Abstract
White-spotted stink bugs, were noticed as a first time in a few numbers of the Egyptian barley cultivar Giza 138 plants in mid of January of 2019/2020 season at soil improvement and conservation Research department at Sakha Agricultural Research station, farm, Kafr El-Sheikh Government, Egypt. Morphological identification and DNA barcoding of mitochondrial gene cytochrome c oxidase subunit I (COI) were used identified the species of collected bugs. The results revealed a new record of Eysarcoris ventralis (Westwood 1837) (Hemiptera: Heteroptera: Pentatomidae). The results of pest insect survey of directly accounts on barley cultivar weekly during two growing winter 2019/2020 and 2019/2021 seasons displayed a significant effect of seasons on the E. ventralis population density. Positive significantly correlation was found between the E. ventralis population density and growth stages of barley under 2019/2020 and 2019/2021 seasons by (0.311 and 0.531) respectively. Regression coefficient, revealed that decrease of temperature by 1 °C increased population of E. ventralis by 0.051 and 0.036 insects per 10 tillers in 2019/2020 and 2019/2021 seasons respectively, while increase of relative humidity by 1% decreased population of E. ventralis by 0.047 and 0.31 insects per 10 tillers in 2019/2020 and 2019/2021 seasons. High population of E. ventralis was found in Anthesis stage of barley. Minor damage was found in immature grain at dough stage. Thus these results are so important to using feature integrated pest management (IPM) programs in barley to avoid the damage of Eysarcoris ventralis (Westwood 1837). Partial DNA sequence of collected sample using cytochrome c oxidase I (COI) mitochondrial gene with clear alignment of 576 bp. The tested sequence more related to Eysarcoris sp. and far from Nezara sp.). For more accuracy, BLAST was used for tested sequence with Eysarcoris sp. and found it closely related to E. ventralis. DNA sequence of COI as type effectively precludes the finding of a new evidence to resolve taxonomic problems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Barley (Hordeum vulgare L.) is a cereal crop that is grown in most countries; it is ranked as fourth in world crop production which is used for animal feed, malts and human food. It is the most important source of carbohydrates and protein for animal and humans [1]. Agricultural crop suffer numerous negative climatic conditions during their growth cycles. Such conditions are involved of biotic stress, including attack by insect pests that reduce yield and quality [2].
Hemiptera is a large and highly diverged order of hemimetabolous which were divided into five suborders: Heteroptera, Cicadomorpha, Fulgoromorpha, Sternorrhyncha and Coelorrhyncha. Out of these five groups, Heteroptera is a highly differentiated taxon and had more than 42,000 species in over 5800 genera and 140 families described worldwide [3].
White-spotted stink bug, Eysarcoris ventralis (Westwood, 1837) belongs to the subfamily Pentatominae, tribe Eysarcorini, commonly known as cereal bug [4] was reported as one of the major damaging pests attacking crops worldwide, which it caused a significant damage by sucking the plant sap, so the damaged leaves become pale yellow, dry, shriveled and fall pre-maturely [5,6,7]. The white spotted stink bug, E. ventralis, is widespread in tropical and subtropical regions of Africa, Asia, Europe and Australia. This species can hibernate as an adult in near the root of grass or under the leaves falling on the ground [8] in Slovakia. In Poland, [9] distribution 28 species from the family Pentatomidae in Northwestern Poland were presented and discussed. In India, [6] reported that E. ventralis has found in many parts of India which been recorded as a polyphagous pest of a variety of host plants including Til, Rice, chrysanthemum, some leguminous plants and some plants of family Lamiaceae. In Iran, [10, 11] reported that white-spotted stink bug found in most regions of Iran which been recorded as a polyphagous pest of a variety of host plants of weeds, grapes, alfalfa and wheat without reference to insect damage. However, [7] found that E. ventralis had caused damage symptoms rice as a first reported.
In Egypt (Sakha station, Kafr Elsheikh Governorate), whites potted stink bug, Eysarcoris inconspicuous (Westwood) was attacked wheat plants in the last of the season during earing stage on a few numbers with causing a slight damage [12]. In rice, green stink bugs (Nezara viridula L.) and white-spotted stink bug (E. ventralis) was attacked rice plants which (bugs adults and nymphs) suck saps of panicles and caused incomplete filled grains of rice [13].
Through the investigation of many specimen series in bugs collected from the different food crop plants; amazing morphological differentiation was noticed in what appeared to be one species. This diversity included both non-genitalic and internal genitalic characters, it could me interspecies matting, the stink bugs (Hemiptera, Pentatomidae) comprise a diverse family of true bugs with polygamous mating system [14]. Traditionally, identification has been completed on the basis of morphological identifies provided by taxonomical studies. But only experts such as taxonomists and trained technicians can identify taxa accurately, as it requires special skills acquired.
DNA sequences was using for taxonomic problems that are difficult to resolve on the basis of morphology alone, its DNA techniques using genomic DNA first is subjected to PCR amplification and then analyzed by sequencing, which molecular data have proven to be realistic for understanding phylogenetic relationships and identifying and defining cryptic species [15].
There were previous studies that using DNA barcoding as an identification tool among Heteroptera in general [16, 17]. DNA sequences of partial DNA sequences of cytochrome c oxidase I (COI) mitochondrial gene have been used to identify new recorded species, a process referred to as DNA barcoding [18] has used COI as a DNA barcode to identify cryptic species, thereby increasing taxonomy-based biodiversity estimates [19] has used COI for species identification by sequencing approximately 650 bp of the cytochrome oxidase subunit I (COI) gene for identify species, and argues.
Currently, morphology identification combined with most recent technology such as and DNA sequences were investigated, whether these morphologically variations is not enough to augment the morphological specimens belong to one or many different species. Thus, DNA sequences included in the investigation, since the collected morphological data were only expressive of species level. Additionally, using DNA sequence data currently is hotly debated and more empirical proof is needed before the importance of sequences for taxonomy can be judged [20,21,22,23].
For barley there no any reports for white-spotted stink bug (E. ventralis) attacked barley plants in the world or Egypt. Thus, the main aims of this study to identify white spotted stink bug, E. ventralis which attacked barley cultivar Giza 138 based on and its population density and DNA sequences of mitochondrial gene cytochrome c oxidase subunit I (COI) as a first record.
2 Materials and methods
2.1 Field experiment evaluation
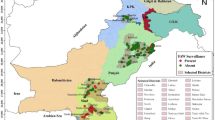
A field experiment was carried out at soil improvement and conservation Research department at Sakha Agricultural Research station, farm, Kafr El-Sheikh Gov., Egypt, located at (31° 05′ 36.28″ N, 30° 56′ 53.56″ E with an elevation 6 m above mean sea level) during two successive winter growing seasons 2019/2020 and 2020/2021 as shown in (Fig. 1). Barley cultivar Giza 138 was sown in (on 4 December and 28 November in 2019 and 2020 respectively) in half karat with three replicates (plot size: 6 m × 7 m log ═ 42 m2). The normal cultural practices for growing barley were applied as recommended according to the Ministry of Agriculture recommendation. No insecticides were used throughout the two studied seasons, to evaluated some agro-morphological traits such as heading data (HD days), maturity data (MD days), plant height (PH cm), number of tillers m−2 (NT m−2), number of grains spike−1 (NGS), 1000 grain weight (TKW) and grain yield (GY ardfad−1).
2.2 First season survey
In the first season of 2019/2020, white-spotted stink bugs, were noticed as a first time in a few numbers in mid of January, and the population density were directly counted weekly on randomly 10 tillers in the field till harvest. The highest number of adult bugs observed on 8 March 2020.
2.3 Insect identification
The identification of white-spotted stink bug has been investigated using morphological identifies has provided by taxonomical studies and DNA barcoding.
2.4 Morphological identification
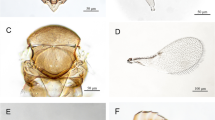
The specimens of white-spotted stink bug were collected from various parts of barley plants after appeared as a first time on 19th 2020 in order to identify the bugs. The white-spotted stink bug classified morphologically to orders Hemiptera, Family Pentatomidae, species Eysarcoris ventralis (Westwood 1837) which their common name is white-spotted stink bug based on outer morphological types defined in taxonomic keys according to [21, 22]. The morphological traits include body (size and color), legs, scutellum, whiskers, styles, wing membrane,, pronotum, anterior and posterior margins. The insect were identified based on assessments of morphological characteristics to confirmed species according to the Department of Survey and Taxonomy department, Plant Protection Research Institute (PPRI), Agriculture Research Center, Cairo, Egypt.
2.5 Molecular DNA sequence
DNA was extracted using Cetyl Trimethyl Ammonium Bromide (CTAB) protocol [23]. DNA concentration and purity were determined spectroscopically at 260 and 280 nm, respectively and DNA samples were stored at −20 °C. The primer pair LCO1490 and HCO2198 was used to amplify a 658 bp fragment of the COI gene [24]. PCR amplifications were done in a 12.5 µl volume including 6.25 µl of 10% trehalose, 2 µl of ultra-pure water, 1.25 µl of 10 × PCR buffer (10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris–HCl (pH 8.8), 2 mM MgSO4, 0.1% Triton X-100), 0.625 µl of MgCl2 (50 mM), 0.125 µl of each primer (10 µM), 0.0625 µl of 10 mM dNTP, 0.06 µl of Taq polymerase and 2 µl of extracted DNA. PCR thermocycling was performed under the following conditions: 2 min at 95 °C; 5 cycles of 40 s at 94 °C, 40 s at 45 °C, 1 min at 72 °C; 35 cycles of 40 s at 94 °C, 40 s at 51 °C, 1 min at 72 °C; 5 min at 72 °C; held at 4 °C. PCR checks and DNA sequencing were carried out using standard methods. Each PCR product was purified, and sequenced in both directions using a Big Dye v. 3 Sequencing Kit. Cycle sequencing was performed at 50 °C in 10 μl reactions with 2 μl of template, 1 μl of Big Dye, 1.75 μl of primer, 3 μl of sequencing buffer and 2.25 μl of H2O.
The sequencing reactions were cleaned with CleanSeq. Sequencing in both directions was done. Chromatograms were edited and contains were assembled using BioEdit and ClusterW. The sequence submitted to NCBI Gene Bank with accession number OP984406.
2.6 Second season survey
In season of 2020/2021, the count of white-spotted stink bug Eysarcoris ventralis (Westwood 1837) attacked 10 tiller/weekly beginning of December at seedling stage of barley growth (sowing data was 24 November 2020) to study the population distribution of E. ventralis attacking barley at different growth stages in (Sakha region, Kafr El-Sheikh Gov.), study associated of their population density with the weather factors and to determine the symptoms of white-spotted stink bug E. ventralis (Westwood 1837) damage on barley.
2.7 The weather factors
The weather factors: temperature °C, relative humidity and rain mm/day were obtained from Meteorological Station of Sakha, Egypt, to evaluate their effect on the population density of E. ventralis (Westwood 1837) on barley during the two winter growing seasons 2019/2020 and 2020/2021.
2.8 Data analysis
2.8.1 Phenotypic data analysis
Data were statistically analyzed as a randomized complete block design (RCBD) following the analysis of variance (ANOVA). Least Significant difference test was used to compare means at 0.05 and 0.01 levels. The partial correlation and regression coefficient between the average weekly prevailing weather factors and population of Eysarcoris ventralis were determine using SPSS software version 16.
2.8.2 Genotypic data analysis
The cluster analysis was performed to produce a dendrogram using neighbor-joining (NJ) trees [25]. DNA sequences for selected closely related four isolates partial DNA were checked and assembled using the ATGC program ver. 4 (GENETYX CORPORATION). Sequence adjusted manually using Bio Edit program [26] (and alignments with CLUSTAL W program [27]. To infer the exact relationship sequences were used to search the GenBank database with the Blast N algorithm to determine relative phylogenetic positions. The alignments of the sequences were done against corresponding nucleotide sequences retrieved from GenBank.
3 Results
3.1 Agro-morphological traits analysis
The analysis of variance of the agro-morphological measured characters of the Egyptian barley cultivar Giza 138 barley cultivar had not significantly varied with seasons. The mean performance of the combined data of agro-morphological traits across the two growing seasons showed that the Egyptian barley Giza 138, was early heading (81.75 days), early maturity (127.95 days), plant height (109.45 cm), had high number of grain spike (72 grain), have high number of tillers (435.4 tiller m2 (455 m2), high thousand kernel weight (49.9 g), high grain yield (18.8 ardfad−1).
3.2 The population density of bug insect
The first appeared of white-spotted stink bug, was on Egyptian barley cultivar Giza 138 at 19 January during season 2019/2020 in (Sakha location, Kafr El-Sheikh governorate, Egypt) as shown Fig. 2A–C, the specimens of stink bug were collected from various parts of barley plants in order to identified and classified it.
3.3 Insect identification
3.3.1 Morphological identification (taxonomic status)
The insect bugs which were collected in 19 January 2020 from barley cultivar Giza 138 at (Sakha area, Kafr El-Sheikh governorate, Egypt) were classified morphologically to orders Hemiptera, Family Pentatomidae, species Eysarcoris ventralis (Westwood 1837) which their common name is white-spotted stink bug. The insect were identified based on assessments of morphological characteristics to confirmed species according to the Department of Survey and Taxonomy department, Plant Protection Research Institute (PPRI), Agriculture Research Center, Cairo, Egypt.
3.3.2 DNA barcoding analysis
Morphological variability was largely continuous among Eysarcoris sp. first step is collect bugs, followed by sorting, and identification of the unruffled bugs. The collection was used to sequence a partial DNA sequences of cytochrome c oxidase I (COI) mitochondrial gene with clear alignment of 576 bp. It was selected due to higher nucleotide substitution rate in mitochondrial DNA results in the rapid accumulation of differences between species; and lack of introns.
For comparison use tested sequence from collected bugs with alignments of Nezara viridula, E: Eysarcoris sp. with diverged species Eysarcoris rosaceus and Stagonomus bipunctatus to identify with species are closely related to our tested sequence, it noticed that tested sequence more related to Eysarcoris sp and far from Nezara viridula and highly diverged from other tested species Eysarcoris rosaceus and Stagonomus bipunctatus Eysarcoris rosaceus and Stagonomus bipunctatus with they have similar DNA sequence from Gene Bank database as showed in Fig. 3. For more accuracy, to which Eysarcoris sp. exactly we run BLAST for tested sequence and as shown in (Fig. 4), the sequence barcodes as Eysarcoris ventralis based on NJ Phylogenetic tree. To barcoding by molecular analysis to other biological knowledge, we requisite reference sequences elucidated by Linnaean taxonomy. In this study, we report the creation of a broad DNA barcodes for the new appear white-spotted stink bug and deliver a new proof of identity tool for insects and bugs. The reference contains mtDNA COI barcodes for partial DNA sequences of cytochrome c oxidase I (COI) mitochondrial gene with clear alignment of 576 bp.
3.4 The population density survey of Eysarcoris ventralis
3.4.1 First season survey
In seasons 2019/2020 at 19 January it was the first appeared of white-spotted stink bug E. ventralis, on Egyptian barley cultivar Giza 138 at (Sakha location, Kafr El-Sheikh governorate, Egypt) with a few numbers was 1.00 insects/10 tillers after 46 days from sowing at Tillering stage of barley with low average weakly temperature (16.6 °C), high relative humidity (67.8) with low rain (1.1 mm/day).The population density were increased gradually on 8 March which the highest numbers of insect 16.00 insects/10 tillers were performed at Anthesies stage with worm average weakly medium temperature (19.6 °C) and medium relative humidity (67.8) with no rain (1.1 mm/day) under filed condition as shown in Table 1.
3.4.2 Second season’s survey
During season 2020/2021, the data in Table 2, showing that the population density of white-spotted stink bug E. ventralis began to appear on 14 December (1 insect/10 tillers) after 21 days from sowing data (24 November) at Tillering stage of barley growth stages with worm average weakly temperature (19.6 °C) and medium relative humidity (69.6) with rain (2.1 mm/day) under filed condition.The infestation of the insect were increased on 8 March which recording highest number of insect (16 insect/10 tillers) at Anthesis stage with average weakly temperature was (16.46 °C) and relative humidity (71.6) with low rain (0.7 mm/day). The adults and eggs of the white-spotted stink bug E. ventralis found on the leave of barley plant at the end of tillering stage as shown in (Fig. 5A, B).
The mean performances of population density of white-spotted stink bug, E. ventralis was high density in season 2020/2021 more than season 2019/2020 was (89 and 59) with average 74 population densities respectively, in general, t-test analysis indicated that the seasonal mean of the insect was higher in second season than first season survey s it was 4.94 ± 0.30 and 4.21 ± 0.17 insects, respectively as shown in Fig. 6.
3.5 Relationships among Eysarcoris ventralis, growth stage and weather factors
To understand the relationships among E. ventralis, growth stage and weather factors correlation, regression coefficient and the principal component analysis (PCA) analysis was applied.
3.6 Correlation and regression coefficient for Eysarcoris ventralis (Westwood, 1837)
The results in Table 3 cleared that the population of E. ventralis was medium positive significantly correlated with barley growth stage under both seasons 2019/2020 and 2020/2021 were (0.311 and 0.531) respectively. However, the population density of E. ventralis a positive with non-significant correlated with temperature and relative humidity in the season of 2019/2020, while had a negative non-significant correlated with temperature and relative humidity in the season 2020/2021. About relative Rain mm/day in both seasons affected insignificantly and negatively population of E. ventralis were found.
3.7 Principal component analysis (PCA)
The loadings of PCA presented in the horizontal axis indicated the direction of association among and weather factors were shown in Fig. 7. The first and two principal components accounted for 72.04% (PCA1 = 50.52% + PCA2 = 21.97%) of the total variability. So, it is noted that growth stage and temperature °C located in the same quarter in the right side (positive) of the horizontal axis according to their positive correlations with E. ventralis that (significant positive correlated with growth stage and non-significant with temperature °C recording (0.207*, 0.236*, 0.633 and 0.728) during seasons 2029/2020 and 2020/2021 respectively.
3.8 The effect of white-spotted stink bug Eysarcoris ventralis on barley plant
Under filed condition two-year survey of E. ventralis population density in fields of Sakah region revealed that adults of E. ventralis were monitored from the middle of January until first April before barley harvested and not appear after harvest as shown in figure.
The population of adult’s E. ventralis was found in different stages of barley growth Tillering, heading stage as shown in Fig. 6A, B. High population was found in Anthesis stage which the adult found on barley spikes in late March and first week of April, which they were feeding on spike (early maturing stage including milky and dough stages) which caused minor damage on the immature grain at dough stage, as shown in Fig. 8. Thus, this is also the first report of the minor damage symptoms caused by E. ventralis on barley in Egypt and the world.
4 Discussion
The Egyptian barley cultivar six rowed; Giza 138 have a good phenology which considers early heading, taller height, precocious, high yield ability high productive in newly reclaimed as found by [28], moderated tolerance to salinity as reported by [29] and moderate resistance to fungi diseases as found by [30]. Also from this study we found that Giza 138 was attacked by white-spotted stink during its tillering and Anthesis stages as susceptible cultivar agents the pest insect E. ventralis.
A two-year survey, 2019/2020 and 2020/2021, the adults of E. ventralis were monitored on barley at Sakha regions, Kafr El-Sheikh Gov., Egypt as a the first report of the attractive symptoms in January 2020 (tillering stage) till end of April 2020 (maturity stage) and 2021 harvested which was identified by DNA barcoding and morphological identification. There no reported for monitored E. ventralis on barley over the world or in Egypt.
In contrast, Although E. ventralis was previously reported at different country on different crops, in Iran [10, 11] reported that E. ventralis found in most regions of Iran which have been recorded as a polyphagous pest of a variety of host plants of weeds, grapes, alfalfa and wheat without reference to insect damage, also [7] found that E. ventralis had caused damage symptoms rice plants as a first reported. In India [6], reported that E. ventralis has found in many parts of India attacking Till, Rice, chrysanthemum, some leguminous plants. In Egypt, other species of whites potted stink bug, Eysarcoris inconspicuous (Westwood) was attacked wheat plants as reported by [12, 13] found white-spotted stink bug E. ventralis attacked rice plants.
The results showed that weather factor and growth stages of barley had a positive effect on appearance of E. ventralis on barley at winter seasons, the results showed that decreasing temperature by 1 °C increased population of E. ventralis by 0.051 and 0.036 insects per 10 tillers in season of 2019/2020 and 2020/2021, respectively, while increasing relative humidity by 1% decreased population of E. ventralis by 0.047 and 0.31 insects per 10 tillers during the two growing seasons, respectively. These results were in good harmony with agreed with [31,32,33] they suggested that climate change (temperature) had a significant effect on appearance of stink bugs on rice plants. This implies that there are other factors influencing the population that have not been considered. This could be due to the differences in current weather condition or natural enemies.
Minor damage of E. ventralis was observed on the spike of barley at early maturing stage including (milky and dough stages), which probably the adults they were feeding on spike which caused minor damage on the immature grain at dough stage. These results were in good harmony with [7] who found that E. ventralis had caused minor damage on rice in Iran and with [12] who found that E.inconspicuous caused minor damage on wheat plants in Egypt.
DNA barcoding showed that he COI interspecific distances among bug species exceeded the intraspecific distances except for the three species in the genus Apolygus. To conservatively test the effectiveness of COI barcodes as identification tools, the levels of intraspecific variation in collected bug were evaluated.
Even though these species were formally identified by key morphological characters Therefore, these three species may represent new synonymy or a very recent divergence, and/or the above morphological key characters might be not enough to discriminate it clearly. To confirm the taxonomies of those collected bug, more sampling of specimens for molecular and morphological analyses is required for future study. To authorize the efficiency of the COI barcodes, we compared the minimum interspecific distance of congeners with the maximum intraspecific divergence in comparison with other bug in gene bank. We focused on species in a relatively large group of bugs.
Most heteropteran species play important ecosystem roles as prey or predators and are also important agriculturally as pests or biological control agents [34]. The patterns of haplotype divergence at the mitochondrial gene cytochrome oxidase I (COI) found in this study, Cytochrome c oxidase subunit I gene (COI) sequences of 576 bp length for various species were generated.
DNA sequence (e.g. DNA taxonomy, DNA barcoding) of COI could be resolve taxonomic problems. Presented result highlights the problems of exclusively relying on DNA sequences for creating DNA-based species level taxonomy. Overall, we believe that this study demonstrates that DNA sequences can indeed be a valuable tool in taxonomy when they are used to obtain additional data to resolve species limits that are difficult to determine solely on the basis of morphology [16]. Moreover, DNA sequence could be supporters of DNA taxonomy [19]. Contrary, and in few studies some species identifications of can easily identified and do not require DNA barcodes as proposed by [20].
Instead, we recommend the use of diagnostic morphological characters with molecular identification to provide species description. Recently [35], presented COI sequences Heteroptera, and concluded that these barcodes can contribute to species identification. COI barcoding is now usually used for identification of new record and molecular studies [36].
4.1 Consolation
This study represents the first published record of E. ventralis on barley at Sakha regions, Kafr El-Sheikh Gov., Egypt. The results showed that there were positive effects of the different growth stages and the weather factors on appearance and increasing the population density of adults of E. ventralis. Minor damage of E. ventralis was observed on the spikes of barley at the end of maturity stage. DNA barcoding using COI is a useful tool for species taxonomy and identification. It is clear that taxonomic approaches integrating DNA sequencing and morphological studies could be achieved maximum efficiency at species identification or new record.
4.2 Future outwork
The obtained results are very important in integrated barley management programs to avoid damage of this insect in the future by using plant breeding programs through first, know why this insect had attack barley plant through studying their chemical composition and study the hypotheses expiation of insect feeding, second defined the resistance and susceptible genotypes through using molecular markers to use them breeding programs along with management programs IPM to control and avoid damage of this insect.
References
FAO STAT (2019) Crops/regions/world list/production quantity for barley
Fedoroff NV, Battisti DS, Beachy RN, Cooper PJ, Fischhoff DA, Hodges CN, Knauf VC, Lobell D, Mazur BJ, Molden D (2010) Radically rethinking agriculture for the 21st century. Science 327:833–834
Park DS, Foottit R, Maw E, Hebert PD (2011) Barcoding bugs: DNA based identification of the true bugs (Insecta: Hemiptera: Heteroptera). PLoS One 6:1–9
Ghahari H, Moulet P, Rider DA (2014) An annotated catalog of the Iranian Pentatomoidea (Hemiptera: Heteroptera: Pentatomomorpha). Zootaxa 3837:1–95
Arias A, Jimenez J, Rodriguez JA, Casado JM, Garcia C, Lancharro AJ, Vazquez J (1998) The pecky rice stink bug, Eysarcoris ventralis West, in Extremadura (Spain): the rice colonization and protection strategies. Boletín Sanidad Veg Plagas 24:79–100
Sonia S, Tara JS (2017) Record of some hemipteran pests of cucurbits from Jammu region of Jammu and Kashmir state. Int J Recent Sci Res 8:18419–18422
Jalaeian M, Zamani S, Farahpour-Haghani A (2019) First report of damage caused by white-spotted stink bug, Eysarcoris ventralis (Westwood) (Hem.: Pentatomidae) on rice in Iran. J Crop Prot 8:4
Hemala V, Cunev J, Franc V (2014) On the occurrence of the stink bug Eysarcoris ventralis (Hemiptera: Heteroptera: Pentatomidae) in Slovakia, with notes on its distribution in neighbouring countries. Klapalekiana 50:51–59
Bunalski M (2020) True bugs (Hemiptera: Heteroptera) of North-Western Poland Part 6. Pentatomoidea. Entomol News (Poland) 39:20–30
Linnavuori RE (2008) Studies on the Acanthosomatidae, Scutelleridae and Pentatomidae (Heteroptera) of Gilan and the adjacent provinces in northern Iran. Acta Entomol Musei Nation Pragae 48:1–21
Linnavuori RE (2012) Studies on Pyrrhocoroidea, Coreoidea and Pentatomoidea of Khuzestan and the adjacent provinces in Iran (Hemiptera: Heteroptera). Acta Entomol Musei Nation Pragae 52(1):67–88
Awadalla SS, Ghanim AA, Abd Allah FE, Abdel-Aziz AA (2018) The main insect pests attacking wheat plants and their associated predators in Sakha District, Kafr Elsheikh Governorate. J Plant Prot Path Mansoura Univ 9:97–101
Hegazy Fatma El-Zahraa H, Hendawy EAS, Mesbah II, Salem FA (2021) The insect pests, the associated predatory insects and prevailing spiders in rice fields. J Plant Protect Pathol Mansoura Univ 12:365–371
Wang Q, Millar JG (1997) Reproductive behavior of Thyanta pallidovirens (Heteroptera: Pentatomidae). Ann Entomol Soc Am 90:380–388
Roe AD, Sperling FAH (2007) Patterns of evolution of mitochondrial cytochrome c oxidase I and II DNA and implications for DNA barcoding. Mol Phylogen Evol 44:325–345
Memon N, Meier R, Manan A, Su KFY (2006) On the use of DNA sequences for determining the species limits of a polymorphic new species in the stink bug genus Halys (Heteroptera: Pentatomidae) from Pakistan. Syst Entomol 31:703–710
Damgaard J (2008) MtDNA diversity and species phylogeny of western Palaearctic members of the Gerris lacustris group (Hemiptera-Heteroptera: Gerridae) with implications for ‘“DNA barcoding”’ of water striders. Insect Systemat Evol 39:107–120
Ward RD, Zemlak TS, Innes BH et al (2005) Barcoding Australia’s fish species. Philos Trans Roy Soc Lond B Biol Sci 360:1847–1857
Tautz D, Arctander P, Minelli A, Thomas RH, Vogler AP (2003) A plea for DNA taxonomy. Trends Ecol Evol 18:70–74
Hebert PDN, Barrett RDH (2005) Reply to the comment by L. Prendini on ‘Identifying spiders through DNA barcodes.’ Can J Zool 83:481–549
Grazia J, Schwertner CF (2008) Review of Parachinavia Roche (Hemiptera, Pentatomidae, Pentatominae). Advances in Heteroptera research. Festschrift in honour of 80th anniversary of Michail Josifov. Pensoft Publishers, Sofia, Bulgaria, pp 159–169
Ferrari A, Schwertner CF, Grazia J (2010) Review, cladistic analysis and biogeography of Nezara Amyot & Serville (Hemiptera: Pentatomidae). Zootaxa 2424:1–41
Doyle JJ, Doyle JL (1990) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Focus 12:13–15
Folmer O, Black M, Hoeh W et al (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from divrse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406
Hall TA (1999) Bio Edit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp 41:95
Thomson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequences alignment aided by quality analysis tools. Nucleic Acids Res 25:4876
Amer KA, Abou El Enein RA, El-Sayed AA, Noaman MM, Ahmed IA, Moselhy EMA, Moustafa KA, Abd El-Hamid M, Megahed MA, El-Bawab AMO, Ashmawy HA, Eid AA, Saad MF, Abbas SI, Badawy AA, El-Nady HA, Ahmed KR, Ali HG, Mansour M, El-Shawy EE, Mariey S, Abdel-Azeem A, El-Wakeel S, Agwa AME, El-Nagar AA, El-Bosely MA, Attya AM, El-Akhdar AA, Ahmed AH, Abdel-Wahab E, Selim A, Khedr R, Mostafa N, El-Rawy AM, Mohamed A (2017) Giza 137 and Giza138, new Egyptian six-rowed barley cultivars for new land. Egypt J Plant Breed 21:380–395
Samah MA, Khaffagy AE, Aiad MA, Khatab IA, Ghareeb ZE (2022) The influence of the salinity and weed control treatments on some barley cultivars and its associated weeds. J Glob Agric Ecol 13:26–50
Mariey SA, Hamza AA, Mahmued EN, Khatab IA (2022) Molecular evaluation and phenol application effects on barley infestation by Rhyzopertha dominica (F.). J Glob Agric Ecol 13:36–51
Kiritani K (2007) The impact of global warming and land-use change on the pest status of rice and fruit bugs (Heteroptera) in Japan. Glob Change Biol 13:1586–1595
Musolin DL (2007) Insects in a warmer world: ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Glob Change Biol 13:1565–1585
Dastorani MT, Poormohammadi S (2016) Mapping of climatic parameters under climate change impacts in Iran. Hydrol Sci J 61:2552–2566
Schuh RT, Slater JA (1995) True bugs of the world (Hemiptera: Heteroptera) classification and natural history. Cornell University Press, Ithaca
Jung S, Duwal RK, Lee S (2011) COI barcoding of true bugs (Insecta, Heteroptera). Mol Ecol Resour 11:266–270
Purty R, Chatterjee S (2016) DNA barcoding: an effective technique in molecular taxonomy. Austin J Biotechnol Bioeng 3:2378–3036
Acknowledgements
The authors extend their thanks to Barley Res., Dept, Field Crops Res. Inst., Plant Protection Research Institute, Soils, Water and Environment Research Institute Agriculture Research Center (ARC), Giza, Egypt and Genetics Dept., Faculty of Agriculture, Kafr El-Sheikh University, Egypt.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [SAM], [IAK], [MAA] and [MRKM]. The first draft of the manuscript was written by [SAM] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mariey, S.A., Khatab, I.A., Aiad, M.A. et al. Barcoding of white-spotted stink bug, Eysarcoris ventralis (Westwood) (Hemiptera: Pentatomidae) attacking Egyptian barley and its population density. J.Umm Al-Qura Univ. Appll. Sci. 9, 230–241 (2023). https://doi.org/10.1007/s43994-022-00021-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-022-00021-6