Abstract
Artemisia pallens, an aromatic and medicinal plant occasionally referred to as Davana is a member of the Asteraceae family. Understanding the physiochemical and therapeutic properties of Davana essential oil (DEO) is the major aim of this study. Essential oil from plant material was extracted using the hydro-distillation method. Examination of the phytochemical components and several plant constituents from the whole oil were detected using GC–MS analysis and some components were Isobutyl propionate, 4,5-Dimethyl-Thiazole, Ligustrazin, Endo-2-Norborneol, Tetradecanoic acid, and Octadecanoic acid. The thermal stability of the oil was tested using thermoanalytical studies such as TG–DTA and DSC. Moreover, to comprehend the biological potential of the oil antimicrobial, antituberculosis, antimalarial, antioxidant, anticancer, and antibiofilm activities were investigated essential oil was tested for antimicrobial activity against 10 bacterial and 7 fungal strains. The antimalarial potential was evaluated against Plasmodium falciparum. Cytotoxicity of the DEO was determined against MCF-7, HeLa, and CHO cell lines employing MTT assay. Meanwhile, the DPPH assay was adopted to assess antioxidant potential, and the ability to suppress biofilm formation was also assessed. The study’s findings reveal that Artemisia pallens is a reservoir of natural compounds and can be used against numerous ailments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the World Health Organisation (WHO), pharmaceutical prescriptions caused 0.5 million deaths in 2019, with opioids accounting for 70% of those deaths and drug overdose accounting for 30%, raising the question of whether any alternative should be established to mitigate these disasters. Overdosage with a variety of medications has already been reported, including tranquilizers, stimulants, and others. Furthermore, resistance has been established as one of the primary reasons for death. Because the target organism no longer reacts to pharmacologically adequate dosages of the medications, drug resistance may result in a loss of entire therapeutic efficacy [1,2,3]. Occasionally, the medicine is given incorrectly, resulting in the development of resistant microorganisms. First-line antibiotics have also been demonstrated to be unsuccessful in treating diseases such as tuberculosis, pneumonia, blood poisoning, gonorrhea food-borne infections, HIV, SARS-CoV-2, and others. According to one research the therapeutic method, agent selection, and duration of antibiotic therapy are inappropriate in 30–50% of cases [4].
Given the severe adverse effects of synthetic medications, natural therapies have spurred a great deal of interest in traditional folk medicine research. Because of their many biological functions, plant extracts are gaining a lot of interest these days. Phytochemicals are in significant demand for primary health care since they are safer, more effective, and have fewer side effects than synthetic pharmaceutical medications [5]. As a result, essential oils and other plant extracts are being tested for physiologically bioactive ingredients for application in food, medicine, aromatherapy, and cosmetics, among other disciplines [6].
Ayurveda is India’s most well-known traditional medicine, having been practiced for millennia. Ayurveda has molded medical research in plenty of ways due to its thorough understanding of natural therapies. Essential oils are also known as natural antibacterial agents, and their broad spectrum of antimicrobial qualities make them an appropriate choice, in addition to playing an important part in a variety of biological processes. Because of its unique mechanism, it does not favor creating resistance [6]. Essential oils play an important part in the medical business today [6].
Recent research suggests that phytochemicals, owing to their antioxidant capacity, could assist in lowering the likelihood of deadly illnesses such as cardiovascular disease, cancer, and others. Through the best of our intentions, we began evaluating and studying Artemisia pallens, often known as ‘Davana’ oil, a member of the Asteraceae family. For bio-prospecting, the genus Artemisia stands at the top of the Asteraceae family. This genus contains around 500 species of plants and shrubs [7]. Davana oil is derived from a common fragrant shrub that is abundantly produced in southern India. Davanite, davan ether, davan furan, and linalool are the main components of davana oil. Other components include methyl cinnamate, ethyl cinnamate, bicyclogermacrene, 2-hydroxyisodavanone, farnesol, geranyl acetate, sesquiterpenes, and germacranolides [8]. Their blooms and foliage are widely recognized for their flavorful aroma and are occasionally used for decoration. Artemisia pallens is a fragrant plant that grows profusely in humid conditions in India [9]. Various studies demonstrate that it is beneficial against Candida albicans fungal infections [10]. Plant extracts have antihelminthic, antipyretic, and tonic qualities, as well as antibacterial, antispasmodic, antifungal, and stimulating characteristics. Davana oil has previously been tested for anti-diabetic, antibacterial, antinociceptive, and wound-healing properties [11].
The current study’s goal is to promote awareness regarding the use of natural medications as a viable alternative to manufactured drugs. Because most studies have not incorporated thermal analysis. As a result, we approached evaluating the various uses and bioactivities of Artemisia pallens essential oil using various techniques and instruments such as Gas Chromatography-Mass Spectrometry (GC–MS), and the thermal properties of the oil were investigated using Differential Scanning Calorimetry (DSC) and TG–DTA. The oil was also studied for its antibacterial, antioxidant, antibiofilm, anticancer, antituberculosis, and antimalarial properties.
2 Materials and methods
2.1 Sample collection, authentication, and extraction of essential oil
The Davana plant (Artemisia pallens) was obtained in the Malshej Ghat foothills (19.340625°N 73.75522°E), Maharashtra (India’s center-west state). It was then transported to the research center. Initially, the plant sample’s genomic DNA was isolated using a CTAB buffer. After completing the quantitative analysis of the collected genomic data using the gel electrophoresis technique. The polymerase chain reaction (PCR) Thermocycler was used to amplify the genomic DNA sample using rbcL universal primers [12] (Forward 5′-ATGTCACCACAAACAGAGACTAAAGC-3′ and Reverse 5′-GTAAAATCAAGTCCACCRCG-3′). Following that, the rbcL gene was sequenced using Sanger sequencing technology at the Eurofins laboratory in India. After that, the sequencing data was sent to the National Centre for Biotechnology Information (NCBI). Finally, the obtained DNA sequence of Artemisia pallens with the GenBank accession number OL581698 was compared to all known rbcL gene sequences of Artemisia species in the NCBI database to understand the evolutionary connection. The sequences were then aligned and a phylogenetic tree was produced using the MEGA 11 tool and the Neighbor-joining technique using bootstrap values based on 1000 replications and the Tamura-Nei model, as shown in Fig. 1. Furthermore, essential oil extraction was carried out, according to Mallavarapu [13]. Fresh herb plant samples weighing 1000 g were dried in the shade for 3 days before being subjected to an 8-h hydro distillation using Clevenger apparatus. The recovered Davana Essential oil (DEO) was dried over Na2SO4 and kept refrigerated at 5 °C until tested.
2.2 GC–MS analysis
The constituents of essential oil isolated from Artemisia pallens were detected through a GC–MS study. The analysis conducted utilized, a Clarus 600C system, which encompassed a Gas Chromatograph associated with a Mass Spectrometer and a GSBP-5 MS column with 5% diphenyl/ 95% dimethyl polysiloxane composition, a 30 m long capillary column with an internal diameter of 0.25 mm and a film thickness of 0.25 μm.
In the GC–MS technique, an electron ionization device in electron impact mode having an ionization energy of 70 eV was adopted. The operation employed helium (99.999%) as a carrier gas, with a uniform flow rate of 14 mL/min and an injection volume of 1 μL, including a split ratio of 80:1. Considering the temperature regulations, the injector temperature and ion-source temperature were held at 250 °C and 220 °C respectively. While the oven temperature was adjusted at 70 °C for 1 min with an isothermal condition. Later, an increase from 190 °C/min to 260 °C with 10 min isothermal condition. The entire analytical procedure was 35 min. Mass spectra in the range of 50 to 650 m/z were obtained at 70 eV. Several constituents of Davana essential oil were determined by evaluating their mass spectra to those from the Wiley and NIST libraries, including those mentioned by Adams [14], and comparing their retention indices.
2.3 Thermogravimetric analysis
The DEO sample was TGA analyzed using STA 2500 equipment purchased from NETZSCH in Germany. The experiments were carried out in a nitrogen gas atmosphere at a flow rate of 300 mL/min. The samples, each weighing 21.4 mg, were properly weighed and placed in the aluminum crucibles. During the process, all samples were heated at a rate of 5.5 °C/min, starting at ambient temperature and escalating to 500 °C.
2.4 Differential scanning calorimetry
The DSC profile of the essential oil was generated using TA Instruments using a DSCQ20 model. In the aluminum crucible, a sample weighing 3 mg was placed. A nitrogen gas flow rate of 40 mL/min was used to test the sample. A dynamic scan was done across a temperature range of 25 °C to 375 °C at a heating rate of 10 °C/min.
2.5 Biological activities
2.5.1 Microbial strains employed
Susceptibility to the DEO of the test specimens were estimated against ten bacterial strains and seven fungal strains. Both gram positive and gram negative nature strains were employed such as Propionibacterium acnes (MTCC 1951), Staphylococcus epidermidis (MTCC 3615), Streptococcus pyogenes (MTCC 1924), Enterococcus hirae (MTCC 2728), Enterococcus faecalis (MTCC 439), Streptococcus pneumoniae (MTCC 655), Pseudomonas aeruginosa (MCC 2080), Proteus Mirabilis (MTCC 1429), Escherichia coli (MCC 2412) and Klebsiella pneumoniae (MCC 2451). Candida albicans (MCC 1151) and certain other isolates of fungi were also employed. Bacterial isolates were grown for 24 h at 37 °C on Mueller Hinton Agar whereas fungal strains on Sabouraud Dextrose Agar. The MTCC and MCC microbial strains were acquired from the Institute of Microbial Technology (IMTECH) in Chandigarh and the National Centre for Cell Science (NCCS) in Pune, respectively, while the remaining bacterial and fungal cultures were procured from existing laboratory test isolates.
2.5.2 Disk diffusion assay
In our study Disk diffusion method suggested by Demo with slight variance was carried out to estimate the potential of DEO against bacterial species [15] Initially, overnight-grown culture was employed, and 200 μL of 106 CFU/mL solution of each test bacterial inoculum was spread over the MH agar plate. Later, 10 μL of the sample was administered over the paper disc with a 6 mm diameter, and the plates were incubated for 24 h at 37 °C.and then the plates were allowed to incubate for 24 h at 37 °C. As a positive control for bacterial isolates, Chloramphenicol (30 μg/mL for each disc) was adopted. However, the potential against the fungal strains was evaluated in the same way with some modifications [16, 17]. For this purpose, fungal strains were cultivated over the Sabouraud Dextrose agar plate, and Ketoconazole (1 mg/mL) was used as a control. Furthermore, the plates were allowed to incubate for 3–7 days at 30 °C. The test was carried out in triplets to obtain satisfactory outcomes.
2.5.3 Determination of minimum inhibitory concentration (MIC) for microbial isolates
The microdilution method was implemented to determine the minimal concentration of DEO critical to inhibit the bacterial cultures, and the process was carried out using 96-well microtiter plates [18]. The bacterial suspension was first formulated, and then it was adjusted with a saline solution containing 1.0 × 105 CFU/mL. To achieve the desired concentrations, the essential oil was diluted in a combination of 5% DMSO and 0.1% polysorbate-80 (1 mg/mL), and then added to a Luria–Bertani medium (100 μL) containing a bacterial inoculum of 1.0 × 104 CFU/mL. Finally, the plates were incubated at 37 °C for 24 h [19]. The MIC of the oil, on the other hand, was calculated in the same manner [20] but with slight variations. In the case of fungal strains, Sabouraud dextrose broth with a final density of 5 × 104 CFU/mL was used. Furthermore, the plates were incubated for 72 h at a temperature of 28 °C, with ketoconazole (1 mg/mL) and chloramphenicol (30 μg/mL for each disc) used as Positive Controls. The MIC was established after incubation as the lowest treatment that stopped detectable microbial growth in the wells.
2.5.4 Antituberculosis activity evaluation
The DEO was tested in vitro against a susceptible strain of Mycobacterium tuberculosis (H37Rv) acquired from the National Institute of Tuberculosis Research in Chennai, Tamil Nadu. The oil was evaluated using a classic procedure that included the L.J. MIC (Lowenstein and Jensen) method. The LJ medium used in this technique was enhanced with salts, malachite green, 2% solution, asparagine, glycerol, and other growth needs. It comprises a homogenized egg solution from fresh hen’s eggs because it is an egg-based media, and the reference medication used as isoniazid. The medium was injected with Mycobacterium suspension strains in various concentrations of DEO (100, 50, 12.5, 6.25. 3.125, 10, 5, 2.5, 1.25, 8, 4, 2, 1, 0.5, 0.25 μg/mL). Finally, incubation was carried out with weekly monitoring [20].
2.5.5 Anti-malarial assay
The activity of the DEO against Plasmodium Falciparum, a malarial pathogen, was investigated in vitro using the microassay procedure described by Rieckmann and colleagues with a minor modification [21]. The experiment began with the cultivation of a drug-sensitive and resistant Plasmodium Falciparum strain in RPMI-1640 medium supplemented with 25 mM HEPES, 0.23% sodium bicarbonate, 1% D-glucose, and 10% heat-inactivated human serum. The cells were treated with 5% D-sorbitol, which caused them to change from asynchronous to synchronous. To create a primary ring stage parasitemia, Jaswant Singh Bhattacharya (JSB) staining was used [22]. Furthermore, 5 mg/mL stock solutions in DMSO of essential oils with concentrations ranging from 0.4 μg/mL to 100 μg/mL were prepared. The test and duplicate wells, which contain parasitized cell preparations, were then filled with exactly 20 μL of diluted material. Culture plates were incubated in a desiccator for 36–40 h at 37 °C. After incubation, blood smears were stained with JSB stain. Microscopic inspection of slides was performed to better understand the development of ring-stage parasites into schizonts and trophozoites. Chloroquine and Quinine were used as reference medicines in the process. Finally, the sample’s MIC value was obtained, and the IC50 value was derived using standard values.
2.5.6 Antioxidant study
In the present study, the antioxidant activity of the DEO was examined by using a DPPH reagent with minor modifications [23, 24]. To proceed, 1 mL from a 0.05 mM methanol solution of the DPPH radical was mixed with 2 mL of DEO sample, and to this 2 mL of 0.1 M, sodium acetate buffer having a pH 5.5 was added. The mixture was properly shaken to dissolve the constituents and was incubated for about 30 min in the dark at room temperature. Post, incubation, the absorbance was measured at 515 nm using a UV Visible spectrophotometer. Methanol was employed as a negative control and Ascorbic acid with varying concentrations was employed as a standard [25].
Later, around 3 mL of this solution was mixed with 100 μL of the essential oil at various concentrations ranging from 100 μg/mL to 1000 μg/mL [26]. Additionally, post-incubation, the absorbance of the sample was taken at 515 nm The control was prepared in the same way mentioned above without the sample.
The % Free radical scavenging activity was calculated using the following formula:
where A blank is the absorbance of the control and A sample is the absorbance of the test compound under study. Thus, essential oil concentration providing 50% inhibition i.e., IC50, was calculated from the graph plotted. The experiment was carried out in a triplet.
2.5.7 Maintenance of cell lines
To assess the essential oil under study’s potential for cytotoxicity, three cell lines were used. MCF-7 and HeLa were cancerous cell lines, on the other hand, CHO was a normal cell line, obtained from National Centre for Cell Science (NCCS), Pune. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Foetal Bovine Serum (FBS), Pen-strep as an antibiotic, and were maintained in regulated conditions at 37 °C, 5% CO2, 95% air, and 100% relative humidity.
2.5.8 Cytotoxic screening
MTT assay was adopted to screen the cytotoxic potential of the DEO as described by Wang et al. [27] with minor variations. The cells were seeded at a density of 5 × 104 cells/well in 96-well microplates and then were incubated in a CO2 incubator at 37° C, 5% CO2 overnight. After being examined under a microscope, the fully confluent cells were treated with the essential oil samples at 6 different concentrations, and they were then allowed to incubate overnight in the presence of the samples in a CO2 incubator at 37 °C, 5% CO2. Following incubation, the cells were examined under a microscope before 10 µL of the 5 mg/mL MTT reagent was added to the wells and incubated for 4 h. The medium with MTT was then flicked off followed by the introduction of formazan crystals which were then dissolved by adding 100 µL of DMSO. Lastly, the 96-well ELISA plate reader used 595 nm to detect the absorbance. The tests were carried out in triplicate, and the average result was noted. Tamoxifen was used as a positive control, while a negative control with no cells and only medium was also used.
The following formula is used to calculate the cell inhibition percentage:
The IC50 value was quantified using graph tools.
2.5.9 Anti-biofilm activity
The essential oil was examined for its potential to inhibit the formation of biofilm [28] produced by various microbial strains; Acinetobacter baumannii, Proteus vulgaris, Escherichia coli, Proteus mirabilis, and Pseudomonas aeruginosa which were obtained from MTCC Chandigarh. Specific colonies of the sample organisms for the study were isolated from a Nutrient agar medium, which were then inoculated into Luria Bertani Broth. The culture suspension was adjusted to 0.5 O.D. according to McFarland Standards.
The crystal violet test was performed in 96-well microtiter plates, 100 µL of sterile LB, and an equal amount of distilled water was added to the well. Following that, 100 µL of DEO was also added in each well except for those wells which were employed for negative and positive control. Instead of the sample, 30 µL of chloramphenicol and 100 µL of sterile distilled water with 200 µL of LB were introduced to the positive and negative control wells, bringing the total capacity to 300 µL. Furthermore, an overnight established bacterial culture in LB broth was diluted, and 20 µL of bacterial suspension with an O.D. of 0.01 measured at a wavelength of 600 nm was introduced into the wells holding the essential oil sample. For bacterial growth and biofilm maturation, the plates were subjected to regulated incubation conditions at 37 °C for 24 h. After which the plates were rinsed thrice and then 400 µL of crystal violet dye was introduced following incubation at room temperature for 30–40 min. Before keeping the plates for drying in a hot air oven at 40 °C for around 15–20 min, they were first rinsed with distilled water multiple times to eliminate unbound dye. Lastly, 400 µL of methanol was added to the wells to take absorbance at 570 nm in the ELISA plate reader as per the method outlined by Cáceres and co-workers [29]. The % inhibition was calculated by the following equation:
3 Results and discussion
3.1 Determination of chemical constituents by GC–MS analysis
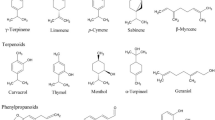
Employing Gas Chromatography-Mass Spectroscopy (GC–MS), the essential oil of Artemisia pallens revealed a wide diversity of chemical compounds [30]. The investigation resulted in the identification of 14 components, constituting the overall content of the oil, which are illustrated in (Table 1) (Fig. 2). The GC–MS chromatogram revealed peaks of the various components present in DEO. The essential oil’s most abundant components were identified to be 4,5-Dimethyl-Thiazole (20.024%), Endo-2-Norborneol (25.561%), Nona-3,6-dien-1-ol (5.378%), 5-Isopropylidene-3,3-dimethyl-dihydrofuran-2-on (4.594%), Hexahydro-3-(2-Methyl propyl)-pyrrolo(1,2-A) Pyrazine-1,4-dione(6.007%) and Octadecanoic acid (5.106%) each of which with a different retention period. DEO has numerous volatile and aromatic components. The presence of isobutyl propionate, Tetradecanoic acid, Octadecanoic acid, Hexahydro-3-(Phenyl methyl)-Pyrrolo[1,2-A] Pyrazine-1,4-dione in Davana essential oil confers antimicrobial action [31, 32]. The presence of Nona-3,6-dien-1-ol, Hexahydro-3-(Phenyl methyl)-Pyrrolo[1,2-A] Pyrazine-1,4-dione and Hexahydro-3-(2-Methyl propyl)-pyrrolo(1,2-A)Pyrazine-1,4-dione, results in anti-oxidant properties [33, 34], as well as, Endo-2-Norborneol, 4,5-Dimethyl-Thiazole both are reported to have anti-cancer property [35, 36]. Ligustrazine derivatives have antiplatelet aggregation activities [37]. Previous studies have reported that DEO also acts as an aromatic agent due to the presence of certain components such as linalool and geraniol, which have a sweet floral scent, whereas phytol, phytyl acetate, and Davanone have a floral balsamic and fruity scent [38], as well as food additive agent due to phytol. Because of its qualities, this essential oil has the potential to be employed in the pharmaceutical industry and, owing to its many capabilities, might become a cosmeceutical product.
3.2 TG–DTA analysis of DEO
To comprehend the thermal behavior of the essential oil under evaluation, it was subjected to synchronous TG–DTA thermal analysis methodologies. In the case of Differential thermogravimetric analysis, the thermogram obtained is displayed in (Fig. 3). It can be observed that the DTA curve initially shows fluctuations from the temperature of 50 °C to 150 °C. In the second temperature region between 200 °C and 220 °C, the phase transition is seen with an onset at temperature 201.1 °C and with a complex peak observed at 214.4 °C, having a complex peak area of − 3.611 J/g. The curve stabilizes after 310 °C indicating an exothermic reaction. The high temperature in the DTA evaluation suggests that perhaps the sample is very stable and has high-temperature endurance. The percentage of mass loss of the sample at different temperatures was understood through TGA analysis. (Fig. 3) represents the thermogram of the DEO. The onset temperature point for the mass loss was observed at 202.2 °C until 350 °C. The curve does, however, stabilize after that. It could be assumed that certain molecules of the essential oil could be thermally decomposed to new substances. The data demonstrate that DEO shows greater mass loss at higher temperatures, which could be due to its strong thermal stability.
3.3 Calorimetry profile of DEO
The calorimetry profiling of DEO through Differential Scanning Calorimetry analysis revealed the thermogram, in (Fig. 4). It can be observed that as the temperature is raised the heat flow within the sample is sharply decreased in a temperature ranging from 25 °C to 178 °C displayed by an endothermic trough, at 174 °C with a heat flow of − 1.401 W/g. As the temperature is further increased, then there is a sudden exponential increase in the heat flow of the sample in a temperature range of 180 °C to 225 °C, showcasing an exothermic shift with a heat flow of − 0.1095 W/g at 207.63 °C. Furthermore, a linear increase is observed till 371.51 °C having a heat flow of 0.2988 W/g. This thermogram shows the heat profile of the essential oil when subjected to escalating temperatures, demonstrating the oil’s thermal endurance.
3.4 Anti-microbial potential
3.4.1 Antimicrobial sensitivity determination
The study evaluated the capability of DEO against 17 microbial species by analyzing the zone of inhibition. The essential oil was shown to have antibacterial action against the strains under investigation, illustrated in (Table 2). The experimental results suggest that DEO was most effective against Klebsiella pneumoniae and Enterococcus faecalis bacterial strains. However, the highest activity was seen in the case of Klebsiella pneumoniae with a zone of inhibition of (21 ± 0.06 mm). There were other bacterial species including Enterococcus hirae, Propionibacterium acne, Proteus Mirabilis, Pseudomonas aeruginosa, and Streptococcus pneumoniae, that showed a moderate zone of clearance. In the study, the least activity was seen in the case of Streptococcus pyogenes, with an inhibitory zone of (13 ± 0.07 mm). Whereas, considering fungal strains; the smallest zone of inhibition was observed in the case of Candida Albicans having an inhibition zone of (12 ± 0.05 mm). The essential oil was highly effective against three fungal species Aspergillus niger, Microsporum gypseum, and Fusarium oxysporum. However, the highest zone of inhibition was observed against the Microsporum gypseum possessing a zone of clearance of (23 ± 0.05 mm).
3.5 Determination of minimum inhibitory concentration
Interpreting the data obtained through the MIC, which is displayed in (Table 3). It can be said that among the various fungal and bacterial species, potential activity was observed against 2 bacterial species: Propionibacterium acnes, and Escherichia coli with MIC values of (31.25 ± 0.04 μg/mL) and (31 ± 0.03 μg/mL), respectively. The least activity of DEO was seen in the case of Klebsiella pneumoniae with a MIC value of (250 ± 0.05 μg/mL). On the other side, Fusarium moniliforme was the most susceptible fungal strain with a MIC of (31.25 ± 0.03 μg/mL), and the highest resistance was shown by Candida Albicans with a minimal concentration of (125 ± 0.02 μg/mL). The activity of DEO against Mycobacterium tuberculosis (H37Rv) was observed at a MIC of 62.5 μg/mL.
The result from the above data suggests that the antimicrobial potential depends on the concertation and types of organisms employed for the study. Previous authors have suggested that essential oil mainly shows antimicrobial action by affecting the cytoplasmic membrane of the cells [39]. Previous research has linked DEO’s antibacterial potential to the presence of Hexahydrofarnesyl acetone [40] and Davanone [41]. Previous studies have also suggested that Geranyl acetate which is present in DEO possesses antifungal potential [42]. Authors have also worked on Artemisia annua species [43], which has demonstrated possible antimicrobial potential against a variety of microbial strains, some of which are similar to ours, with positive findings.
3.6 Anti-malarial potential of DEO
The genus Artemisia includes key therapeutic plants that are currently considered the focus of phytochemical research, owing to their biological and chemical complexity, as well as their capacity to make essential oils [44]. Malaria is one of the major public health problems in many developing countries today, and it is one of the deadliest infections produced by Anopheles mosquitoes [45]. The anti-plasmodial activity of Artemisia pallens essential oil was examined against drug-resistant and drug-sensitive strains of Plasmodium falciparum, and the IC50 value was quantified. The IC50 value of Davana essential oil is mentioned in (Table 4). The minimal inhibitory concentration value against drug-sensitive Plasmodium falciparum was 1.34 μg/mL, whereas the drug concentration predicted to suppress 50% of the cells against the drug-resistant strain was 1.98 μg/mL. Survival vs. essential oil concentration is depicted schematically in (Fig. 5). In other research findings, Artemisia annua was shown to be a potential antimalarial agent against chloroquine-resistant malaria, and the dichloromethane extracts of both Artemisia spicigera and Artemisia scoparia have been found to have an antimalarial action with an IC50 of 0.7780 mg/mL [45].
3.7 Antioxidant activity of DEO
In the current study, the potential of DEO to scavenge free radicals was examined through the DPPH (2,2′-diphenyl-1-picrylhydrazyl) assay. The essential oil neutralizes free radicals in a dose-dependent manner, as given in (Table 5). It can be seen that the oil showed the least activity of 61.52 ± 0.03% at the 0.1 mg/mL concentration range. Meanwhile, the highest capacity to capture free radicals was exhibited at a concentration of 1 mg/mL with (80.78 ± 0.04%) inhibition. Figure 6 illustrates the graphical representation of the antioxidant capacity along with the IC50 value of 0.55 mg/mL. The antioxidant potential of the DEO was owing to the presence of certain molecules like Davanone [46] and Dimethyl fumarate [47], along with components such as Hexahydro-3-(Phenyl methyl)-Pyrrolo[1,2-A] Pyrazine-1,4-dione which were confirmed by our GC–MS analysis. Previously, studies have been conducted on similar lines to demonstrate the antioxidant capacity of the methanol extract of Artemisia pallens with an IC50 value of 292.7 µg [48].
3.8 Cytotoxic potential to inhibit cell lines
The potential of the DEO against three cell lines was evaluated using MTT Assay, where these cell lines were subjected to varying concentrations of the essential oil (5, 10, 20, 40, 60, 80, and 100 µg/mL), under-regulated conditions. The DEO showed anticancer activity against cancerous cell lines, HeLa, and MCF-7 with an IC50 value of 45.4 µg/mL and 37.1 µg/mL, respectively. However, the action towards CHO, a non-cancerous cell line was observed to have an IC50 value of 21.6 µg/mL, which is illustrated in (Table 6). Figure 7 depicts the graphical format of DEO activity towards the cell lines taken under study. The capacity of the DEO to inhibit the cell lines could be attributed to the presence of Geranyl acetate [49], Dimethyl fumarate [50], and Davanone [51].
Previously, studies on Artemisia Indicia essential oil have been conducted which revealed its potential cytotoxic activity against cancer cell lines [52]. Other studies have shown the cytotoxic significance of ethanolic extract, bioactive fractions, and sub-fractions of Artemisia nilagirica towards 3 cell lines out of which MCF-7 is common with our study [53]. Numerous studies revealed that a large proportion of sesquiterpenes seems to be accountable for the anticancer effect [54].
3.9 Capability of the DEO to inhibit biofilm formation
The ability of the essential oil to prevent the formation of biofilm made by various microbial strains was examined by using a crystal violet assay. The essential oil’s activity against the range of organisms was seen with different efficiencies, which is depicted in (Table 7). If the percentage inhibition findings are between 0 and 100%, biofilm inhibition takes place, according to currently known standards [55]. Meanwhile, if the figure is less than 0%, this indicates the development of biofilm. If the inhibition values are above 50%, the action is regarded as good, however, if the percent inhibition is within the range of 0% and 50%, it is considered bad. From the table, it can be estimated that the biofilm inhibition percentage is above 50% at concentrations of 80 and 100 μg/mL for every selected microbe, which signifies that the DEO is an effective biofilm development inhibitor. The capacity to prevent biofilm formation was seen against Proteus vulgaris with the highest % inhibition of 82.31%. However, the least effectiveness was observed against Proteus mirabilis with 69.76% biofilm inhibition. The potential to inhibit 50% of biofilm development as IC50 value was quantified and it was noticed that the least IC50 value of 49.5 µg/mL was seen for Proteus vulgaris, which portrays that the DEO inhibits the development of biofilm against it most efficiently when compared with other test microbes. (Fig. 8), depicts the graphical representation of biofilm inhibition along with IC50 values. From this experiment, we may infer that the type of organism used with the same oil affects how much biofilm inhibition occurs.
These essential oils are thought to prevent the growth of biofilms by interfering with their growth processes. Previous studies have shown that essential oils with high concentrations of monoterpenes or phenylpropanoids can effectively suppress the growth of biofilm [56].
4 Conclusion
On a brief note, our study investigated the potential of historically useful Artemisia pallens essential oil as a potential replacement for synthetic drugs that are known to have adverse consequences on an individual’s health. Essential oil under study demonstrated moderate antioxidant capacity and potential antimicrobial activity against 17 microbial strains, which showcases its effectiveness on a range of microorganisms. Promising outcomes were observed when the cytotoxic potential of the essential oil against HeLa, MCF-7, and CHO cell lines was determined. The effectiveness of the oil was also evaluated against two pathogens namely, Plasmodium Falciparum and Mycobacterium tuberculosis with positive findings. Thermal analysis of the oil established evidence for its thermal stability, while the GC–MS study recognized bioactive components with pharmacological relevance. Taking everything into account, the essential oil extracted from Artemisia pallens has a huge potential to be employed as a pharmacological entity and it might also be beneficial in other industries owing to its numerous activities.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DEO:
-
Davana Essential Oil
- GC–MS:
-
Gas chromatography-mass spectrometry
- DSC:
-
Differential Scanning Calorimetry
- TG–DTA:
-
Thermogravimetric, and Differential Thermal Analysis
- MIC:
-
Minimum Inhibitory Concentrations
- DPPH Assay:
-
2,2′- Diphenyl-1-picrylhydrazyl Assay
- LB:
-
Luria–Bertani
- RT:
-
Room temperature
- HeLa:
-
Human cervical cancer cell line
- CHO:
-
Chinese hamster ovary
- MCF-7:
-
Michigan Cancer Foundation-7
- MTT Assay:
-
3-(4,5- Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay
- FBS:
-
Foetal bovine serum
References
Jha V et al (2022) Chemical composition, bioactive potential, and thermal behaviour of cyperus scariosus essential oil. Chem Sci Int J. https://doi.org/10.9734/csji/2022/v31i230276
Jha V et al (2022) Evaluation of physiochemical properties, thermal behavior and phytopharmaceutical potential of citrus aurantium’s essential oil. Eur J Med Plants. https://doi.org/10.9734/ejmp/2022/v33i730479
Hazlehurst L, Hacker M (2009) Drug resistance, 1st edn. Elsevier Inc., Amsterdam. https://doi.org/10.1016/B978-0-12-369521-5.00015-4
Abraham EP (1963) The antibiotics. Compr Biochem 11(4):181–224. https://doi.org/10.1016/B978-1-4831-9711-1.50022-3
Deshpande R et al (2018) Comparative evaluation of antimicrobial properties of two different extracts of Artemisia Pallens (Davana) and 0.2% Chlorhexidine against acidogenic salivary microflora in mixed dentition age group. Res J Pharm Biol Chem Sci 9(1):545–549
Jaiswal YS, Williams LL (2017) A glimpse of Ayurveda—the forgotten history and principles of Indian traditional medicine. J Tradit Complement Med 7(1):50–53. https://doi.org/10.1016/j.jtcme.2016.02.002
Pandey AK, Singh P (2017) The genus artemisia: a 2012–2017 literature review on chemical composition antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 4(3):68. https://doi.org/10.3390/medicines4030068
Pujar PP, Sawaikar DD, Rojatkar SR, Nagasampagi BA (2000) A new germacranolide from Artemisia pallens. Fitoterapia 71(5):590–592. https://doi.org/10.1016/S0367-326X(00)00168-4
R. Shetty (2014) Influence of integrated nutrient management on dry matter production and flowering in davana (Artemisia Pallens Wall.). 4:760–762
Obistioiu D et al (2014) Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem Cent J. https://doi.org/10.1186/1752-153X-8-6
Vengala N (2017) Antihypertensive activity of methanolic extract of Artemisia pallens wall in renal hypertensive diabetic rats. Res Rev BioSci 12(2):1–11
Swarna J et al (2015) Characterization of Talinum triangulare (Jacq.) Willd. germplasm using molecular descriptors. S Afr J Bot 97:59–68. https://doi.org/10.1016/j.sajb.2014.12.012
Mallavarapu GR, Kulkarni RN, Baskaran K, Rao L, Ramesh S (1999) Influence of plant growth stage on the essential oil content and composition in Davana (Artemisia pallens Wall.). J Agric Food Chem 47(1):254–258. https://doi.org/10.1021/jf980624c
Adams RP, Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy Mexican Cypresses View project Phylogeny and phylogeography of the genus Juniperus in Iran View project. [Online]. Available: https://www.researchgate.net/publication/283650275
Demo M, Oliva MDLM, López ML, Zunino MP, Zygadlo JA (2005) Antimicrobial activity of essential oils obtained from aromatic plants of Argentina. Pharm Biol 43(2):129–134. https://doi.org/10.1080/13880200590919438
Tolba H, Moghrani H, Benelmouffok A, Kellou D, Maachi R (2015) Essential oil of Algerian Eucalyptus citriodora: chemical composition, antifungal activity. J Mycol Med 25(4):e128–e133. https://doi.org/10.1016/j.mycmed.2015.10.009
Jha V et al (2022) Pratyusha Mane, Aishwarya Marath. exploration of probiotic potential of lactic acid bacteria isolated from different food sources. Am J BioSci 10(3):118–130. https://doi.org/10.11648/j.ajbio.20221003.14
Tsukatani T et al (2012) Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J Microbiol Methods 90(3):160–166. https://doi.org/10.1016/j.mimet.2012.05.001
Shin S, Kang C-A (2002) Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. Lett Appl Microbiol 36:111–115. https://doi.org/10.1046/j.1472-765X.2003.01271.x
Patel RV, Kumari P, Rajani DP, Chikhalia KH (2011) Synthesis and studies of novel 2-(4-cyano-3-trifluoromethylphenyl amino)-4-(quinoline-4-yloxy)-6-(piperazinyl/piperidinyl)-s-triazines as potential antimicrobial, antimycobacterial and anticancer agents. Eur J Med Chem 46(9):4354–4365. https://doi.org/10.1016/j.ejmech.2011.07.006
Jha V et al (2022) Exploration of chemical composition and unveiling the phytopharmaceutical potentials of essential oil from fossilized resin of Pinus succinefera. Int Res J Pure Appl Chem. https://doi.org/10.9734/irjpac/2022/v23i330464
Rieckmann K et al (1978) Drug sensitivity of plasmodium falciparum. An in-vitro microtechnique. The Lancet 1(8054):22–23. https://doi.org/10.1016/S0140-6736(78)90365-3
Jha V et al (2022) Streptomyces peucetius M1 and Streptomyces lavendulae M3 soil isolates as a promising source for antimicrobials discovery. J Pharm Res Int. https://doi.org/10.9734/jpri/2022/v34i50b36438
Thusoo S et al (2014) Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. Biomed Res Int. https://doi.org/10.1155/2014/614187
Jha V et al (2022) ‘GC-MS Analysis and investigation of bioactive potential of essential oil from Citrus aurantium var. amara. Int J Pharm Chem 8(3):29–39. https://doi.org/10.11648/j.ijpc.20220803.11
Devare S et al (2013) Antioxidant potential of Artemisia pallens roots. Int J PharmTech Res 5(3):1360–1363
Wang W, Li N, Luo M, Zu Y, Efferth T (2012) Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 17(3):2704–2713. https://doi.org/10.3390/molecules17032704
Haney EF, Trimble MJ, Cheng JT, Vallé Q, Hancock REW (2018) Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules. https://doi.org/10.3390/biom8020029
Cáceres M, Hidalgo W, Stashenko E, Torres R, Ortiz C (2020) Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics. https://doi.org/10.3390/antibiotics9040147
Sharma V, Singh B, Gupta RC, Dhaliwal HS, Srivastava DK (2014) In vitro antimicrobial activity and GCMS analysis of essential oil of Artemisia maritima (Linn.) from Lahaul & Spiti (Cold Desert) region of North-Indian higher altitude Himalayas. Journal of Medicinal Plants Studies Year, 2(2). [Online]. Available: www.plantsjournal.comwww.plantsjournal.com
Hui Pu Z et al (2010) Antibacterial activity of 9-octadecanoic acid-hexadecanoic acid-tetrahydrofuran-3,4-diyl ester from neem oil. Agric Sci China 9(8):1236–1240. https://doi.org/10.1016/S1671-2927(09)60212-1
Kiran GS, Priyadharsini S, Sajayan A, Ravindran A, Selvin J (2018) An antibiotic agent pyrrolo[1,2-: A] pyrazine-1,4-dione, hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv 8(32):17837–17846. https://doi.org/10.1039/c8ra00820e
Ben Bakrim W et al (2022) Phytochemical study and antioxidant activity of the most used medicinal and aromatic plants in Morocco. J Essent Oil Res 34(2):131–142. https://doi.org/10.1080/10412905.2022.2029777
Ser HL et al (2015) Presence of antioxidative agent, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- in newly isolated Streptomyces mangrovisoli sp. nov. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00854
Calvo-Martín G et al (2022) Norbornene and related structures as scaffolds in the search for new cancer treatments. Pharmaceuticals. https://doi.org/10.3390/ph15121465
Cherniienko A et al (2022) Antimicrobial and Odour Qualities of Alkylpyrazines Occurring in Chocolate and Cocoa Products. Appl Sci 12(22):11361. https://doi.org/10.3390/app122211361
Cheng XC, Liu XY, Xu WF, Guo XL, Ou Y (2007) Design, synthesis, and biological activities of novel Ligustrazine derivatives. Bioorg Med Chem 15(10):3315–3320. https://doi.org/10.1016/j.bmc.2007.03.033
Bail S et al (2008) GC-MS-analysis, antimicrobial activities and olfactory evaluation of essential davana (Artemisia pallens Wall. ex DC) oil from India. Nat Prod Commun 3(7):1057–1062. https://doi.org/10.1177/1934578X08003007
Diao WR, Hu QP, Zhang H, Xu JG (2014) Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 35(1):109–116. https://doi.org/10.1016/j.foodcont.2013.06.056
Abd-Elgawad AM, Elshamy AI, Al-Rowaily SL, El-Amier YA (2019) Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant heliotropium curassavicum. Plants. https://doi.org/10.3390/plants8110482
Aati HY et al (2020) Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia. Arab J Chem 13(11):8209–8217. https://doi.org/10.1016/j.arabjc.2020.09.055
Gonçalves MJ et al (2012) Composition and biological activity of the essential oil from Thapsia minor, a new source of geranyl acetate. Ind Crops Prod 35(1):166–171. https://doi.org/10.1016/j.indcrop.2011.06.030
Bilia AR, Santomauro F, Sacco C, Bergonzi MC, Donato R (2014) Essential oil of artemisia annua L.: an extraordinary component with numerous antimicrobial properties. Evid based Complement Altern Med. https://doi.org/10.1155/2014/159819
Nigam M et al (2019) Bioactive compounds and health benefits of Artemisia species. Nat Prod Commun. https://doi.org/10.1177/1934578X19850354
Bisht D, Kumar D, Kumar D, Dua K, Chellappan DK (2021) Phytochemistry and pharmacological activity of the genus artemisia. Arch Pharmacal Res 44(5):439–474. https://doi.org/10.1007/s12272-021-01328-4
Younessi-Hamzekhanlu M et al (2020) Evaluation of essential oil from different artemisia fragrans willd. Populations: chemical composition, antioxidant, and antibacterial activity. J Essent Oil Bear Plants 23(6):1218–1236. https://doi.org/10.1080/0972060X.2020.1854129
Rosito M, Testi C, Parisi G, Cortese B, Baiocco P, di Angelantonio S (2020) Exploring the use of dimethyl fumarate as microglia modulator for neurodegenerative diseases treatment. Antioxidants 9(8):1–22. https://doi.org/10.3390/antiox9080700
Ruikar A et al (2011) Studies on aerial parts of Artemisia pallens wall for phenol, flavonoid and evaluation of antioxidant activity. J Pharm Bioallied Sci 3(2):302–305. https://doi.org/10.4103/0975-7406.80768
Zhang G, Qi F, Yan Q, Zheng Z, Liu J, Chen Y (2018) Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. JBUON 23(2):346–352
Chen K et al (2021) Dimethyl fumarate induces metabolic crisie to suppress pancreatic carcinoma. Front Pharmacol. https://doi.org/10.3389/fphar.2021.617714
Tian S, Cai J, Zhong Y (2020) Naturally occurring davanone exhibits anticancer potential against ovarian cancer cells by inducing programmed cell death, suppression of cell migration and invasion and modulation of PI3K/AKT/MAPK signaling pathway. JBUON 25(5):2301–2307
Rashid S, Rather MA, Shah WA, Bhat BA (2013) Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem 138(1):693–700. https://doi.org/10.1016/j.foodchem.2012.10.102
Sahu N et al (2018) Extraction, fractionation and re-fractionation of Artemisia nilagirica for anticancer activity and HPLC-ESI-QTOF-MS/MS determination. J Ethnopharmacol 213:72–80. https://doi.org/10.1016/j.jep.2017.10.029
Elgamal AM, Ahmed RF, Abd-Elgawad AM, el Gendy AENG, Elshamy AI, Nassar MI (2021) Chemical profiles, anticancer, and anti-aging activities of essential oils of pluchea dioscoridis (L.) dc. and erigeron bonariensis l. Plants. https://doi.org/10.3390/plants10040667
Famuyide IM, Aro AO, Fasina FO, Eloff JN, McGaw LJ (2019) Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement Altern Med. https://doi.org/10.1186/s12906-019-2547-z
Rossi C, Chaves-López C, Serio A, Casaccia M, Maggio F, Paparella A (2020) Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: an updated review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2020.1851169
Acknowledgements
The researcher would like to acknowledge Editors as well as reviewers for their invaluable contributions throughout the administration of the submitted papers.
Author information
Authors and Affiliations
Contributions
All the authors listed have made enormous practical and cognitive contributions to the research and have provided their approval for it to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors assert that no commercial or financial links existed during the research that may be regarded as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jha, V., Kadam, P., Jain, T. et al. Investigation of physico-chemical properties and evaluation of the biological potential of essential oil extracted from Artemisia pallens. J.Umm Al-Qura Univ. Appll. Sci. 9, 494–507 (2023). https://doi.org/10.1007/s43994-023-00059-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00059-0