Abstract
Abrus precatorius (AP) is a medicinal plant rarely studied for its beneficial effects against diabetes mellitus (DM) type-1. We estimated DM type-1 related parameters—total protein (TP), direct bilirubin (DB), urea, creatinine, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and serum glucose (SG) after treatment with methanol extracts of AP leaves (APMLE) for 21 days, followed by histopathological analysis of kidney and liver sections. AP leaf bioactives (ALPBs) were collected from GCMS fractions, database, and literature; common targets were intersected with annotated DM type-1 genes from the experimental GSE14503 microarray dataset and genecard database. Overlapping differentially expressed genes were collected, and their protein–protein interaction network was analyzed using various bioinformatics tools: Enrichr, SRplot, GSEA, and Cytoscape, to provide insight into the potential molecular basis of APLBs in DM-type-1. 15 compounds were identified from GCMS analysis of APMLE. Antidiabetic potential of APMLE was observed with significant (p < 0.05) normalization of SG, TP, DB, ALT, AST, ALP, urea and creatinine while hepatorenal photomicrographs indicated moderate safety. Erucic acid, oleic acid, phytol and stigmasterol interacted with 25 type-1 DM biomarkers enriched in lipid and prostaglandin metabolic processes, neuroactive ligand receptor interaction, PPAR signaling pathway, diabetic cardiomyopathy, and cAMP signaling pathway. Furthermore, PPARalpha (peroxisome proliferator-activated alpha) and SCD (stearoyl-coenzyme A desaturase) were revealed as core biotargets interacting with APLBs via hydrogen bond, hydrophobic interaction, and van der Waals forces from the docking study. Future interests may provide additional experimental data into the mechanisms by which APLBs elicit this remarkable ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes mellitus (DM) is a disease affecting about 537 million people and projected to increase to 643 million by 2030 worldwide [1]. DM type-1 is metabolically distinguished by hyperglycemia resulting from an autoimmune process whereby insulin-producing cells are attacked as a result of genetic or environmental predispositions [1], with treatment options involving daily insulin procedures and regular monitoring of blood glucose.
Biological and pharmacological potentials have been attributed to medicinal plants. Nanu et al. [2] acknowledged that proven ethnobotanical data underpinned the roles of potent bioactives of medicinal plants in the treatment of various pathological conditions, including DM. One such promising medicinal plant is Abrus precatorius (AP), a plant from the Fabaceae family, which is a significantly underutilized food legume with widespread distribution across the tropics. Its antidiabetic potential is attributed to certain bioactive compounds with potential antidiabetic properties. These compounds include flavonoids, alkaloids, and other phytochemicals [3,4,5,6,7].
Computational biology, artificial intelligence, bioinformatics, and big data science have provided a foundational shift from the conventional single-isolation modes of traditional medicine research to a more holistic approach where there is much more reference to the whole. This provides a better understanding of the molecular mechanisms of multicomponent medicinal plants in drug discovery, design, and development. The construction of biomolecular networks of bioactive targets exerts significant visualization of these physiological mechanisms, hence elucidating the therapeutic targets of the complex and multifaceted botanical drug candidates. [8]
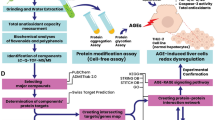
Boye et al. [9], Gaddala and Nataru [10], Boye et al. [11], and Pokharkar et al. [12] have provided some insights into the type II antidiabetic potentials of A. precatorius extracts in murine models as well as their bioactive components; however, this current study expanded the exploration of the potency of the methanolic extract of the plant’s leaf against alloxan-induced DM type-1 in Wistar rats. We also aimed to provide insight into the potential molecular basis by which AP leaf bioactives (APLBs) elicit this biological effect in vivo from a computational point of view. We administered the methanolic extract of AP leaf (APMLE) to experimental rats for in vivo antidiabetic efficacy elucidation and thereafter obtained a library of the plant’s bioactives via GCMS and literature searches. We performed a network pharmacology study along with other bioinformatics analyses to elucidate the plant’s potential molecular basis—a novel, committed approach to the best of our understanding. This provides a useful foundation for scientific evidence of AP use in traditional medicine.
2 Materials and methods
2.1 Chemicals and drugs
Alloxan monohydrate and methanol were purchased from Sigma-Aldrich Co., St. Louis, MO, USA; glibenclamide was a product of May & Baker, Nigeria; and diagnostic kits were products of Randox, UK. All other chemicals and solvents used in the study were of analytical grade.
2.2 Plant collection, identification and authentication
A. precatorius leaves were harvested in August 2022 from an uncultivated local neighborhood (Latitude: 7.081374 and Longitude: 3.3041158) of Harvarde College of Science, Business, and Management Studies (HCSBMS), Obada campus, and were subsequently identified by a taxonomist, Mr. O.A. Oropo of the Botany Unit, HCSBMS, where a voucher number (HCSBMS/001/012) was deposited for future reference.
2.3 Preparation of APMLE
Harvested and authenticated AP leaves were fully shade-dried, milled into fine powder, and passed through 60 mesh before storage in airtight containers (plastics) for further procedures. 150 g of the powdered leaf were soaked in 800 mL of methanol for 72 h while covered with cheesecloth and mildly stirred four times per day. A Whatman filter paper (No. 1) was utilized for filtering the content at the end of 72 h, followed by the use of a rotary evaporator (Heidolph Laborato 400) on a water bath to recover the methanol. This left a dark-green liquid extract, which was dried using a desiccator until completely dried. The procedures were repeated to obtain a sufficient yield of APMLE, which was then stored for further use.
2.4 Gas Chromatography – Mass Spectrometry (GC–MS) of plant extract
The GC–MS analysis of the plant extract was carried out using a Hewlett-Packard Agilent 6890 gas chromatograph with a 5973 mass selective detector. The column was fused with a silica capillary column, HP-5 (30 m × 0.25 µm film thickness, CA, USA). Helium gas was used as the carrier gas at a flow rate of 1.0 ml/min, and the oven temperature was programmed from 50 °C (2 min hold) to 280 °C (10 min hold) at a 20 °C/min rate. Respective injection and interface temperatures were set at 250 °C and 280 °C. Splitless injection of 1 ml of the sample followed, and analysis was done in full MS scan mode. The spectrums of the bioactives were compared with the GC–MS National Institute Standard and Technology [NIST 11] library, which contains spectra of known components.
2.5 Ethical clearance
All animal protocols followed in the study were reviewed and approved by the Institutional Review Board on Animal Experimentation (Approval ID: HCSBMS/AEAN/005). We followed the National Institutes of Health, NIH, OPRR Public Health Service Policy on Humane Care and Use of Laboratory Animals. Rockville, MD: NIH/Office for Protection from Research Risks, 1996 (http://grants.nih.gov/grants/olaw/olaw.htm) guidelines on human care and use of animals in scientific experimentation. We also strictly adhered to the European Commission Directive 86/609/EEC for animal experiments [13].
2.6 Acquisition and care of animals
A total of 30 six-week-old male Wistar rats weighing between 120 and 170 g were obtained from the laboratory animal housing and care facility of the Biochemistry Unit of HCSBMS. Rats were housed in standard plastic cages of dimensions 50 cm × 36 cm × 60 cm and fed with standard rodent chow (Vital Feeds Nig. Ltd.). Dry wood shavings were used as bedding while they had unrestricted access to water and were maintained under ambient conditions of temperature, humidity, and a normal dark/light cycle. At the end of the first week, the fasting blood glucose (FBG) levels of the animals were ascertained via tail clip sampling to ensure that only animals with FBG levels not above 100 mg/dl were retained for the study.
2.7 Induction of diabetes mellitus type-1and experimental design
For induction of DM type-1, the rats were fasted overnight, and diabetes was induced by intraperitoneal injection of a single dose of 200 mg/kg b.wt. alloxan monohydrate. The rats were exposed to a 10% glucose solution post-diabetes induction while blood was collected after 48 h from the tail vein to confirm diabetic status (blood glucose ≥ 250 mg/dl). The current study randomly assigned the rats to six experimental groups of five rats each, as follows:
Control: normal (5 ml/rat/day; po) + rodent chow + water
Model: Alloxan monohydrate (200 mg/kg; ip) + rodent chow + water
Glibenclamide: Alloxan monohydrate (200 mg/kg; ip) + Glibenclamide (0.5 mg/kg; po) + rodent chow + water
APMLE (100 mg/kg): Alloxan monohydrate (200 mg/kg; ip) + APMLE (100 mg/kg; po) + rodent chow + water
APMLE (200 mg/kg): Alloxan monohydrate (200 mg/kg; ip) + APMLE (200 mg/kg; po) + rodent chow + water
APMLE (400 mg/kg): Alloxan monohydrate (200 mg/kg; ip) + APMLE (400 mg/kg; po) + rodent chow + water
The treatment was administered for 21 days at doses not exceeding the LD50 of plant extract [14], while final body weight and final blood glucose were recorded.
2.8 Blood collection and analysis of biochemical parameters in serum and isolation of organs
Blood was collected into plain tubes via ocular puncture before sacrifice using capillary tubes and centrifuged at 1006 × g (Eppendorf centrifuge 5702R, 4 °C) for 5 min to obtain the serum (supernatant) which was dispensed into labelled tubes. This was followed by the assay of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), creatinine (CREA), urea, serum glucose (GLC), direct bilirubin (DB) and total protein (TP) parameters in the obtained serum using diagnostic kits (Randox, UK) with adherence to manufacturer’s instruction. Liver and kidney from each group were collected and preserved in 10% buffered formalin for histopathological procedure.
2.9 Histopathology examination of liver and kidney tissues
A portion of each of these tissues was observed and cut into small pieces not more than 4 mm thick into pre-labeled cassettes. These were further immersed in 10% formal saline for 24 h to cure. Tissue processing was carried out using the automatic tissue processor (Leica TP 1020) before embedding in paraffin wax, followed by sectioning of the tissues at 4 µm (ribbon section), and floating on a water bath (Raymond lamb) set at 55 °C. Drying at 60 °C for 1 h was followed by staining using hematoxylin and eosin techniques.
2.10 Collection of A. precatorius bioactives and targets
We created a library of AP chemical ingredients from the identified fractions by GCMS analysis. The library was expanded by searching for reported APLB from the traditional Chinese medicine systems pharmacology database (TCMSP) [15]. The chemical ingredients were filtered by the integration of pharmacokinetic properties: oral bioavailability (OB) ≥ 20 and drug-likeness (DL) ≥ 0.1. Next, the SMILES specifications of the bioactives were obtained from the PUBCHEM database [16] and fed into the SwissTarget prediction database [17] for the elucidation of their Homo sapiens biotargets. Targets with a probability score ≥ 0.1 were retained for further studies.
2.11 Determination of therapeutic biotargets of DM Type-1 and common target screening
To create a therapeutic target library of DM type-1, the gene expression omnibus database [18] (GEO) [http://www.ncbi.nlm.nih.gov/geo/] and the genecard database [19] (http://www.genecard.org/) were utilized. The experimental DNA microarray dataset, GSE14503 [20], a human gene expression microarray profile of type-1 diabetes mellitus, was downloaded from GEO. GSE14503 was profiled on the Affymetrix Human Genome U133 Plus 2.0 Array platform GPL570 (Affymetrix, Inc., Santa Clara, CA, USA). Stored. CEL files generated by the Affymetrix platform were downloaded and processed using the Bioconductor package in the R software program [21, 22]. Using the Bioconductor R Package Oligo, the data underwent format conversion, missing data filling, background correction, and data normalization by the robust multiarray average (RMA) method [19]. Furthermore, annotation of the dataset was carried out using the Bioconductor Limma package via the GEO2R tool [23]. All the expression probes were referenced against gene symbols with the removal of duplicate transcripts. The genes showing a log-fold change (FC) expression difference ≥ 1.1 and which cleared Benjamini and Hochberg’s false discovery rate (FDR) with an adjusted p-value ≤ 0.05 were noted as differentially expressed genes (DEGs) [24]. DEGs from the GSE14503 dataset and those from the Genecard database were intersected with those obtained for APLBs using the Venny 2.1 webtool (www.bioinfogp.cnb.csic.es/tools/venny) to finally obtain a library of overlapped genes (OGEs) as therapeutic targets as expressed by A. precatorius.
2.12 Network construction and functional enrichment analysis
A protein–protein interaction (PPI) network of the OGEs was constructed using the StringApp of the cytoscape software [25] for studying both the indirect and direct interactions of OGEs. To understand the enriched molecular functions, cellular components, and biological processes of the OGEs, we carried out gene ontology (GO) terms with KEGG pathway enrichment analysis using the Enrichr webserver [26]. A summary of the GO terms and the removal of redundancies were carried out using the REVIGO tool [27]. This was followed by the visualization of the results using the SRPlot webtool (http://www.bioinformatics.com.cn/en).
2.13 Gene set enrichment analysis (GSEA)
GSEA is a potent computational tool which provides biological information on the link between gene sets and a disease phenotype. In the current study, the GSEA of GSE14503 dataset was run using JAVA GSEA 4.3.2 software [28, 29] at default [except permutation type = gene set; gene set database = h.all.v2023.1.Hs.symbols.gmt and chip platform = Human_AFFY_HG_U133_MSigDB.v2023.1.Hs.chip]. Expression dataset and phenotype labels were provided in gct, and cls formats.
2.14 Functional clustering core ppi subnetwork extraction
A Cytoscape plug-in, MCODE, was used for clustering the protein network. The parameters were set at default (degree cutoff: 2, cluster finding: haircut, node score cutoff: 0.2, max. depth: 100, K-Core: 2). For the extraction of the core PPI subnetwork via in-depth topological analyses, we used CytoNCA, a Cytoscape plug-in, for the network centrality analysis. The filtering criteria were set to be above the average of closeness, betweenness, and subgraph centralities of the preliminary network and the corresponding subnetworks extracted, respectively. The merge function of the Cytoscape software was thereafter used to isolate the core PPI subnetwork (core proteins) with the sorting out of the key nodes in the preliminary PPI network.
2.15 Molecular docking of key targets
To simulate the interaction of the core targets with the APLBs, crystal structures of the key targets were downloaded in PDB format and were prepared in Discovery Studio analyzer [30] software by removing water molecules. Pyrx [31] was utilized for adding charge, and parameterizing for molecular docking via AutoDock Vina-based platform of APLBs on binding sites of crystal structures elucidated using the protein–ligand interaction identifier tool (https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index). The corresponding affinity energy values were recorded with the best pose taken as the pose with the lowest binding energy in kcal/mol. Docking results were then exported away from PyRx into Discovery Studio software for receptor-ligand interactions analysis.
2.16 Statistical analysis
Analyses of the biochemical parameters were conducted in triplicates and the results were expressed as mean ± SD. Student’s t-test was used to analyze statistical differences in weight and FBG changes assayed before and after treatment while one-way ANOVA was used to determine the statistical difference between the treatments and the control group at 95% level of confidence (p < 0.05) using IBM SPSS (v.23). Post-hoc analysis was done using post Tukey’s post-hoc test.
3 Results
3.1 Gas Chromatography – Mass Spectrometry (GC–MS) of plant extract
15 compounds were identified from the GC–MS analysis of APMLE. They included (1) 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (phytol); (2) oleic acid; (3) erucic acid; (4)11,13-Dimethyl-12-tetradecen-1-ol; (5) Morpholine; (6) 2-Methyl-Z,Z-3,13-octadecadienol; (7) Oxiraneundecanoic acid, 3-pentyl-, methyl ester, cis-; (8) Cyclopropaneoctanal, 2-octyl-; (9) Piperidine, 1-methanesulfonyl-4-methoxy-; (10) Benzeneethanamine, N-[(pentafluorophenyl)methylene]-.beta.,3,4-tris[(trimethylsilyl)oxy]-; (11) Cycloheptasiloxane, tetradecamethyl-; (12) cumidine; (13) Cyclohexasiloxane, dodecamethyl-; (14) Benzenamine, N-ethyl-3-methyl- and (15) Benzofuran, 2,3-dihydro-.
3.2 Body weight and blood glucose post diabetes mellitus type-1 induction
Table 1 showed that there were significant (p < 0.05) differences between the initial and final body weights of the experimental rats. The percentage differences in weight were between -3.099 and 12.676 after a 21-day period of the study. The initial and final FBG levels after 21 days of the study were also observed to be significantly different (p < 0.05), indicating the hypoglycemic ability of APMLE against DM type 1. The percentage differences in the FBG levels across the experimental groups were between -1.854 and 81.854, as shown in Table 1.
3.3 Effect of APMLE on serum biochemical parameters
The effect of APMLE administration on serum biochemical parameters compared to the model, control, and glibenclamide groups is as observed in Table 2. The table showed that, except for the value obtained for the TP, all serum biochemical parameters studied had higher values for the model group compared to the other groups. Further noted in the table are the significant differences (p < 0.05) in the obtained values of TP, ALT, AST, and ALP in all the APMLE-treated groups compared to the glibenclamide group. Although significantly different, these differences fall within the normal ranges of TP and the liver marker enzymes. Table 2 additionally indicated that the data obtained for DB levels in the serum of experimental animals following administration of 200 mg/kg b.wt. and 400 mg/kg b.wt. APMLE were not statistically different (p < 0.05) from the glibenclamide group. This trend was similar to the observation obtained in the DB values for the 100 mg/kg b.wt. APMLE-treated group and the normal group. Table 2 further showed that administration of 200 mg/kg b.wt. APMLE did not result in statistical differences in urea levels in these groups compared to the glibenclamide-treated groups, an observation conversely noted for the 100 mg/kg b.wt. and 200 mg/kg APMLE-treated groups. Creatinine values obtained after 21 days of APMLE treatment at 100 mg/kg b.wt. were compared favorably (p < 0.05) with the control group. While the values obtained for the 200 mg/kg b.wt. treated group were not statistically different (p < 0.05) from the glibenclamide group, a similar observation was noted for the data obtained for the 100 mg/kg b.wt. treated group compared to the 400 mg/kg b.wt. treated group. Serum glucose levels obtained after 21 days of 100 mg/kg b.wt. and 400 mg/kg b.wt. APMLE administration were not significantly different (p < 0.05) from the data obtained for the model and control groups. These ranges of values obtained, which were observed to be within normal ranges compared to the model group, indicated the hypoglycemic ability of APMLE against DM Fig. 1.
3.4 Histopathological effect of APMLE on kidney tissues
Histopathological effects on the kidneys following APMLE treatment compared to the control, model and glibenclamide groups are as shown in Fig. 2A–F. Here, the renal cortex showed normal glomeruli with normal mesangial cells and capsular spaces (white arrow), while the renal tubules also appeared normal (blue arrow). In 2A and 2C, the interstitial spaces appear normal (slender arrows). Apparently, in 2B, the interstitial spaces showed congested, dilated vessels (slender arrow). In 2D-F, the interstitial spaces showed mild vascular congestion in D and much less congestion in the dilated vessels of E and F (slender arrows), indicating the progressive corrective effect of the administration of A. precatorius type-1 DM in rats.
3.5 Histopathological effect of APMLE on liver tissues
Figure 3A–F shows the photomicrograph of a liver section stained by hematoxylin and eosin. Figure 3A shows a normal central venule (yellow arrow) and normal portal veins with mild congestion (white arrow). The morphology of the hepatocytes appears normal (blue arrow), as does that of the sinusoids (black arrow). Similar observations were noted in 3C-F. Figure 3B shows a congested portal vein (white arrow), cytoplasmic infiltration of fat (blue arrow), hepatic steatosis, and necrotized hepatocytes (green arrow). Treatment with APMLE showed progressive attenuation of this effect (Fig. 3F).
3.6 Collection of A. precatorius bioactives and targets
We obtained a total of 105 targets (probability score ≥ 0.1) from the Swisstarget prediction software for APLBs obtained from the GCMS, TCMSP database, and literature that passed the screening criteria (OB ≥ 20 and DL ≥ 0.1). These bioactives included erucic acid, stigmasterol, oleic acid, and phytol (E,7R,11S)-3,7,11,15-tetramethylhexadec-2-en-1-ol) (Fig. 4 A–D).
3.7 Therapeutic biotargets of diabetes mellitus type-1 and common target screening
The intersection of annotated DEGs genes from the microarray dataset, GSE14503, DM Type-1 genes from the genecard database, and AP expressed biotargets resulted in 25 significantly regulated (adjusted p-value ≤ 0.05; logFC ≥ 1.1) OGEs, 12 of which were up-regulated and 13 down-regulated, as shown in Table 3.
3.8 Network construction and functional enrichment analysis
Using the String App of the cytoscape software, a preliminary protein–protein interaction (PPI) network of the OGEs was generated characterized by 25 nodes connected to 24 edges (Fig. 5) and have average clustering coefficient of 0.404, network density of 0.242, heterogeneity of 0.646 and centralization of 0.346. Using the Enrichr tool, the top GO enriched terms (p < 0.05) – Biological process, Cellular component and Molecular function are presented in Bubble plots (Fig. 6). The enriched KEGG pathways are as shown in the Bubble plot combined with Sankey diagram in Fig. 7.
Figure 6 showed that biological processes for which the OGEs were significantly enriched (p < 0.05) included regulation of lipid metabolic processes, regulation of primary metabolic processes, transcription initiation from the RNA polymerase II promoter, regulation of cholesterol storage, and so on. Go terms enriched for cellular components included mostly integral components of the plasma membrane, followed by the peroxisome, endoplasmic reticulum membrane, and voltage-gated calcium channel complex. For molecular function, the enriched GO terms were oxidoreductase activity, acting on the CH-OH group of donors; NAD or NADP as acceptor; prostaglandin E receptor activity; prostaglandin receptor activity; and so on. The top KEGG pathway for which OGEs were enriched as obtained in this study (FDR < 0.05) included neuroactive ligand-receptor interaction, the PPAR signaling pathway, diabetic cardiomyopathy, the cAMP signaling pathway, chemical carcinogenesis, etc. (Fig. 7).
3.9 Gene set enrichment analysis of OGEs
Figure. 8A–E showed the gene set enrichment analysis of OGEs. GSEA results showed core enrichment of OGEs in UV response (NES = 2.19), androgen response (NES = 1.71), heme metabolism (NES = 1.5), and xenobiotic metabolism (NES = 1.41) and hypoxia (NES = 1.31).
3.10 Functional clustering core PPI subnetwork extraction
As proteins show a propensity for forming different clusters while eliciting their functions in vivo, we used the MCODE plugin of cytoscape software to cluster the OGEs and obtained three clusters. (Fig. 9). Further topological analysis of the preliminary PPI using the CytoNCA plugin of cytoscape software provided additional insight into the core proteins that define the PPI network. The topology of the PPI network suggests that nodes occupying critical locations usually have significant values of centrality, which include subgraph centrality, betweenness centrality, and closeness centrality. We first calculated the average values of these centrality measures and obtained 5.7186, 11.36, and 0.0617, respectively. We went further to extract these networks by setting the threshold to be the obtained averages to obtain the subnetworks (Fig. 9 A, B, C). Additionally, we used the intersectional merge function of the cytoscape software to extract the core proteins involved in our PPI network. We obtained PPARalpha (peroxisome proliferator-activated alpha) and SCD (stearoyl-coenzyme A desaturase) as the core protein targets of APLB in DM type-1 Fig. 10.
3.11 Molecular docking of key targets
The docking of PPARA and SCD against APLBs is presented in Fig. 11. In the current study, more hydrophobic interactions were observed compared to other interaction types, with binding energies ranging from − 8.4 to − 5.6 kcal/mol (Fig. 11), with lower binding energies implying more affinity. Hydrogen bonds, on the other hand, were the second most encountered interactions in our protein–ligand complexes (Fig. 11). PPARA docking (Fig. 11A–D) showed binding site residues’ interaction with erucic acid involves one hydrogen bond with SER 280 and hydrophobic alkyl and pi-alkyl interactions with MET 320 and PHE 218, respectively. With oleic acid, the interactions were hydrophobic alkyl interactions with MET 220, VAL 324, and LEU 331; with stigmasterol, van der Waals interactions as well as hydrophobic alkyl interactions with PRO 424, ILE 420, and LEU 412; and finally, with phytol, the observed interactions were pi-sigma (TYR 334), alkyl (MET 220, VAL 324, ILE 317, LEU 321, LEU 331), and pi-alkyl (PHE 218) hydrophobic interactions. Docking with SCD (Fig. 11 E–H) showed binding site residues’ interaction with erucic acid produces van der Waals interaction, hydrophobic alkyl (MET 79, VAL 182, LEU 186) and pi-alkyl (HIS 83) interactions. On the other hand, its binding with oleic produces one hydrogen bond (HIS 83) and one pi-alkyl interaction (PHE 178). With stigmasterol, the binding produces van der Waals interaction as well as hydrophobic alkyl (LYS 162, ILE 280, PRO 282, PRO 305), hydrophobic pi-alkyl (HIS 125, HIS 161, HIS 302) interactions. Binding with phytol produces one pi-sigma (TRP 153), one carbon hydrogen bond (GLN 147), hydrophobic alkyl (ILE 115, LEU 185, VAL 264, ALA 292), pi-alkyl (HIS 120, HIS 157, HIS 171, TRP 184, LEU 185, HIS 298) interactions.
4 Discussion
Medicinal plants elicit their pharmacological and therapeutic effects due to the presence of various potent bioactives or natural products. These are elucidated using methods such as GC–MS, LC–MS, LC-NMR, NMR, HPLC, and so on. In this study, we have utilized GCMS for APLBs constituent determination. There is strong evidence supporting the effects of natural products on glucose metabolism and diabetes-related parameters [32,33,34,35,36]. Weight gain in treated groups suggests enhancement of glucose metabolism in the APMLE-treated groups compared to the model group. The remarkable hypoglycemic activity of APMLE observed in this study, as also reported by Boye et al. [11] and Pokharkar et al. [12], could be linked to stimulation of insulin release following pancreatic beta cell repair by the extract.
Normal total protein level is an important parameter for ascertaining health and diagnosing disease. During diabetes, for example, increased protein catabolism increases amino acid flow into the liver. With glucagon responses being abnormal in type 1 diabetes, this enhanced proteolysis in uncontrolled diabetes might explain the observed decrease in the total protein content of the model group. Conversely, APMLE administration significantly increased the total protein levels to about normalcy, possibly inhibiting the increased proteolysis observed in DM type 1. Liver inflammation might have been indicated in the model group due to the increase in serum levels of bilirubin and marker enzymes (ALT, AST, and ALP). An increase in the activities of these enzymes in the serum might have occurred due to leakage from the breached cell membrane structure of the model rat groups. The cell membrane compromise could be attributed to an increase in free radical generation in response to alloxan induction, causing leakage into extrahepatic tissues. Oral administration of APMLE abated the observed elevated activities of the enzymes. This protective effect of the plant extract in reversing liver damage due to diabetes was also reported by Uroko et al. [37] using a methanolic extract of A. precatorius leaf in CCl4-induced diabetic rats. DB estimation is a good indication of liver health. Elevated DB in the serum of model animals in the present study may be due to reduced clearance arising from liver disease. Treatment with APMLE reversed this condition in treatment groups, thereby lowering the DB level. The increase in serum levels of urea and creatinine in the model group is not unexpected. This is because insulin absence and attendant glucose starvation in extrahepatic tissues stimulate the gluconeogenic pathway as an alternative in the route of glucose supply and, consequently, increased proteolysis. This results in an increased urea level in the blood due to the deamination of free glucogenic amino acids in the liver. Urea levels in the plasma therefore increase as a result of an increase in proteolysis. Creatinine is a degradative metabolite of muscle creatine phosphate, and its serum concentration is usually fairly constant. Its concentrations, however, become elevated in conditions of compromised renal function. Elevated creatinine levels in model animals suggest renal function impairment. Treatment with APMLE, however, clearly attenuated this effect, signifying better clearance in the event of treatment with APMLE. In type-1 diabetes, interstitial space accumulation causes impaired oxygen delivery to issues (cellular hypoxia) and is associated with dysfunction of the mitochondria, which increases oxidative stress as well as hyperglycemia [38]. Hyperglycemia induces activation of the transcription factors—carbohydrate response element-binding protein (ChREBP) and sterol regulatory element-binding protein 1c (SREBP-1c) [39], hence the hepatic steatosis and necrosis observed in the model group, which are cleared in APMLE-treated groups. This indicates the restorative potential of the plant extract and implies that APMLE helps clear fats infiltrating the cytoplasm of the hepatocytes as well as aiding cellular recovery from the effect of necrotized hepatocytes. Enrichment of APLBs in the lipid catabolic process could suggest that APLBs potentially correct lipid abnormalities due to hyperglycemia associated with type-1 diabetes. Promoting the lipid catabolic process improves glucose uptake at a cellular level and improves whole-body insulin sensitivity [40,41,42,43]. The top enrichment of APLBs in the prostaglandin metabolic process suggests the plant extract potentially corrects altered prostaglandin metabolism implicated in the occurrence and advancement of vascular complexity in type 1 DM. Enrichment in the neuroactive ligand receptor interaction pathway, which contains G protein-coupled receptors (GPCRs) for dopamine and serotonin, could mean that APLBs elicit physiological effects via correcting altered dopamine and serotonin metabolism in type-1 DM. SCD and PPARA have been revealed as core targets of APLBs in this study. SCD is the rate-limiting enzyme in monounsaturated fatty acid biosynthesis and is therefore demonstrated in this study as a risk factor for diabetes. Brenner (2006) [44] submitted that type 1 diabetes depresses unsaturated fatty acid biosynthesis via depressing the mRNAs and activities of SCD, while PPARA, on the other hand, is activated by unsaturated fatty acids. This potentially suggests that APLBs elicit ameliorative action in type-1 DM via regulation of the biosynthesis of unsaturated fatty acids, which play important and critically varied roles in human health [45].
The varied interactions of APLBs with core type-1 DM biomarkers revealed in this study have been shown to include hydrogen bonds, van der Waals forces, weak hydrogen bonds (carbon hydrogen bonds), and hydrophobic interactions (alkyl, pi-alkyl, and pi-sigma) at the proteins’ binding sites. Hydrophobic interactions are formed between ligand lipophilic groups and non-polar amino acid side chains of proteins when they come in close proximity. They are the major driving forces in drug-receptor/protein–ligand interactions [45] and help to bury methyl groups in solvent-exposed locations into the hydrophobic pocket of the protein or receptor. Hydrophobic interactions help intercalate the ligands at the receptor binding sites. Hydrogen bonds, on the other hand, provide directional interactions underpinning protein structure, folding, and molecular recognition [32]. Vander Waals forces as well as weak hydrogen bonds observed in the current study provided further support for stabilizing protein structures between non-polar surfaces [45].
5 Conclusion
The potential of APLBs against diabetes mellitus type-1 has been demonstrated in this study. The potential basis by which APLBs elicited this activity was elucidated computationally and was shown to involve 25 overlapping genes (OGEs), of which in-depth analysis revealed SCD and PPARalpha as core targets of APLBs. This provides a prospective foundation for consideration of this medicinal plant in the fight against type-1 diabetes conditions. It is noteworthy that this study provided insight into the in vivo anti-diabetes type-1 activity of APLBs while also computationally providing a basis for this observed effect. Future studies may provide additional quantification methods such as HPLC, HPTLC, LCMS, etc. in standardizing APLBs as well as exploring robust experimental validation of gene expression data analysis.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on necessary request.
References
Federation ID. IDF Diabetes Atlas, tenth. International Diabetes. 2021
Nanu R, Raghuveer I, Chitme HR, Chandra R (2008) Antidiabetic activity of Nyctanthes arbortristis. Pharmacogn Mag 4:335–340
Bhakta S, Das SK (2020) The medicinal values of Abrus precatorius: a review study. J Adv Biotechnol Exp Ther 3:84–91
Omoboyowa DA, Singh G, Fatoki JO, Oyeneyin OE (2023) Computational investigation of phytochemicals from Abrus precatorius seeds as modulators of peroxisome proliferator-activated receptor gamma (PPARγ). J Biomol Struct Dyn 41:5568–5582
Alayande KA, Sabiu S, Ashafa OT (2017) Medicinal properties of Abrus precatorius L. leaf extract: antimicrobial, cytotoxicity and carbohydrate metabolising enzymes’ inhibitory potential. Trans R Soc South Afr 72:242–250
Gautam DN (2017) Ethnomedicinal, toxicity and pharmacological study of Abrus precatorious: a critical review. Res J Pharm Technol 10:3621–3627
Qian H, Wang L, Li Y, Wang B, Li C, Fang L, Tang L (2022) The traditional uses, phytochemistry and pharmacology of Abrus precatorius L: a comprehensive review. J Ethnopharmacol 296:115463
Lai X, Wang X, Hu Y, Su S, Li W, Li S (2020) Network pharmacology and traditional medicine. Front Pharmacol 11:1194
Boye A, Acheampong DO, Gyamerah EO, Asiamah EA, Addo JK, Mensah DA, Brah AS, Ayiku PJ (2020) Glucose lowering and pancreato-protective effects of Abrus precatorius (L.) leaf extract in normoglycemic and STZ/Nicotinamide–Induced diabetic rats. J Ethnopharmacol 258:112918
Gaddala B, Nataru S (2015) Synthesis, characterization and evaluation of silver nanoparticles through leaves of Abrus precatorius L.: an important medicinal plant. Appl Nanosci 5:99–104
Boye A, Barku VY, Acheampong DO, Ofori EG (2021) Abrus precatorius Leaf Extract Reverses Alloxan/Nicotinamide-Induced Diabetes Mellitus in Rats through Hormonal (Insulin, GLP-1, and Glucagon) and Enzymatic (α-Amylase/α-Glucosidase) Modulation. Biomed Res. Int.
Pokharkar R, Saraswat R, Bhavare V, Kanawade M (2011) GCMS studies of Abrus precatorius. Pharmacol Online 2:1178–1189
Louhimies S (2002) Directive 86/609/EEC on the protection of animals used for experimental and other scientific purposes. Altern Lab Anim 30:217–219
Ogbuehi IH, Ebong OO, Obianime AW (2015) Oral acute toxicity (LD50) study of different solvent extracts of Abrus precatorius Linn leaves in wistar rats. Eur J Exp Biol 5:18–25
Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X (2014) TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 6:1–6
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J (2016) PubChem substance and compound databases. Nucleic Acids Res 44(D1):D1202-1213
Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V (2014) SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res 42(W1):W32-38
Clough E, Barrett T (2016) The gene expression omnibus database. Stat Genom Methods Protoc. https://doi.org/10.1007/978-1-4939-3578-9_5
Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, Sirota-Madi A (2010) GeneCards Version 3: the human gene integrator. Database. https://doi.org/10.1093/database/baq020
Chen BZ, Yu SL, Singh S, Kao LP, Tsai ZY, Yang PC, Chen BH, Shoei-Lung Li S (2011) Identification of microRNAs expressed highly in pancreatic islet-like cell clusters differentiated from human embryonic stem cells. Cell Biol Int 35:29–37
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315
Ritchie ME, Silver J, Oshlack A, Holmes M, Diyagama D, Holloway A, Smyth GK (2007) A comparison of background correction methods for two-colour microarrays. Bioinformatics 23:2700–2707
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:1–6
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Xie Z, Bailey A, Kuleshov MV, Clarke DJ, Evangelista JE, Jenkins SL, Lachmann A, Wojciechowicz ML, Kropiwnicki E, Jagodnik KM, Jeon M (2021) Gene set knowledge discovery with Enrichr. Curr Protoc 1(3):e90
Supek F, Bošnjak M, Škunca N, Šmuc T (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6:e21800
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N (2003) PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273
Studio D. Discovery studio (2008) Accelrys [2.1].
Dallakyan S, Olson AJ. Small-Molecule Library Screening by Docking with PyRx. In: Hempel J, Williams C, Hong C (2015), eds. Chemical Biology. Methods in Molecular Biology. New York, NY:Humana Press. 243–250
Gong X, Xiong L, Bi C, Zhang B (2021) Diosmetin ameliorate type 2 diabetic mellitus by up-regulating Corynebacterium glutamicum to regulate IRS/PI3K/AKT-mediated glucose metabolism disorder in KK-Ay mice. Phytomedicine 87:153582
Kang C, Kim E (2010) Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food Chem Toxicol 48:2366–2373
Karkute SG, Koley TK, Yengkhom BK, Tripathi A, Srivastava S, Maurya A, Singh B (2018) Anti-diabetic phenolic compounds of black carrot (Daucus carota Subspecies sativus var. atrorubens Alef.) inhibit enzymes of glucose metabolism: an in silico and in vitro validation. Med Chem 14:641–649
Seo YS, Shon MY, Kong R, Kang OH, Zhou T, Kim DY, Kwon DY (2016) Black ginseng extract exerts anti-hyperglycemic effect via modulation of glucose metabolism in liver and muscle. J Ethnopharmacol 190:231–240
Yan F, Zhang J, Zhang L, Zheng X (2016) Mulberry anthocyanin extract regulates glucose metabolism by promotion of glycogen synthesis and reduction of gluconeogenesis in human HepG2 cells. Food Funct 7:425–433
Uroko RI, Sangodare RS, Muhammad KH, Asadu CL (2015) Effect of methanol extract of Abrus precatorius leaves on male Wistar albino rats induced liver damage using carbon tetrachloride. J Biol Sci 15:116–123
Khoo J, Hagemeyer CE, Henstridge DC, Kumble S, Wang TY, Xu R, Gani L, King T, Soh SB, Puar T, Au V (2021) Effects of water stably-enriched with oxygen as a novel method of tissue oxygenation on mitochondrial function, and as adjuvant therapy for type 2 diabetes in a randomized placebo-controlled trial. PLoS ONE 16:e0254619
Ortega-Prieto P, Postic C (2019) Carbohydrate sensing through the transcription factor ChREBP. Front Genet 10:472
Kim NH, Jegal J, Kim YN, Heo JD, Rho JR, Yang MH, Jeong EJ (2018) Chokeberry extract and its active polyphenols suppress adipogenesis in 3T3-L1 adipocytes and modulates fat accumulation and insulin resistance in diet-induced obese mice. Nutrients 10:1734
Lahrita L, Kato E, Kawabata J (2015) Uncovering potential of Indonesian medicinal plants on glucose uptake enhancement and lipid suppression in 3T3-L1 adipocytes. J Ethnopharmacol 168:229–236
Lee S, Libman I, Hughan K, Kuk JL, Jeong JH, Zhang D, Arslanian S (2019) Effects of exercise modality on insulin resistance and ectopic fat in adolescents with overweight and obesity: a randomized clinical trial. J Pediatr 206:91–98
Zeng XY, Zhou X, Xu J, Chan SM, Xue CL, Molero JC, Ye JM (2012) Screening for the efficacy on lipid accumulation in 3T3-L1 cells is an effective tool for the identification of new anti-diabetic compounds. Biochem Pharmacol 84:830–837
Brenner R (2006) Antagonism between Type 1 and Type 2 diabetes in unsaturated fatty acid biosynthesis. Future Lipidol 1:631–640
de Freitas RF, Schapira M (2017) A systematic analysis of atomic protein–ligand interactions in the PDB. Medchemcomm 8:1970–1981
Acknowledgements
We would like to appreciate Mr. Odedele from Accurate Medical Diagnostics and Mr. Rahman Samson from Soar Biological and Diagnostic Laboratory for their advice and timely technical support during the course of this research work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. Methodology, investigation, and data collection were performed by FOA, Precious OA, DVD, FTO, JEA, EOJ, SOA, MAA. Data analyses were performed by OOT, OMO, LAS, and SOS. The first draft of the manuscript was written by OOT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taofeek, O.O., Oyewole, O.M., Sulaimon, L.A. et al. Abrus precatorius leaf bioactives: invivo anti-diabetes mellitus type-1 activity, PPARA and SCD as novel targets. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-023-00113-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-023-00113-x