissue-derived stem cells induce proapoptotic gene expression in breast tumor cell line. Int J Mol Sci 25:2190. https://doi.org/10.3390/ijms25042190
Lappin T, Cheng T (2021) An urgent need for standardization of stem cells and stem cell-derived products toward clinical applications. Stem Cells Transl Med 10:S1–S3. https://doi.org/10.1002/sctm.21-0269
Tragoonlugkana P, Chitchongyingcharoen N, Pruksapong C et al (2024) The use of human platelet lysate as a coating substance for adipose-derived stem cell expansion. Front Biosci Landmark 29:88. https://doi.org/10.31083/j.fbl2902088
Greenwood V, Clausen P, Matuska AM (2022) Micro-fragmented adipose tissue cellular composition varies by processing device and analytical method. Sci Rep 12:16107. https://doi.org/10.1038/s41598-022-20581-1
Taha S, Akova E, Saller MM et al (2022) Early transcriptional changes of adipose-derived stem cells (ADSCs) in cell culture. Medicina (B Aires) 58:1249. https://doi.org/10.3390/medicina58091249
Schipper BM, Marra KG, Zhang W et al (2008) Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 60:538–544. https://doi.org/10.1097/SAP.0b013e3181723bbe
Aksu AE, Rubin JP, Dudas JR, Marra KG (2008) Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg 60:306–322. https://doi.org/10.1097/SAP.0b013e3180621ff0
van Harmelen V, Skurk T, Röhrig K et al (2003) Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord 27:889–895. https://doi.org/10.1038/sj.ijo.0802314
Ryu Y, Ha H, Lee C et al (2016) ADSC from younger donors have more abundant initial ADSC yield. Cytotherapy 18:S79. https://doi.org/10.1016/j.jcyt.2016.03.164
Abbo O, Taurand M, Monsarrat P et al (2017) Comparison between pediatric and adult adipose mesenchymal stromal cells. Cytotherapy 19:395–407. https://doi.org/10.1016/j.jcyt.2016.11.012
Ock S-A, Lee Y-M, Park J-S et al (2016) Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source. J Vet Med Sci 78:987–995. https://doi.org/10.1292/jvms.15-0596
Altamirano-Samaniego F, Enciso-Benavides J, Rojas N et al (2022) First report of canine morbillivirus infection of adipose tissue-derived stem cells from dogs with distemper. Vet World. https://doi.org/10.14202/vetworld.2022.1835-1842
Firriolo JM, Condé-Green A, Pu LLQ (2022) Fat grafting as regenerative surgery: a current review. Plast Reconstr Surg 150:1340e–1347e. https://doi.org/10.1097/PRS.0000000000009710
Shetty AK (2020) Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)- induced pneumonia. Aging Dis 11:462–464. https://doi.org/10.14336/AD.2020.0301
Alcayaga-Miranda F, Cuenca J, Khoury M (2017) Antimicrobial activity of mesenchymal stem cells: current status and new perspectives of antimicrobial peptide-based therapies. Front Immunol 8:339. https://doi.org/10.3389/fimmu.2017.00339
Al-Ghadban S, Bunnell BA (2020) Adipose tissue-derived stem cells: immunomodulatory effects and therapeutic potential. Physiology 35:125–133. https://doi.org/10.1152/physiol.00021.2019
Zhou L, Wang H, Yao S et al (2022) Efficacy of human adipose derived mesenchymal stem cells in promoting skin wound healing. J Healthc Eng 2022:6590025. https://doi.org/10.1155/2022/6590025
Randelli PS, Cucchi D, Fossati C et al (2022) Arthroscopic rotator cuff repair augmentation with autologous microfragmented lipoaspirate tissue is safe and effectively improves short-term clinical and functional results: a prospective randomized controlled trial with 24-month follow-up. Am J Sports Med 50:1344–1357. https://doi.org/10.1177/03635465221083324
el Zarif M et al (2020) Corneal stroma cell density evolution in keratoconus corneas following the implantation of adipose mesenchymal stem cells and corneal laminas: an in vivo confocal microscopy study. Investigative Opthalmology & Visual Science 61:22. https://doi.org/10.1167/iovs.61.4.22
Zheng C, Chiu I, Chen Y et al (2022) Allogeneic adipose tissue-derived stem cells ELIXCYTE ® in chronic kidney disease: a phase I study assessing safety and clinical feasibility. J Cell Mol Med 26:2972–2980. https://doi.org/10.1111/jcmm.17310
Ferracini R, Alessio-Mazzola M, Sonzogni B et al (2022) Age and synovitis affect the results of the treatment of knee osteoarthritis with microfragmented autologous fat tissue. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-022-07139-4
Behrangi E, Moradi S, Ghassemi M et al (2022) The investigation of the efficacy and safety of stromal vascular fraction in the treatment of nanofat-treated acne scar: a randomized blinded controlled clinical trial. Stem Cell Res Ther 13:298. https://doi.org/10.1186/s13287-022-02957-2
Lynggaard CD, Grønhøj C, Jensen SB et al (2022) Long-term safety of treatment with autologous mesenchymal stem cells in patients with radiation-induced xerostomia: primary results of the MESRIX phase I/II randomized trial. Clin Cancer Res 28:2890–2897. https://doi.org/10.1158/1078-0432.CCR-21-4520
Ceccarelli S, Pontecorvi P, Anastasiadou E et al (2020) Immunomodulatory effect of adipose-derived stem cells: the cutting edge of clinical application. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.00236
Acknowledgements
The author thank Dr. Darren Smith for kindly providing editorial assistance while preparing the manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
The corresponding author states that there is no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Skrypnyk, M. Current progress and limitations of research regarding the therapeutic use of adipose-derived stem cells: literature review. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00147-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00147-9