Abstract
Thermal water in northeastern Algeria (Setif) is a promising, sustainable resource of energy. To date, thermal water has not been used as a source of renewable energy in Algeria as no clear strategy has yet been developed for its use. In this study, eight samples of thermal water springs distributed within the area of Setif city were assessed, and their physical and chemical parameters (such as temperature, potential of hydrogen, electrical conductivity, and major ionic composition, including the K+, Na+, Ca2+, Mg2+, Cl−, SO42− and HCO3−) were measured. The results show that the temperature, potential of hydrogen and the electrical conductivity range between 30 and 52 °C (as measured in field), 6.92 and 7.35, and 1170 and 3160 µS/cm, respectively. Furthermore, the results indicate the existence of four hydrogeochemical facies dominating the hydrogeological system, which are SO4–Ca, Na–SO4, HCO3–Na and Cl–Na. The interaction with Jurassic limestone and the existence of evaporates along the upwelling system, respectively, control this. Due to the high variation of temperature, different geothermometers were used to estimate the geothermal reservoir temperature, the application of silica geothermometer (Quartz), because it is the most suitable in our case, gives temperatures estimated at the origin of the order of 80° C. It is higher than that measured at emergence, it reflects an average dissipation of 48° C. According to an analysis of these findings and the geothermal gradient in the area, two aquifer systems of the thermal complex are supplied by a reservoir that is stored in fissured Jurassic limestone that is more than 2600 m deep.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Geothermal energy has received a lot of attention recently, and the most recent focus is on the intensive use of hot springs. It is a significant source of renewable energy in the world. In Algeria, there are more than 240 hot springs. According to certain geological and thermal considerations, three geothermal areas were identified in Algeria: the Albian sandstone reservoir in the Algerian Sahara, the Carbonate formations in the northeastern part of the country, and the dolomites in the Tlemcen province in the country's northwest [16, 17].
The objectives of this study are as follows: to get greater knowledge of the chemistry of water and how it interacts with host rock; to determine the reservoir's temperature (in depth) using various geothermometers; and, ultimately, to identify the source of thermal waters [1].
The discharge area is located in an area where a sophisticated fault network is present. A variety of sources produce water at rates ranging from 1 to 55 l/s, with temperatures between 30 and 52 °C, an average electrical conductivity of 1170–3160 uS/cm, and a mineralization of around 2022.6 mg/L.
2 Geological, geophysical and hydrogeological settings
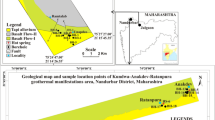
With a total area of 6550 km2, Setif city represents one of Algeria's most significant geothermal zones. It is a part of North Africa's Maghreb-Alpine chain and is situated northeast of Algiers. It is a part of a peri-Mediterranean Alpine region of Tertiary age. It belongs to Tellian series that are part of the external zones, which is the basement of the northern Algeria structures, (Fig. 1). Regionally, between Tellian series in the north and Hodna series in the south rough sea facies are interposed very shallow, which defines the Setif organization formed by a row of small carbonate reliefs and sandstone [18]. The intensity of tectonic accidents affecting the area, permitted the installation of a series of closed depressions draining the majority of surface water and groundwater in the region.
On the lithostratigraphique plan (Fig. 2), the formations encountered are shown in the basal part of dolomitic limestone and sandstone of the Lower Lias, and by carbonate and sandstone formations, with thick marl layers of the Eocene in its uppermost part sometimes. However, the center is filled by lacustrine limestones, and overlain conglomerates which are overcome by red clay that is sometimes gypsums, with silt of the Mio-Pliocene–Quaternary age [19]. The area also has several Triassic outcrops, formed by a variety of sediments (gypsum pile crushed to small calcareous-dolomitic cubes, greenish sandstone, and greenstone with magmatic nature of extrusions belonging to the Paleozoic basement. The whole packed in colored clays [18, 19].
Based on geophysical interpretation (seismic profiles) allows us to confirm the main points of Hydrogeological and structural hypothesis as they are mentioned below: (1) Two types of aquifers. The first is superficial with interstitial porosity in the formations of Mio-Pliocene–Quaternary. The second, in carbonate karstified formations and sandstone in permeability fractures which constitute a huge reserve [3,4,5]. (2) The existence of principal fault, which affecting the area from the NW to SE and producing tow hydrothermal system, one on the West and the other one on the East. (3) Set of rosettes, according to lineament detailed studies in order to demonstrate the primary direction of faults.
3 Materials and methods
3.1 Sampling and analytical methods
The use of water for geothermal energy implies that we can determine its temperature in the thermal field in great detail, based on the chemical composition of water arriving at emergence, height samples of hot springs water were collected during January 2013, from different locations in the studied area. Measurements of temperature, electrical conductivity, total dissolved solids (TDS) and pH were made in the field meanwhile. The cations and the anions were analyzed at the laboratory of the university. The ions chlorides and sulphates were proportioned by chromatography in liquid phase, the ions bicarbonates, calcium and magnesium by volumetry, sodium and potassium by photometry of flame. The ionic balance error of all samples was determined to be within the acceptable limit of ± 5%. The hydrochemical calculations were performed using diagrams program [8, 9].
To address this issue, numerous solutions have been put up [13, 14] they were called geothermometric methods. Several geothermometers are applied, including traditional chemical geothermometers, based on equilibrium relationships between elements in solutions and minerals from the host rock [8, 9]. The methods used are as follows:
Chemical concentrations as reported in Table 1 are expressed in mg / l and β = 4/3, T is expressed in ° C (
3.1.1 Silica geothermometers
3.1.2 Geothermometers Na/K
3.1.3 Geothermometer Na–K–Ca
4 Results and discussion
4.1 Structural analysis and fracturing
Several thermal studies showed the close relationship between faults and Thermalism. Almost, all hot reassembled springs are facilitated by one or more significant fracture often overlapping and affecting, more or less favorable to the reliefs, sources installation [15]. In order to define the most prevailing direction of fractures, much research has been done, the first one on the laboratory, based on the review of the geological map, the second one, concentrates on the measurements of faults parameters (observations made at the rock surface) such us: vertical extent, the spacing or density and the orientation of individual fractures. As a result of these steps, the global rose diagram (Fig. 3) allows us to conclude the existence of two fault families types, however, the most important one is NW–SE [8, 9].
4.2 Hydrochemistry of the thermal water
4.2.1 Geothermometry
Various techniques, including chemical procedures, are employed by numerous authors to estimate the geothermal reservoir temperature. These techniques are predicated on how water and minerals interact. According to [10], the reaction between water and minerals approaches equilibrium slowly at low temperatures [12], and considerably more quickly at high temperatures (reservoirs). Different geothermometers have been designed to determine the reservoir temperature for the research region due to the intricacy of the water–mineral reaction. As a result, estimates of the reservoir temperature were made by several writers. Eleven different geothermometers were utilized in our investigation to determine the reservoir temperature.
4.2.2 Silica geothermometers
The use of silica geothermometers on all of the hot springs will lead us to emphasize that the water of the sources are in equilibrium with quartz and to a lesser extent with chalcedony, but not with cristobalite (T4 and T5) or amorphous silica (T6). We can also see that for all of the research area’s sites, the temperatures predicted by quartz geothermometers T1 and T2 do not surpass 100 °C (Table 2). Furthermore, the Boutara and Ouled Yelles sources (Boutara & Ouled Yelles) could signify either a warmer region of the reservoir that is in contact with the basement and is isolated from the aquifers traversed in the research area (NW–SE fault). This idea demonstrates the viability of a fault running from northwest to southeast that would transport water into the basement and raise its temperature [13, 14].
4.2.3 Geothermometers Na/K
By using geothermometers Na/K, we have been able to determine that these thermometers provide temperatures that are greater than those calculated by geothermometers silica (Table 3), they consistently exceed 100 °C and only seldom do so for the geothermometers' calculated temperatures (T 9).
4.2.4 Geothermometers Na–K–Ca
Temperatures calculated using the silica geothermometers were clearly lower than those obtained using the Na–K–Ca geothermometer (Table 4). There may be unusually high temperatures if there are considerable levels of free CO2 when water is extracted from calcium carbonate. However, one may also acknowledge their amount of sodium intake from chlorinated water in relation to the gypsiferous complex equation, which would reduce the computed values of log (Na/K) + log (Ca/Na), which is what caused the excessive values [2, 4].
4.3 Diagram of the International Institute of Research Geothermal
The basis for this method’s operation is the sum of the (+) cations and (−) anions expressed in meq/l and the ratios of the concentrations of the major elements. As a result, the following six parameters (A, B, C, D, E and F), without unit, are defined and normalized between − 100 and + 100 as follows:
Using these diagrams, we were able to come to the conclusion that the graphic representation of reference patterns created by Amore et al. 1983 is much closer spectrum (γ) (Fig. 4), indicating that the majority of sources are of deep origin (contact with socl), with a net change in the parameters D, F and B, E. However, the surface carbonate formations and terrigenous salt formations were affected by the faults it reached along its circuit. These parameters’ (D and F) variation is connected to a flow through the Jurassic calcareous-dolomitic reservoir. B and E fluctuating values suggest a movement across terrigenous salt deposits that are rich in evaporates [5].
4.4 Map establishing
The geometrical component of a reservoir determines how the physical parameters (temperature and depth) evolve while interpreting its iso-values. To do this, the architecture must be determined using maps of the depth and geothermal gradient silica (Quartz). In order to transplant a new Hammam in the future, it is important to study the form, gradient, and depth of the most perspective places, which are depicted on these maps.
4.4.1 Iso-gradient geothermal map
This map (Fig. 5) demonstrates how crucial the gradient values are to the west, center, and southern regions. Although they diminish as you move east and north, the highest values are found close to the sources H, Ouled Yelles (89.89) and H, Ouled Boutara (85.88). The lowest value is noted close to source H, Dehamcha (73.60).
4.4.2 Iso-depth map
This map (Fig. 6) demonstrates how important depth values are in the region's western, central, and southern regions. However, they drop down when traveling east and north; the highest readings are found close to the sources H, Ouled Yelles (3150 m) and H, Ouled Boutara (3010 m). The lowest value is noted close to source H, Dehamcha (2590 m).
4.5 Structural flow model of the thermal water springs into the surface
As a result of the deep penetration of meteoric waters and subsequent heating by a heat flow circuit (Fig. 7), i.e. in contact with the basement, our results provide the first description of the structural flow model of hot water from the basement into the surface [13, 14]. The faults allowed the hot water to rise to the surface where they combined with the cold water to form thermal water springs in various locations [20]. Further, the flow circuit shows that the decrease in source temperatures and depth values are interchangeably related, supporting the following two main hypotheses: (1) the existence of two deep reservoirs, one in the West (Jurassic limestone) and the other in the Eastern Part (terrigenous salt formation), and (2) the middle (Hot Spring of Boutara) as the second point of contact between the Basement and water [12, 19]. The first point of interaction, however, is located in the western portion of the study region. In this instance, the interaction between water and rock and the length of the flow channels between the water supply area and the emerging zones determine the waters' chemical composition [6, 15].
5 Conclusion
The investigation into the use of geothermometers has been quite successful, according to the findings. A rigorous hydrochemical examination of geothermal interest was conducted using spring water major element analysis.
There are four groundwater facies known: SO4–Ca, SO4–Na, HCO3–Na and Cl–Na. Meaning that the meteoric water infiltrated, heated, and acquired their initial carbonate mineralization before ascending and heating for a second time through the fault NW–SE [7], in contact with a terrigenous salt formation, to obtain a secondary facies enriched in chloride.
Through a complex series of deep faults, groundwater is liberated. The spring's temperature ranges from 30 to 52 degrees Celsius. The silica geothermometers used in our situation indicate origin temperatures estimated to be about 80 °C, which is indicative of a low enthalpy geothermal system. It exceeds the value recorded at emergence, indicating an average dissipation of 48 °C. A combination with cold water, thermal diffusion connected to the considerable distance traveled, and other factors could all contribute to this dissipation. The fissured Jurassic limestone is identified as the thermal reservoir where the waters have been maintained and have circulated at depths of 2600 m.
Data availability
This manuscript has no associated data.
References
Benamara A, Kherici-Bousnoubra H, Bouabdallah F (2017) Thermomineral waters of Hammam Meskoutine (north-east Algeria): composition and origin of mineralization. J Water Land Dev 34:47–57. https://doi.org/10.1515/jwld-2017-0037
Boudoukha A et al (2015) Etude Du potentiel hydrothermal De Hammam Sokhna, Est Algérien. Larhyss J 24:161–174
Boudoukha A, Athamena M (2012) Caracterisation des eaux thermales de l’ensemble Sud setifien. Est alge´rien. Rev Sci Eau 25:103. https://doi.org/10.7202/1011602ar
Boudoukha A, Messaid B (2014) Caractérisation électrique des formations aquifères de L’Est Algérien. Courrier du Savoir, No 18:77–82
Belhai M, Fujimitsu Y, Nishijima J, Bersi M (2017) Hydrochemistry and gas geochemistry of the northeastern Algerian geothermal waters. Arab J Geosci 10:743. https://doi.org/10.1007/s12517-016-2790-2
Bouchareb-Haouchine FZ et al (2012) Hydrogéochimie et géothermométrie: apports à l’identification du réservoir thermal des sources de Hammam Righa, Algérie. Hydrol Sci J 57(6):1184–1205
Chulli B et al (2011) Une approche multidisciplinaire pour la prospection des bassins hydrogéothermiques : cas du Sahel de Sfax (Tunisie orientale). Hydrol Sci J 56:507–520
Djemmal S (2018) Étude des sources thermominérales de la région de Sétif dans leur contexte structural (Est Algérien), Thèse Doctorat d'état en science, Département de Géologie, Université El Hadj Lakhdar, Batna 2, Fesdis, Algérie
Djemmal S, Menani MR, Chamekh K, Baali F (2017) The contribution of fracturations in the emergence of the thermal springs in Setif city, Eastern Algeria. Carbonates Evaporites 12:141. https://doi.org/10.1007/s13146-017-0375-0
Dib-Adjoul H (1985) Le thermalisme de l’Est algérien. Thèse de Doctorat, Univ. Alger, Algérie, p 281
Dib H (2008) Guide pratique des sources thermales de l’Est algérien. Mémoire du Service Géologique national, vol 1, Editions du Service Géologique national, Alger
Han DM, Liang X, Jin MG, Currell MJ, Song XF, Liu CM (2010) Evaluation of groundwater hydrochemical characteristics and mixing behavior in the Daying and Qicun geothermal systems, Xinzhou Basin. J Volcanol Geotherm Res 189:92–104. https://doi.org/10.1016/j.jvolgeores.2009.10.011
Issaadi A (1992) Le thermalisme dans son cadre géostructurale, apports à la connaissance de la structure profonde de l’Algérie et de ses ressources géothermales. Thèse de Doctorat, Univ. Alger, Algérie, p 274
Issaadi A (1996) Mécanismes de fonctionnement des systèmes hydrothermaux. Application aux eaux thermominérales algériennes et aux eaux de Hammam Bou-Hadjar. Bull Ser Géol Algér 7–71:85
Rachid N (1987) Etude hydrogéologique et hydrochimique des eaux thermales du centre algérien (Nord). Geochemistry. Université Scientique et Médicale de Grenoble, Grenoble, p 154
Saibi H (2009) Geothermal resources in Algeria. Renew Sustain Energy Rev 13:2544–2552. https://doi.org/10.1016/j.rser.2009.06.020
Saibi H (2015) Geothermal resources in Algeria. Proceedings World Geothermal Congress, Melbourne, Australia, pp 20–25
Verdeil P (1982) Algerian thermalism in its geostructural setting—how hydrogeology has helped in the elucidation of Algeria’s deep-seated structure. J Hydrol 56:107–117. https://doi.org/10.1016/0022-1694(82)90060-9
Vila JM (1980) La chaine alpine d’Algérie orientale et les confins algéro-tunisiens. Thèse de Doctorat, Univ. Pierre et Marie Curie, Paris VI, France, pp 665
Yazdi M, Taheri M, Navi P (2015) Environmental geochemistry and sources of natural arsenic in the Kharaqan hot springs, Qazvin. Iran Environ Earth Sci 73:5395–5404. https://doi.org/10.1007/s12665-014-3794-4
Author information
Authors and Affiliations
Contributions
Mr Djemmal Samir is in charge of the article’s writing and geothermal research, with Khemissi Chamekh undertaking the structural analysis.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samir, D., Khemissi, C. Application of geothermometric and hydrochemical methods to the investigation of thermal water of sources in the Northeastern of Algeria case of Setif city. J.Umm Al-Qura Univ. Appll. Sci. 8, 69–78 (2022). https://doi.org/10.1007/s43994-022-00005-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-022-00005-6