Abstract
Due to chronic inflammation, elevated cyclooxygenase (COX-2) level leads to tumorigenesis, proliferation, invasion, angiogenesis and metastasis. Therefore, suppression of COX-2 enzyme is a fascinating approach in cancer treatment. In the present study, natural product eugenol was modified to develop new 1,2,4-triazole derivatives as antiCOX-2 and antiproliferative agents. The structures of newly prepared derivatives were established using sophisticated analytical techniques. The antiproliferative result showed compound 10 to be equipotent to doxorubicin towards MDA-MB 231 and PC-3 cancer cells with IC50 1.42 and 5.69 μM, respectively and potent COX-2 inhibitor with IC50 0.28 μM. Compound 10 was also non carcinogenic, non mutagenic with good drug likeness property as depicted by in silico physicochemical and pharmacokinetic studies. The docking results against COX-2 protein showed highest binding energy for compound 10 which was found to be in consistent with the cytoxicity and COX-2 results. In conclusion, compound 10 could harness COX-2 and cell proliferation and could be a promising candidate in cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chronic inflammation and cancer are closely linked as inflammation can cause tumorigenesis, cellular transformation, proliferation, invasion, angiogenesis and metastasis [1, 2]. The main risk factors for various cancers notably gastric, cervical or hepatocellular are caused due to infection with Helicobacter pylori (H. pylori), Hepatitis B virus (HBV) and Human Papillomavirus (HPV), respectively [3, 4]. It has been observed that elevated COX-2 level result in cell proliferation and metastasis [5], therefore suppression of COX-2 enzyme is an important strategy for killing cancer cells. Moreover, the antiproliferative effect by COX-2 inhibitors has been observed in cancers of gastric, colorectal, rectum and cervical in various clinical trials [6, 7]. Therefore, application of COX-2 inhibitors in cancer treatment is a fascinating approach to treat this debilitating disease.
Eugenol is an aromatic phenolic constituent of Syzigium aromaticum, endowed with important pharmacological activities such as anticancer, antiviral, antidiabetic, antiparasitic, antiinflammatory, antimicrobial and antiinflammatory [8,9,10,11,12,13,14,15]. This promising natural product inhibits COX-2, lipooxygenase (5-LOX) [16], DNA synthesis [17], increases reactive oxygen species (ROS) production, arrests cell cycle and induces apoptosis (Fig. 1) [18]. Various semisynthetic derivatives of eugenol conjugated with different heterocycles have been reported over the past years with better bioactivities and broad mode of actions [19,20,21,22,23,24,25,26,27,28,29]. Due to its safety and multidirectional effect, Eugenol has been explored in the present study.
1,2,4-triazole is an important nitrogen bearing heterocycle in medicinal chemistry which possess broad spectrum of biological activities including antimicrobial [30], anticancer [31, 32], antiinflammatory [33], antidiabetic [34], antioxidant [35], antiviral [36] and anticonvulsant [37]. This scaffold is capable of forming hydrogen bonds with the biological targets [38] leading to enhanced bioactivities, lipophilicity and improved physicochemical properties [39, 40]. The common drugs bearing 1,2,4-triazole ring such as antiviral ribavirin, antifungal tebuconazole, itraconazole and fluconazole, anticancer vorozole, anastrazole, and antidepressant alprazolam (Fig. 1) has gained a lot of interest in drug discovery [41]. The biological importance of 1,2,4- triazole, eugenol and our efforts to develop new antiproliferative agents, herein we report the synthesis and pharmacological evaluation of 1,2,4-triazole incorporated eugenol derivatives as dual anticancer and anti COX-2 agents.
2 Results and discussion
2.1 Chemistry
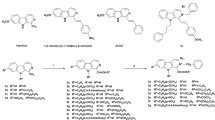
The intermediates 2–4 were synthesized using the previously published work [42]. Compound 4 refluxed with ethyl bromoacetate and hydrazine hydrate yielded compound 5 (83%), which when reacted with different aromatic aldehydes (equimolar) in ethanol and glacial acetic acid (few drops) afforded compounds 6–11 with 65–85% yield (Scheme 1). The newly prepared eugenol-1,2,4-triazole derivatives were characterized using sophisticated spectroscopic techniques. The 1H NMR spectrum of intermediate 5 showed aliphatic protons signals of propyl, methoxy, thiomethylene and -NH2 groups in the range 0.94–5.38 ppm, while in 13C NMR, appeared at 13.84, 24.66, 37.76, 39.86, 55.85 and 62.56 ppm, respectively whereas the downfield peak at 168.90 ppm was attributed to carbonyl carbon. The conversion of compound 5 to final compounds 6–11 was supported by the absence of NH2 protons signals and presence of new signal in the range 7.86–8.84 ppm that corresponded to azomethine proton (N = CH), supporting the formation of Schiff base. The structures were also supported by their 13C NMR which displayed additional azomethine carbon in the range 144.16–145.58 ppm and aromatic carbons of new phenyl ring, besides the previously observed signals in compound 5. Lastly, the confirmation of the final eugenol-1,2,4-triazole derivatives 6–11 were evidenced from their mass spectra displaying desired molecular ion peaks. For instance, in 1H NMR of compound 9 (Figs. 2, 3 and 4), aliphatic protons of propyl chain i.e. methyl (CH3), methylene (–CH2–), and methylene attached to phenyl ring (CH2–Ar) appeared as multiplets in the range 0.89–2.60 ppm for seven protons, while four singlets at 3.74 ppm, 3.96 ppm, 5.36 ppm and 5.59 ppm attributed to methoxy (–O–CH3), thio-methylene (S–CH2–), oxy-methylene (O–CH2–) and NH2 protons, respectively. These signals in 13C NMR were observed at 13.81 (CH3–), 24.62 (CH3–CH2–CH2–), 37.76 (CH3–CH2–CH2–Ar), 40.02 (–S–CH2–), 55.66 (–O–CH3), and 63.56 (–O–CH2–). The multiplets of seven aromatic protons appeared in the range 6.99–7.59 ppm and the three downfield signals at 8.75 ppm, 9.93 ppm and 11.04 ppm corresponded to azomethine (N = CH–), CONH and Ar–OH protons, respectively. The characteristic carbon signals for C = O, C–OH, C = N of oxadiazole ring and azomethine carbon were observed at 164.74 ppm, 159.82 ppm, 158.54 ppm, 151.04 ppm and 144.28 ppm, respectively in 13C NMR. The ESI mass spectrum of compound 9 revealed molecular ion peak at 471.17, confirming its formation.

Reagents and reaction conditions: i. Dry Acetone, K2CO3, Ethyl bromoacetate, reflux, 8 hrs; ii.Methanol, hydrazine hydrate, reflux, 3 hrs; iii. a. Ethanol, KOH, CS2, 0-30 0C 14 hrs, b. Reflux, 16 hrs iv. Acetone, K2CO3, ethyl bromoacetate, hydrazine hydrate, reflux, 4 hrs; v. Absolute EtOH, aromatic aldehydes, acetic acid, 60-70 0C, 6-12 hrs.
2.2 Biological activities
2.2.1 In vitro antiproliferative activity
The eugenol-1,2,4-triazole derivatives (6–11) were tested against different human adenocarcinomas, particularly breast (MDA-MB 231), colon (HCT-116) and prostate (PC-3) by MTT method. The results are presented in the form of IC50 in Table 1.
Compounds bearing hydroxyl group such as 9 and 10 were found to be the most cytotoxic among the tested compounds. Compound 10 was equipotent to doxorubicin towards PC-3 and MDA-MB 231 carcinomas with IC50 5.69 and 1.42 μM, respectively. Whereas compound 9 displayed cytotoxicity with IC50 2.11 (MDA MB-231), 10.36 (HCT-116) and 8.39 μM (PC-3), compared to doxorubicin with IC50 1.39, 2.36 and 5.51 μM against breast (MDA-MB 231), colon (HCT-116) and prostate (PC-3), respectively. The presence of N,N-dimethyl phenyl substituent caused decrease in activity to IC50 8.25, 21.92 and 10.32 μM while bromo phenyl and piperonal moieties further diminishes the cytotoxicity to 12.69 & 21.42 (MDA-MB 231), 18.36 & 48.44 (HCT-116) and 21.47 & 56.93 (PC-3), respectively. The weaker antiproliferative activity was demonstrated by electron donating groups such as OCH3 in compound 7 with IC50 in the range 49.32–81.59 μM, compared to doxorubicin.
Compared to previously published work, 1,2,4-triazole-3-thiol derivative I [43] and 1,2,4-triazole-linked thiourea conjugate II [44] have exhibited cytotoxic effect with EC50 of 4.5 μM and IC50 of 9.28 μM, respectively against multidrug-resistant breast MDA-MB-231 cell line while 1,2,4‑triazole‑N‑arylamide hybrid III [45] and Eugenol-1,2,3-triazole hybrid IV [29] were found to be highly susceptible to the same cell line with IC50 of 3.61 μM and 6.91 μM, respectively. El-Sharief et al. reported 1,2,4-triazole derivative V [46] with cell growth inhibitory GI value of 87% against breast MDA-MB-231. Against prostate PC-3 cancer cells, 1,2,4‐triazole derivative VI [47] have shown cytotoxicity with IC50 of 26.0 μM and Eugenol derivatives VII [28] and VIII [27] bearing oxadiazole scaffold displayed promising cytotoxicity with IC50 0.26 μM and 1.1 μM, respectively. Towards colon HCT-116 adenocarcinoma,1,2,4-triazolo[1,5-a]pyridinyl pyridines IX (IC50 0.60) and X (0.74 μM) [48] and 1,2,4-triazole-3-thiol derivative XI (IC50 4.16 μM) [49] displayed potent antiproliferation whereas 1,2,4-triazole analogue XII [50] and 4-(1H-1,2,4-triazol-1-yl)benzoic acid hybrid XIII [51] as moderate antiproliferative agents with IC50 19.89 μM. and 23.9 μM, respectively. The weaker cytotoxic compounds XIV and XV with IC50 of 83.0 μM and 90.5 μM, respectively were reported by Zeinet al [52].
2.2.2 COX-2 inhibition assay
It has been reported that cyclooxygenase (COX-2) over expression results in development and progression of various cancers such as prostate, lung, colon, pancreatic, bladder, cervical, breast, etc. COX-2 inhibitors have shown increase in survival rates of cancer patients by boosting angiogenesis, triggering apoptosis, increasing chemo and radiotherapy efficiency. Therefore, the final compounds (6–11) were screened for COX-2 inhibitory assay to know the probable mechanism of these compounds as COX-2 inhibitors. It was interesting to see that these compounds inhibited COX-2 enzyme significantly. Compounds 9 and 10 showing promising cytotoxicity also displayed potent inhibition on COX-2 with IC500.32 and 0.28 μM, respectively which was comparable to reference COX-2 inhibitor, celecoxib (IC500.25 μM). The moderate COX-2 inhibition was observed by compound 11 (IC500.53μM) whereas mild COX-2 inhibition was noted for compounds 6,7 and 8 with IC50values in the range 1.71–2.52 μM (Table 2). These results were found to be in parallel with the cytotoxicity results, indicating that these eugenol-1,2,4-triazole derivatives could hinder proliferation by suppressing COX-2 enzyme for cancer treatment.
2.3 In silico Physicochemical and pharmacokinetics studies.
The high cost, risk, hurdles and a little success rate has always been a challenge in the discovery of any drug. Mostly, the drug candidates fail in various clinical trials due to their unacceptable flaws, efficiency, efficacy and safety issues. Hence, advent of computational studies has resulted in enhancement of pharmacokinetic and toxicity properties by cost reduction and providing information about probable success rate leading to drug development efficiently [53].
The final Eugenol-1,2,4-triazole derivatives have been tested for physicochemical and pharmacokinetic properties using Swiss ADME software. For an efficient drug, the molecule has to follow certain rules, namely Lipinski and Veber rules, which are considered as hallmarks in drug development and discovery [54]. The lead hit must have molecular weight fewer than 500, lipophilicity (iLogPo/w) < 5, hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) fewer than 10 and 5, respectively. Besides, number of rotatable bonds which signify molecule flexibility and polar surface area (PSA) should be less than 10 and 140 Å2, respectively.
The synthesized derivatives as presented in Table 3 showed good physicochemical properties. All the derivatives displayed molecular weight less than 500 except 7 and 10 which due to bulky groups possess MW more than 500. The HBA and HBD were in desired range while all the derivatives were found to be lipophilic as depicted by their good octanol–water coefficient. The molecules were observed to be non flexible as they exhibited number of rotatable bonds more than 10. All the derivatives except 7 and 10 followed Lipinski rule stating that they possess qualities of a good drug candidate.
The blood brain barrier (BBB) and gastrointestinal absorption (GI) are other important parameters which decide the fate of oral drugs. The drugs are absorbed from the small intestine or colon upon oral administration and the blood brain barrier (BBB) prevents the entry of a drug into the brain from the blood therefore large molecules could not pass this barrier and prevents brain from harmful effects of the drug. As shown in Boiled Egg diagram (Fig. 5), all the Eugenol derivatives were unable to cross the blood brain barrier (yellow yolk) and showed low gastro intestinal absorption percentage from 53.04% to 60.02% (white albumin).
The toxicity prediction using in silico approaches provide an overview about the unwanted or undesirable effects and toxicity of the synthesized molecules. The important toxicity parameters such as LD50, chronic and AMES are pivotal toxicological parameters in both drug innovation and environmental assessment. As per the guidelines by NICEATM, EPA and NCCT, a molecule can be highly toxic (LD50 ≤ 50 mg/kg), moderately toxic (LD50 in range 50 ≤ 500 mg/kg) or non toxic (LD50 > 5000 mg/kg). As LD50 of eugenol-1,2,4-triazole derivatives were observed in the range 2.10–2.61 mol/kg, they are non toxic and were devoid of AMES toxicity indicating they are non-carcinogens and non mutagens (Table 4). Furthermore, chronic toxicity was also found to be in safer zone in the range 0.70–2.70 logmg/kgb.w./day and did not caused skin sensitization.
2.4 Molecular docking studies
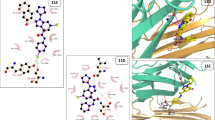
Molecular docking studies was performed to acquire an insight about the interaction ability of the eugenol-1,2,4-triazole derivatives with the COX-2 protein. For the present study, COX-2 protein (PDB 3NT1) was chosen and the docking procedure applied were previously reported [55].The inhibition behavior of the derivatives were expressed in terms of binding energy (BE) and compared with celecoxib, a reported COX-2 inhibitor.
From Table 5, the strong interactions between the synthesized derivatives and COX-2 protein was observed. Compounds 10 and 9 displayed highest binding energy of -8.81 kcal/mol and -8.02 kcal/mol, respectively, and were deeply buried into the binding pocket of COX-2 protein. Compound 10 was hydrogen bonded with Thr199 while compound 9 with Asn67 and His94 residue of the protein. These amino acids interactions were also appeared in celecoxib (Thr199, His94 and Gln92) indicating that these compounds have similar interaction like celecoxib (Fig. 6). The docking results were in favor of in vitro COX-2 inhibitory and cytotoxicity data suggesting that these new eugenol-1,2,4-triazole derivatives could harness COX-2 and cell proliferation.
3 Experimental
3.1 Chemistry
The reagents and required solvents were procured from Sigma Aldrich and Across. The different analytical methods like Bruker NMR, FT-IR (Thermo Scientific iS50) and mass spectra were recorded on Thermo Scientific LCQ Fleet (LCF10605) spectrometer for characterization of final compounds. The purity was checked using TLC, elemental analysis using LEECO Elementar Analyzer and melting points using Stuart SMP40 apparatus. Compounds (2–4) were prepared according to the previously reported method [42].
Preparation of 2-[(5-{[2-methoxy-4-(prop-2-en-1-yl)phenoxy]methyl}-1,3,4-oxadiazol-2-yl)sulfanyl]acetohydrazide (5).
Compound 4 (0.01 mmol), potassium carbonate (0.01 mmol) and ethyl bromoacetate (0.012 mmol) was added into 250 mL round bottom flask and heated in acetone at 40–50 0C for 3 h. After reaction completion, the reaction mixture was filtered, the filtrate was completely dried to get acetone free crude product. To this crude product, methanol (150 mL) followed by hydrazine hydrate (0.025 mmol) were added and refluxed for 4 h. After completion of the reaction, the reaction mixture was concentrated to 50 mL, kept at room temperature for 6 h, the solid crystals were filtered, washed with methanol–water (1:1; 25 mL), dried to get compound 5 as light yellowish brown solid.
Yield:86%; m.p.85–87 °C; IR:3536, 3307, 3066, 2928, 1682, 1617, 1510, 1467, 1399, 1237, 1257, 1185, 1019, 1002, 786; 1H NMR (CDCl3, 800 MHz): 0.94 (t, J = 8.0 Hz, 3H, CH3–), 1.60–1.63 (m, 2H, –CH2–), 2.53 (t, J = 8.0 Hz, J = 8.0 Hz, –CH2–Ar), 3.79–3.82 (m, 5H, –O–CH3, S–CH2–), 5.22 (s, 2H, O–CH2–), 5.38 (s, 2H, –NH2), 6.70–6.71 (m, 2H, Ar–H), 6.97 (s, 1H, Ar–H), 7.28 (s, 1H, –CO–NH–); 13C NMR (CDCl3, 201.21 MHz): 13.84 (CH3–), 24.66 (CH3–CH2–CH2–), 37.76 (CH3–CH2–CH2–Ar), 39.86 (–S–CH2–), 55.85 (–O–CH3), 62.56 (–O–CH2–), 112.27, 116.26, 120.74, 138.31, 144.62, 149.68, 152.38 and153.61 (triazole ring carbon), 168.90 (–C = O). Mass (ESI, + ve): 366.43 [M + 1]+. Elemental analysis for C15H22N6O3S, Calcd: C (49.17), H (6.05), N (22.93), S (8.75); Obsd: C (49.13), H (6.06), N (22.90), S (8.77).
General procedure for synthesis of compound 6–11
Compound 5 (1 mmol) was added to 100 mL round bottom flask, then added 50 mL absolute ethanol with few drops of glacial acetic acid and heated to get clear solution. To this reaction mixture, different aromatic aldehydes were added and the reaction mixture was stirred at 60–70 °C for 6–12 h. When reaction was completed, the reaction mixture was concentrated to around 15–20 mL and kept at room temperature for 4–12 h, the solid mass precipitated or crystallized out were filtered, washed with ethanol and dried. The dried products were again recrystallized either by ethanol or isopropyl alcohol.
(E)-2-((4-amino-5-((2-methoxy-4-propylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-N'-(4-methoxybenzylidene)acetohydrazide (6):
Yield:86%; m.p.150–152 °C; IR:3355, 3072, 2929, 1683, 1608, 1511, 1463, 1376, 1255, 1236, 1138, 1001, 786; 1H NMR (CDCl3, 800 MHz): 0.94 (t, J = 8.0 Hz, 3H, CH3-), 1.61–1.66 (m, 2H, –CH2–), 2.52 (t, J = 8.0 Hz, 2H, –CH2–Ar), 3.82–3.90 (m, 6H, –O–CH3), 3.92 (s, 2H, S–CH2–), 5.30 (s, 2H, O–CH2–), 5.37 (s, 2H, –NH2–), 6.72–6.92 (m, 3H, Ar–H), 6.99–7.04 (m, 4H, Ar–H), 7.86–7.87 (m, 1H, –CO–NH–), 8.70 (s, 1H, N = CH–); 13C NMR (CDCl3, 201.21 MHz): 13.82 (CH3–), 24.63 (CH3–CH2–CH2–), 37.78 (CH3–CH2–CH2–Ar), 37.88 (–S–CH2–), 55.46 (–O–CH3), 62.46 (–O–CH2–), 112.14, 112.34, 114.06, 114.33, 117.56, 21.19, 1129.48, 136.31, 144.62 (C = N) 149.70 and 161.66 (triazole ring carbon), 170.45 (–C = O), Mass (ESI, + ve): 484.57 [M + H]+. Elemental analysis for C23H28N6O4S, Calcd: C (57.01), H (5.82), N (17.34), S (6.62); Obsd: C (56.96), H (5.84), N (17.30), S (6.61).
(E)-2-((4-amino-5-((2-methoxy-4-propylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-N'-(4-bromobenzylidene)acetohydrazide (7)
Yield:86%; m.p.185-187 °C; IR:3356, 3096, 2957, 1705, 1485, 1604, 1591, 1515, 1371, 1255, 1233, 1217, 1168, 1140, 1072, 1002, 814, 795; 1H NMR (CDCl3, 800 MHz): 0.95 (t, J = 8.0 Hz, 3H, CH3–), 1.60–1.64 (m, 2H, –CH2–), 2.53 (t, J = 8.0 Hz, 2H, –CH2–Ar), 3.82 (s, 3H, –O–CH3), 4.09 (s, 2H, S–CH2–), 5.33 (s, 2H, O–CH2–), 5.47 (s, 2H, NH2–), 6.71–6.72 (d, J = 8.1 Hz, 2H, Ar–H), 6.98–6.99 (m, 1H, Ar–H), 7.46–7.51 (m, 2H, Ar–H), 7.61–7.74 (m, 2H, Ar–H), 8.16 (s, 1H, –CO–NH–), 8.62 (s, 1H, N = CH–); 13C NMR (CDCl3, 201.21 MHz): 13.82 (CH3–), 24.62 (CH3–CH2–CH2–), 37.79 (CH3–CH2–CH2–Ar), 37.79 (–S–CH2–), 55.68 (–O–CH3), 62.88 (–O–CH2–), 112.39, 117.22,120.91, 124.96, 125.87, 129.18, 129.96, 132.13, 132.88, 139.24, 144.16 (C = N), 149.89 and 161.23 (triazole ring carbon), 164.55 –C = O). Mass (ESI, + ve): 533.08 [M + H]+,535.08 [M + 1 + 2H]+. Elemental analysis for C22H25BrN6O3S, Calcd: C (49.53), H (4.72), N (15.75), S (6.01); Obsd: C (49.46), H (4.73), N (15.69), S (6.03).
(E)-2-((4-amino-5-((2-methoxy-4-propylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-N'-(benzo[d][1,3]dioxol-5-ylmethylene)acetohydrazide (8)
Yield:86%; m.p.162–164 °C; IR:3354, 3094, 2957, 1701, 1592, 1515, 1500, 1447, 1362, 1252, 1218, 1141, 1037, 999, 937, 795; 1H NMR (CDCl3, 800 MHz): 0.89–0.96 (m, 3H, CH3–), 1.60–1.63 (m, 2H, –CH2–), 2.53–2.54 (m, 2H –CH2–Ar), 3.82 (m, 3H, –O–CH3,), 4.41 (s, 2H, S–CH2–), 5.41 (s, 2H, O–CH2–), 5.53 (s, 2H, NH2–), 6.11–6.14 (m, 2H, Ar–CH2–O–CH2–Ar), 6.96 (d, 2H, Ar–H), 7.37–7.45 (m, 4H, Ar–H), 8.14 (s, 1H, N = CH–); 8.84 (s, 1H, –CO–NH–); 13C NMR (CDCl3, 201.21 MHz): 13.51 (CH3–), 24.40 (CH3–CH2–CH2–), 37.52 (CH3–CH2–CH2–Ar), 41.02 (–S–CH2–), 55.70 (–O–CH3),61.83 (–O–CH2–), 102.12 (Ar–CH2–O–CH2–Ar), 106.95, 108.38, 113.32, 115.16, 117.34, 122.67, 128.73, 131.60, 134.28, 137.28, 143.33, 145.58 (–C = N), 145.83 and 151.23 (Ar–C–O), 153.72 and 158.72 (triazole ring carbon),168.65 (–C = O), Mass (ESI, + ve): 498.55 [M + H]+. Elemental analysis for C23H26N6O5S, Calcd: C (55.41), H (5.26), N (16.86), S (6.43); Obsd: C (55.48), H (5.28), N (16.83), S (6.40).
(E)-2-((4-amino-5-((2-methoxy-4-propylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-N'-(2-hydroxybenzylidene)acetohydrazide (9)
Yield:86%; m.p.168–170 °C; IR: 3327, 3198, 2969, 1676, 1612, 1571, 1488, 1370, 1324, 1271, 1196, 1152, 1023, 970, 747; 1H NMR (CDCl3, 800 MHz): 0.89–0.98 (m, 3H, CH3–), 1.60–1.66 (m, 2H, –CH2–), 2.57–2.60 (m, 2H, –CH2–Ar), 3.74 (s, 3H, –O–CH3), 3.96 (s, 2H, S–CH2–), 5.36 (s, 2H, O–CH2–), 5.59 (s, 2H, NH2–), 6.99–7.07 (m, 4H, Ar–H), 7.38–7.59 (m, 3H, Ar–H), 8.75 (s, 1H, N = CH–), 9.93 (s, 1H, –CON–H), 11.04 (s, 1H, Ar–OH);13C NMR (CDCl3, 201.21 MHz): 13.81 (CH3–), 24.62 (CH3–CH2–CH2–), 37.76 (CH3–CH2–CH2–Ar), 40.02 (–S–CH2–), 55.66 (–O–CH3), 63.56 (–O–CH2–), 112.36, 117.17, 119.76, 120.84, 131.10, 131.88, 132.58, 133.48, 137.02, 144.28 (C = N), 149.86, 151.04 and 158.54 (triazole ring carbons), 159.82 (Ar–C–OH), 164.74 (C = O). Mass (ESI, + ve): 471.17 [M + H]+. Elemental analysis for C22H26N6O4S, Calcd: C (56.16), H (5.57), N (17.86), S (6.81); Obsd: C (56.20), H (5.60), N (17.80), S (6.79).
(E)-2-((4-amino-5-((2-methoxy-4-propylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-N'-((2-hydroxynaphthalen-1-yl)methylene)acetohydrazide (10)
Yield:86%; m.p. 142–144 °C; IR:3341, 3139, 2993, 1695, 1596, 1544, 1464, 1412, 1361, 1324, 1221, 1181, 1131, 954, 814, 740; 1H NMR (CDCl3, 800 MHz): 0.81 (t, 3H, CH3–), 1.45–1.48 (m, 2H, –CH2–), 2.37 (t, J = 8.0 Hz, 2H, –CH2–Ar), 3.69 (s, 3H, –O–CH3), 3.92 (s, 2H, S–CH2–), 5.17 (s, 2H, O–CH2–), 5.57 (s, 2H, NH2–), 6.57–6.58 (m, 2H, Ar–H), 6.85 (d, J = 8.0 Hz, 1H, Ar–H), 7.04 (d, J = 8.0 Hz, 1H, Ar–H), 7.19–7.20 (m, 2H, Ar–H), 7.33–7.34 (d, J = 8.0 Hz, 1H, Ar–H), 7.60–7.63 (m, 2H, Ar–H), 7.86 (s, 1H, N = CH–), 9.11(s, 1H, –CO–N–H–),9.61(s, 1H, Ar–OH);13C NMR (CDCl3, 201.21 MHz): 13.80 (CH3–), 24.59 (CH3–CH2–CH2–), 37.71 (CH3–CH2–CH2–Ar), 40.02 (–S–CH2–), 55.64 (–O–CH3), 62.69 (–O–CH2–), 107.95, 112.30, 116.68, 119.25, 120.16, 120.74, 123.45, 123.92, 127.53, 128.87, 132.05, 132.82, 133.10, 134.98, 144.38 (C = N), 147.42, 149.80 and 159.07 (Triazole ring carbons), 163.88 (C = O). Mass (ESI, + ve): 521.25 [M + H]+. Elemental analysis for C26H28N6O4S, Calcd: C (59.98), H (5.42), N (16.14), S (6.16); Obsd: C (59.91), H (5.44), N (16.17), S (6.13).
(E)-2-((4-amino-5-((2-methoxy-4-propylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-N'-(4-(dimethylamino)benzylidene)acetohydrazide (11)
Yield:86%; m.p.178–180 °C; IR:3378, 2997, 1705, 1602, 1578, 1531, 1431, 1407, 1361, 1223, 1169, 1124, 1065, 812; 1H NMR (CDCl3, 800 MHz): 0.94 (t, 3H, CH3–), 1.60–1.63 (m, 2H, –CH2–), 2.52 (t, J = 8.0 Hz, 2H, –CH2–Ar), 3.04 (s, 6H, –N(CH3)2), 3.74 (s, 3H, –O–CH3), 3.97 (s, 2H, S–CH2–), 5.22 (s, 2H, O–CH2–),5.52 (s, 2H, NH2–), 6.69–6.71 (m, 4H, Ar–H), 7.01 (d, J = 8.0 Hz, 1H, Ar–H), 7.67–7.70 (m, 2H, Ar–H), 8.04 (s, 1H, N = CH–), 8.70 (s, 1H, –CO–NH–). Mass (ESI, + ve): 498.22 [M + H]+. Elemental analysis for C24H31N7O3S, Calcd: C (57.93), H (6.28), N (19.70), S (6.44); Obsd: C (57.99), H (6.30), N (19.67), S (6.42).
3.2 Biological evaluation
3.2.1 In vitro antiproliferative activity
The cytotoxicity of eugenol–1,2,4–triazole derivatives was tested on human adenocarcinomas, notably breast (MDA-MB 231), colon (HCT-116) and prostate (PC3) cancer cells adopting standard MTT protocol using previously published work [56].
3.2.2 In vitro COX-2 inhibitory activity
It was performed as previously published work [55].
3.3 Molecular docking studies
The molecular docking was done as previously published [55].
4 Conclusions
In the present study, natural product eugenol was utilized to develop new eugenol based 1,2,4-triazole derivatives as antiCOX-2 and antiproliferative agents. From the antiproliferative study, compound (E)-2-((4-amino-5-((2-methoxy-4-propylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-N'-((2-hydroxynaphthalen-1-yl)methylene)acetohydrazide (10) emerged to be equipotent to doxorubicin with IC505.69 μMand1.42, towards PC3 and MDA-MB 231 carcinomas, respectively and inhibted COX-2 with IC500.28 μM. Compound 10 was also non carcinogenic, non mutagenic with good drug likeness property as depicted by in silico physicochemical and pharmacokinetic studies. The docking results against COX-2 protein showed highest binding energy for compound 10 which was in favor of its highest cytoxicity and COX-2 result. In conclusion, compound 10 could harness COX-2 and cell proliferation and could be a promising molecule in cancer therapy.
Availability of data and materials
The data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB (2019) Inflammation and cancer. Ann Afr Med 18:121–126
Khandia R, Munjal A (2020) Interplay between inflammation and cancer. Adv Protein Chem Struct Biol 119:199–245
Wang D, Dubois RN (2010) The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29:781–788
Parkin DM, Hammer L, Ferlay J, Kantelhardt EJ (2020) Cancer in Africa 2018: the role of infections. Int J Cancer 146:2089–2103
Karim A, Mccarthy K, Jawahar A, Smith D, Willis B, Nanda A (2005) Differential cyclooxygenase-2 enzyme expression in radiosensitive versus radioresistant glioblastoma multiforme cell lines. Anticancer Res 25:675–679
Baron JA, Sandler RS (2000) Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med 51:511–523
Zhao X, Xu Z, Li H (2017) NSAIDs use and reduced metastasis in cancer patients: results from a meta-analysis. Sci Rep 7:1875–1881
Nisar MF, Khadim M, Rafiq M, Chen J, Yang Y, Wan CC (2021) Pharmacological properties and health benefits of Eugenol: a comprehensive review. Oxid Med Cell Longev 2021:1–14
Buurma HA, Buurma BJ (2020) The effect of smear layer on bacterial penetration through roots obturated using zinc oxide eugenol-based sealer. BMC Oral Health 20:88–94
Kotani S, Irie S, Izumizaki M, Onimaru H (2018) Effects of eugenol on respiratory burst generation in newborn rat brainstem-spinal cord preparations. Pflugers Arch Eur J Physiol 470:385–394
Gulcin I (2011) Antioxidant activity of eugenol: a structure-activity relationship study. J Med Food 14:975–985
Lane T, Anantpadma M, Freundlich JS, Davey RA, Madrid PB, Ekins S (2019) The natural product Eugenol is an inhibitor of the Ebola virus In Vitro. Pharm Res 36:104–114
Sehajpal S, Prasad DN, Singh RK (2019) Novel ketoprofen-antioxidants mutual codrugs as safer nonsteroidal anti-inflammatory drugs: synthesis, kinetic and pharmacological evaluation. Arch Pharm (Weinheim) 352:1–15
Zari AT, Zari TA, Hakeem KR (2021) Anticancer properties of Eugenol: a review. Molecules 26:7407–7423
Rohane SH, Chauhan AJ, Fulori NK, Fulori S (2020) Synthesis and in vitro antimycobacterial potential of novel hydrazones of eugenol. Arab J Chem 13:4495–4504
De Andrade FDCP, Mendes AN (2020) Computational analysis of eugenol inhibitory activity in lipoxygenase and cyclooxygenase pathways. Sci Rep 10:16204–16217
Shin SH, Park JH, Kim GC (2007) The mechanism of apoptosis induced by eugenol in human osteosarcoma cells. J Korean Oral MaxillofacSurg 33:20–27
Al-Sharif I, Remmal A, Aboussekhr A (2013) Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 13:600–609
Da Silva FFM, Monte FJQ, de Lemos TLG, Do Nascimento PGG, Costa AKM, De Paiva LMM (2018) Eugenol derivatives: synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem Central J 12:34–43
Maurya AK, Agarwal K, Gupta AC, Saxena A, Nooreen Z, Tandon S, Ahmad A, Bawankule DU (2020) Synthesis of eugenol derivatives and its anti-inflammatory activity against skin inflammation. Nat Prod Res 34:251–260
Hidalgo ME, Rosa CD (2009) Antioxidant capacity of eugenol derivatives. Quim Nova 32:1467–1470
Alvarenga DJ, Matias LMF, Finotti C, de Souza TB, Lavorato SN, Pereira MGAG, Dias DF, Carvalho DT (2022) Synthesis of eugenol-derived glucosides and evaluation of their ability in inhibiting the angiotensin converting enzyme. Nat Prod Res 36:2246–2253
Eyambe G, Canales L, Banik B (2011) Antimicrobial activity of eugenol derivatives. Heterocyc Lett 1:154–157
Fernandes MJG, Pereira RB, Pereira DM, Fortes AG, Castanheira EMS, Goncalves MST (2020) New Eugenol derivatives with enhanced insecticidal activity. Int J Mol Sci 21:9257–9271
Teixeira RR, Gazolla PAR, da Silva AM, Borsodi MPG, Bergmann BR, Ferreira RS, Vaz BG, Vasconcelos GA, Lima WP (2018) Synthesis and leishmanicidal activity of eugenol derivatives bearing 1,2,3-triazole functionalities. Eur J Med Chem 146:274–286
Anjum NF, Shanmugarajan D, Shivaraju VK, Faizan S, Naishima NL, Prashantha Kumar BR, Javid S, Purohit MN (2022) Novel derivatives of eugenol as potent anti-inflammatory agents via PPARγ agonism: rational design, synthesis, analysis, PPARγ protein binding assay and computational studies. RSC Adv 12:16966–16978
Nazreen S, Elbehairi SEI, Malebari AM, Alghamdi N, Alshehri RF, Shati AA, Ali NM, Alfaifi MY, Elhenawy AA, Alam MM (2023) New natural Eugenol derivatives as antiproliferative agents: synthesis, biological evaluation, and computational studies. ACS Omega 8:18811–18822
Alam MM, Elbehairi SEI, Shati AA, Hussein RA, Alfaifi MY, Malebari AM, AsadM EAA, Asiri AM, Mahzari AM, Alshehri RF, Nazreen S (2023) Design, synthesis and biological evaluation of new eugenol derivatives containing 1,3,4-oxadiazole as novel inhibitors of thymidylate synthase. New J Chem 47:5021–5032
Alam MM (2023) Synthesis and anticancer activity of novel Eugenol derivatives against breast cancer cells. Nat Prod Res 37:1632–1640
Kumari M, Tahlan S, Narasimhan B, Ramasamy K, Lim SM, Shah SAA, Mani V, Kakkar S (2021) Synthesis and biological evaluation of heterocyclic 1,2,4-triazole scaffolds as promising pharmacological agents. BMC Chem 15:5–20
Alsenani NI (2023) Design, synthesis and antitumor activity of new naproxen based1,2,4-triazole-Schif base derivatives. J Umm Al-Qura Univ Appll Sci 9:294–303
Kaur R, Dwivedi AR, Kumar B, Kumar V (2016) Recent developments on 1,2,4-triazole nucleus in anticancer compounds: a review. Anticancer Agents Med Chem 16:465–489
Paprocka R, Kołodziej P, Wiese-Szadkowska M, Helmin-Basa A, Bogucka-Kocka A (2022) Evaluation of anthelmintic and anti-inflammatory activity of 1,2,4-triazole derivatives. Molecules 27:4488–4501
Gani RS, Timanagouda K, Madhushree S, Joshi SD, Hiremath MB, Mujawar SBH, Kudva AK (2020) Synthesis of novel indole, 1,2,4-triazole derivatives as potential glucosidase inhibitors. J King Saud Univ 32:3388–3399
Pachuta-Stec A (2022) Antioxidant activity of 1,2,4-triazole and its derivatives: a mini-review. Mini Rev Med Chem 22:1081–1094
Simurova NV, Maiboroda OI (2021) Antiviral activity of 1,2,4-triazole derivatives (microreview). Chem Heterocycl Comp 57:420–422
Kamboj VK, Verma PK, Dhanda A, Ranjan S (2015) 1,2,4-triazole derivatives as potential scaffold for anticonvulsant activity. Cent Nerv Syst Agents Med Chem 15:17–22
Shalini K, Kumar N, Drabu S, Sharma PK (2011) Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J Org Chem 7(1):668–677
Gao F, Wang T, Xiao J, Huang G (2019) Antibacterial activity study of 1,2,4-triazole derivatives. Eur J Med Chem 173:274–281
Kapron B, Luszczki JJ, Plazinska A, Siwek A, Karcz T, Grybos A, Nowak G, Makuch-Kocka A, Walczak K, Langner E, Szalast K, Marciniak S, Paczkowska M, Cielecka-Piontek J, Ciesla LM, Plech T (2019) Development of the 1,2,4-triazole-based anticonvulsant drug candidates acting on the voltage-gated sodium channels. Insights from in-vivo, in-vitro, and in-silico studies. Eur J Pharm Sci 129:42–57
Aggarwal R, Sumran G (2020) An insight on medicinal attributes of 1,2,4-triazoles. Eur J Med Chem 205:1–47
Mamatha SV, Belagali SL, Bhat M (2020) Synthesis, characterisation and evaluation of oxadiazole as promising anticancer agent. SN Appl Sci 2:882–893
SermuksnyteA KK, Jonuskiene I, Tumosiene I, Petrikaite V (2022) The effect of 1,2,4-triazole-3-thiol derivatives bearing hydrazone moiety on cancer cell migration and growth of melanoma, breast, and pancreatic cancer spheroids. Pharmaceuticals 15:1026–1046
Tokala R, Bale S, Janrao IP, Vennela A, Kumar NP, Senwar KR, Godugu C, Shankaraiah N (2018) Synthesis of 1,2,4-triazole-linked urea/thiourea conjugates as cytotoxic and apoptosis inducing agents. Bioorg Med Chem Lett 28:1919–1924
Turky A, Bayoumi AH, Sherbiny FF, El-Adl K, Abulkhair HS (2021) Unravelling the anticancer potency of 1,2,4-triazole-N-arylamide hybrids through inhibition of STAT3: synthesis and in silico mechanistic studies. Mol Divers 25:403–420
El-Sherief HAM, Youssif BGM, Abbas Bukhari SN, Abdelazeem AH, Abdel-Aziz M, Abdel-Rahman HM (2018) Synthesis, anticancer activity and molecular modeling studies of 1,2,4-triazole derivatives as EGFR inhibitors. Eur J Med Chem 156:774–789
Han MI, Bekci H, Uba AI, Yıldırım Y, Karasulu E, Cumaoglu A, Karasulu HY, Yelekci K, Yılmaz O, Kucukguzel SG (2019) Synthesis, molecular modeling, in vivo study, and anticancer activity of 1,2,4-triazole containing hydrazide-hydrazones derived from (S)-naproxen. Arch Pharm (Weinheim) 352:1–14
Wang XM, Xu J, Li YP, Li H, Jiang CS, Yang GD, Lu SM, Zhang SQ (2013) Synthesis and anticancer activity evaluation of a series of [1,2,4]triazolo[1,5-a]pyridinylpyridines in vitro and in vivo. Eur J Med Chem 67:243–251
Abdelrehim EM (2021) Synthesis and screening of new [1,3,4] Oxadiazole, [1,2,4] Triazole, and [1,2,4]Triazolo[4,3-b][1,2,4]triazole derivatives as potential antitumor agents on the colon carcinoma cell line (HCT-116). ACS Omega 6:1687–1696
Al Sheikh Ali A, Khan D, Naqvi A, Al-Blewi FF, Rezki N, Aouad MR, Hagar M (2020) Design, synthesis, molecular modeling, anticancer studies, and density functional theory calculations of 4-(1,2,4-Triazol-3-ylsulfanylmethyl)-1,2,3-triazole derivatives. ACS Omega 6:301–316
Abuelizz HA, Awad HM, Marzouk M, Nasr FA, Alqahtani AS, Bakheit AH, Naglah AM, Al-Salahi R (2019) Synthesis and biological evaluation of 4-(1H–1,2,4-triazol-1-yl)benzoic acid hybrids as anticancer agents. RSC Adv 9:19065–19074
Zein N, Shaban SM, Shafek SM, Baghi H, Aiad I, Omran M (2021) Synthesis and characterization of new 1,2,4-triazole anticancer scaffold derivatives. In Vitro study Egypt J Chem 64:4005–4015
Nazreen S (2021) Design, synthesis, and molecular docking studies of thiazolidinediones as PPAR-γ agonists and thymidylate synthase inhibitors. Arch Pharm (Weinheim) 354:1–12
Almalki ASA, Nazreen S, Elbehairi SEI, Asad M, Shati AA, Alfaifi MY, Alhadhrami A, Elhenawy AA, Alorabi AQ, Asiri AA, Alam MM (2022) Design, synthesis, anticancer activity and molecular docking studies of new benzimidazole derivatives bearing 1,3,4-oxadiazole moieties as potential thymidylate synthase inhibitors. New J Chem 46:14967–14978
Alam MM, Alsenani NI, Abdelhamid AA, Ahmad A, Baothman OA, Hosawi SA, Altayeb H, Nadeem MS, Ahmad V, Nazreen S, Elhenawy AA (2023) New paracetamol hybrids as anticancer and COX-2 inhibitors: synthesis, biological evaluation and docking studies. Arch Pharm. https://doi.org/10.1002/ardp.202300340
Nazreen S, Almalki ASA, Elbehairi SEI, Shati AA, Alfaifi MY, Elhenawy AA, Alsenani NI, Alfarsi A, Alhadhrami A, Alqurashi EA, Alam MM (2022) Cell cycle arrest and apoptosis-inducing ability of benzimidazole derivatives: design, synthesis, docking, and biological evaluation. Molecules 27:6899–6912
Acknowledgements
The author thanks Al-Baha University for the required facilities to complete this task.
Funding
No funding was received for conducting this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alam, M.M. New 1,2,4-triazole based eugenol derivatives as antiCOX-2 and anticancer agents. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00127-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00127-z