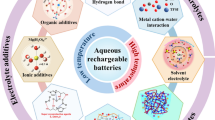
Abstract
Sodium-ion batteries are considered one of the perspective alternatives to lithium-ion batteries due to their affordability and plentiful supply of sodium. However, traditional sodium-ion batteries that use organic electrolytes pose a threat to public safety and the ecological environment. As a result, aqueous electrolytes with high safety and cost-effectiveness are becoming more popular. Unfortunately, typically aqueous electrolytes face limitations in ionic conductivity and have relatively high freezing points, which hinder their ability to function at extremely low temperatures. These issues can be resolved with an easy-to-use method called electrolyte additive. The research on electrolyte additives for subzero-temperature aqueous sodium-ion batteries has not been systematically reviewed at present. This review aims to provide a comprehensive summary of the electrolyte additives for subzero-temperature aqueous sodium-ion batteries. Furthermore, the potential development paths of electrolyte additives to promote the advancement of electrochemical energy storage are also explored.
Graphical Abstract

Highlights
• Challenges on current subzero-temperature aqueous sodium-ion batteries are described.
• Three strategies for aqueous electrolyte additives are summarized.
• Potential development directions for future aqueous electrolyte additives are outlined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
To achieve the goal of “carbon peak” by 2030 and “carbon neutrality” by 2060, developing green, efficient, sustainable, and cost-effective electrochemical energy storage technologies (EEST) has become a consensus [1, 2]. Up to now, commercial lithium-ion batteries (LIBs) as the most successful and advanced EEST, have been extensively utilized in smart grids, electric vehicles, 5G base stations, and other fields due to their high energy density, large operating voltage, and long cycle life. However, the limited reserves of lithium in the earth’s crust, high cost, and susceptibility to explosion pose challenges for the widespread application of LIBs in the future [3,4,5,6]. Therefore, the EEST with inexpensive, abundant reserves, environment-friendly, and good safety performance urgently needs to be developed. On the other hand, sodium-ion batteries (SIBs), which utilize sodium, an element belonging to the same group as lithium in the periodic table, are emerging as a suitable substitute for LIBs in energy storage applications. SIBs possess the advantages of low cost and widespread availability [7,8,9,10,11,12]. Moreover, employing aluminum foil as current collectors for both positive and negative electrodes in SIBs, due to the absence of alloying reactions between sodium and aluminum, effectively reduces overall cost [13, 14]. Promoting the commercial development of rechargeable SIBs can significantly contribute towards achieving the “carbon peak and carbon neutrality” target.
The electrolyte is an indispensable component in SIBs as it facilitates the transport of Na+ between the anode and cathode, determines the electrochemical stability window (ESW), and triggers the formation of solid electrolyte interface (SEI) film on the electrode surface [15, 16]. Organic electrolytes are the main electrolytes used in SIBs due to their high energy density, good chemical stability, excellent rate performance, and long cycle life, which meet the demands of EEST. However, they are toxic and flammable, and their safety can be compromised in the presence of large quantities, leading to explosion accidents [17, 18]. In contrast, aqueous electrolytes that use water as a solvent are non-poisonous, non-combustible, and environmentally friendly, offering high ionic conductivity and excellent chemical stability at an affordable cost [19,20,21]. Despite the immense potential of aqueous sodium-ion batteries (ASIBs) in EEST, their practical application in high latitude or cold regions is limited due to the high freezing points of water. In some regions where temperatures drop below -50 °C, electronic products and electric vehicles face significant challenges in maintaining optimal functionality. This poses a particularly daunting task for specific industries such as deep-sea mining and aerospace, where batteries must operate efficiently even below -40 °C.
To cope with extreme conditions while fulfilling safety and environmental requirements, extensive systematic research is necessary for subzero-temperature aqueous sodium-ion batteries (SASIBs) [22]. Several challenges currently exist in SASIBs research: (1) Narrow electrochemical stability window: due to the thermodynamic and kinetic properties of water, the ESW of water is relatively narrow, only 1.23 V, which limits the voltage range and thus affects the energy density of SASIBs. If the voltage exceeds this range during the charging process, the electrolytes may decompose, leading to a decreased performance of SASIBs. Furthermore, severe hydrogen evolution and oxygen evolution reactions occur on the electrode surface, leading to surface corrosion and structural damage of the electrode materials, ultimately reducing the lifespan of SASIBs. Due to the limited ESW, there are relatively few electrode materials that can function stably within this window, which restricts the choice of electrode materials. Finding new electrode materials may be necessary to meet practical needs [23, 24]. (2) Electrolyte solidification and Low ionic conductivity: when the temperature approaches the freezing points, water molecules bind together through strong hydrogen bonds, reducing fluidity, enhancing electrolytes viscosity, and hindering the mass transfer process [25]. The resulting frozen electrolytes are incapable of efficiently transporting ions and reducing the diffusion rate of ions. This causes poor contact between the electrolyte and electrode interface, preventing normal reactions on the electrode surface, increasing the concentration polarization, and decreasing the ionic conductivity of the SASIBs [24]. (3) Salt precipitation: the solubility of sodium salts can decrease due to the aggregation and increased order of solvent molecules at low temperatures. This can lead to their deposition and potentially cause corrosion of certain components in the battery, resulting in damage to its structure and performance, ultimately leading to short circuits or failure [26]. (4) Dendrite growth: in a low-temperature environment, the movement speed of ions slows down and the efficiency of charge transfer decreases. The transport rate of sodium ions in an aqueous electrolyte is faster than their embedding in the anode electrode materials, leading to the accumulation of a significant amount of Na+ deposited on the surface of the anode. The uneven deposition of sodium can form sodium dendrites that may puncture the separators, causing a short circuit in SASIBs and compromising their safety performance [23, 27]. Improving the output voltage, energy density, and cycle life of SASIBs is crucial. Numerous studies have aimed to achieve this by regulating the interfacial electrochemical reaction process of aqueous electrolytes, inhibiting side reactions at the electrode interface, and broadening its ESW.
Although modified electrode materials have been developed to address the issue of electrolyte freezing points in SASIBs, the fundamental problem of high freezing points remains unresolved [28]. Thus, the current challenge facing SASIBs is to regulate the electrolytes [29]. Despite numerous studies on SASIBs, there is currently a dearth of comprehensive reviews that analyze their characteristics and potential applications. To fill the vacancy, our review aims to provide a summary of the latest advancements in the additives of SASIBs. This review focuses on four aspects: no additive, organic additive, inorganic additive, and organic–inorganic hybrid additives. Additionally, we have included Table 1, which integrates various parameters for SASIBs, and have proposed potential strategies to tackle the challenges that arise in this field. We anticipate that our review will not only spark more interest in SASIBs but also stimulate further research in this area.
2 Subzero-temperature aqueous electrolyte without additive
The impact of sodium salt on the characteristics of SASIBs has been extensively investigated by many researchers. Chen’s group designed a novel aqueous sodium-ion hybrid battery with Na+ and ClO4− ions as carriers [30]. The battery configuration consisted of a nano/microstructured Ni(OH)2 (NNH) cathode (Fig. 1a), a carbon-coated Na3V2(PO4)3 (NTP@C) anode, and 2 M NaClO4 aqueous solution. The charge storage mechanism in the NNH electrode was confirmed through ex-situ Fourier transform infrared spectroscopy (FT-IR) and density functional theory (DFT). Meanwhile, the full cell showed stable capacity output under all climatic conditions. Even at -20 °C, the full battery remained with a capacity retention of 85 % after 10,000 cycles (Fig. 1b).
Moreover, the increased concentration of sodium salt was found to suppress water activity and further broaden the ESW and cycle performance of aqueous electrolytes [42]. Concurrently, organic materials showed excellent stability in SASIBs [43]. In 2019, Tao et al. constructed a new cell with phenazine (PNZ) as the negative electrode (Fig. 1c), Na0.44MnO2 (NMO) (Fig. 1d) as the positive electrode and 10 M NaOH as the electrolyte [31]. The absence of color change in the 10 M NaOH solution indicated the inhibitory effect on dissolution, showing excellent stability of the system (Fig. 1e).
Subsequently, the rate performance of this cell was tested at different temperatures from -20 °C to 50 °C. Initially, the button cell was placed in a thermostat at 20 °C and exhibited a specific capacity of 155 mA h g−1 at a current density of 10 C. When the temperature dropped to -20 °C, it maintained a specific capacity of 67 mA h g−1. Upon returning to 20 °C, the capacity approached its original value, indicating a high degree of reversibility across different temperatures. Moreover, the coulomb efficiency was close to 100 % at all temperatures. The battery system also exhibited excellent long-term cycle life at varying temperatures. Even at the current density of 4 C, there was no decay in specific capacity after 2000 cycles at-20 °C to 50 °C.
To extend the ESW and maintain the advantages of traditional aqueous electrolytes, Wu’s group constructed a high-pressure aqueous planar Na ion microcell with high energy density and favorable low-temperature performance. This was accomplished by leveraging the properties of the high-concentration electrolyte, such as low flammability and low solvent activity [32]. They utilized Na3V2(PO4)3 as both the positive and negative electrodes, along with a 17 M NaClO4 electrolyte, which facilitated efficient electrons and ions transfer within the electrodes. Remarkably, the cell demonstrated 88 % capacity retention at room temperature, which was maintained even at a frigid temperature of -40 °C.
Reducing the presence of free water molecules in high-concentration salt electrolytes and facilitating the formation of an SEI through solvent and salt decomposition on the anode surface can greatly expand the ESW of aqueous electrolytes. However, the practical application of SASIBs is hindered by their high cost, increased viscosity, and reduced ionic conductivity. Therefore, it would be beneficial to design low-salt concentration aqueous electrolytes that possess a wide ESW, and this can promote the practical implementation of SASIBs.
3 Subzero-temperature aqueous electrolyte additive
Electrolyte additives are one of the most effective methods to improve the performance of SASIBs. They are mainly divided into three types: organic additive, inorganic additive, and organic–inorganic hybrid additives. The following points should be considered in selecting the appropriate electrolyte additives based on specific requirements:
-
(1)
Chemical stability: Different electrolyte additives possess various chemical properties and compositions. In general, organic additives exhibit lower stability compared to inorganic additives.
-
(2)
Conductivity: Electrolyte additives should have high conductivity and effectively reduce the internal resistance of the battery.
-
(3)
Safety: Electrolyte additives should have good safety to prevent issues such as battery over-charge, over-discharge, and short circuits.
-
(4)
Environmental protection: It is important for electrolyte additives to have no adverse impact on the environment or human health, ensuring that they do not cause pollution.
3.1 Organic additive
The utilization of high-concentration aqueous electrolytes is regarded as an effective method to enhance the cycle stability of the battery. Nevertheless, practical applications still present challenges in terms of cost and safety. Given that a significant number of organic functional groups possess strong hydrogen bonding capabilities with water molecules, employing organic solvents as co-solvents represents a feasible and convenient strategy [44]. This strategy involves disrupting the hydrogen bond association between water and ions, thereby modifying the solvation structure. Consequently, organic electrolyte additives have progressively played a crucial role in improving the performance of aqueous batteries at low temperatures [45].
DMSO (Dimethyl sulfoxide) as one of the organic solvents, features high polarity, a high boiling point, and excellent thermal stability, consequently, it can dissolve various inorganic salts and mix with water in any ratio by forming hydrogen bonds with water. Therefore, extensive research has been conducted to improve the performance of electrolytes at low temperatures through the introduction of DMSO additive [46]. Chen et al. successfully developed an electrolyte with a freezing point below -130 °C by incorporating DMSO with a molar fraction of 0.3 into a 2 M NaClO4 aqueous solution (Fig. 2a) [33]. The ionic conductivity of the electrolyte at -50 °C reached 0.11 mS cm−1, while the capacity at -50 °C was 61 % of that at 25 °C (Fig. 2b). Furthermore, the mechanism was elucidated through a combination of spectral studies and molecular dynamics simulations (Fig. 2c).
a The Polarized light microscope images, b rate performance and (c) conformation of DMSO as an additive. d Comparison of the physicochemical properties of different organic solutions used in SASIBs. e and f The MD and DFT calculations, (g) long cycle life, and (h) smartphone charging diagram of FA as an additive
Qian’s group expanded the voltage window of the cell to 2.8 V by using a multi-component electrolyte containing NaClO4, H2O, urea, and N, N-Dimethylformamide (DMF), which was close to the voltage window for highly concentrated “Water-in-Salt” electrolyte (WiSE), surpassing the 1.23 V limit for water [34]. By modifying the solvation structure of Na+ and the composition of the SEI, organic additives such as urea and DMF could reduce the number of free water molecules and improve the stability and reversibility of the NVP/NTP SIBs, expanding the battery’s working temperature range from -50 °C to 50 °C.
Jiao et al. reconstructed the hydrogen bond network between carbon, amino, and hydroxyl groups in a combined solution to achieve ultra-low freezing points (< -50 °C). [35]. They first employed strongly polar formamide (FA) as a cosolvent. Compared with other common organic additives, FA was non-flammable in the air and had a very essential value in practical applications (Fig. 2d). Noticeably, the (NaClO4)1.7- (H2O)5.5- (FA)5.81 exhibited a broader ESW (> 3.0 V) and a higher ionic conductivity (1.75 mS cm−1) at -50 °C due to the average interaction energy between water molecules and FA (Fig. 2e and f). The assembled pouch cell demonstrated outstanding cycle performance at -50 °C after 6000 cycles at a current density of 4 C (Fig. 2g). The research also considered practical applications, and it was found that three pouch batteries could recharge a smartphone at -50 °C, while two pouch batteries connected in series could easily light an LED lamp (Fig. 2h).
Li’s group successfully extended the ESW of the mixed electrolyte to 2.7 V using glycerin as an additive [36]. The SIBs provided a capacity of 40 mA h g−1 at -10 °C. Additionally, they used adiponitrile (ADN) to build a hyper-concentrated sodium electrolyte aqueous solution (Na+/H2O = 1), which confined nearly all water molecules to the primary solvated shell of Na+ and reduced the activity of water molecules (Fig. 3a) [37]. The optimal ionic conductivity was achieved in the hybrid electrolyte with a ratio of sodium bis (trifluoromethane sulfonyl) imide (NaTFSI): H2O: ADN is 1:1:2.5 (denoted as AWE) (Fig. 3b). This unique solvation structure not only extended the ESW of the electrolyte to 2.75 V (Fig. 3c) but also significantly alleviated the SEI dissolution and the formation of the NaF-rich SEI layer on the electrode surface (Fig. 3d). Compared with the 9 M NaTFSI electrolyte, the improved electrolyte significantly reduced the solubility of vanadium (Fig. 3e) and exhibited no noticeable capacity fading at -20 °C after 100 cycles (Fig. 3f). Huang’s group developed an aqueous ionic gel electrolyte by co-polymerized poly(ethylene glycol) methyl ether methacrylate (PEGMA) with bisphenol A ethoxylate dimethacrylate (BEMA) [38]. The primary chain structure of PEGMA was the same as PEG, which promoted the hydrogen bonds formation with water molecules. The inclusion of fluoride carbonate additive and -OCH3 improved the compatibility of the electrolyte with hydrophobic electrode materials, and created a stable SEI and cathode electrolyte interface (CEI), which could finally reach a high voltage of 3 V.
a Solvation structure of the AWE electrolyte obtained from MD simulation. b Ionic conductivity with different ratios of NaTFSI, H2O and ADN. c Electrochemical stability window in AWE and 9 m NaTFSI electrolytes. d XPS profiles of F1s spectra of NTP electrodes after 50 cycles in AWE. e Vanadium ions’ dissolve amount and (f) Cycle performance of NVP/NTP full in two different electrolytes
Functional groups in organic electrolyte additives, serving as hydrogen bond donors and/or acceptors, react with water molecules to form hydrogen bonds. This disrupts the original hydrogen bond networks among water molecules and effectively prevents the formation of ice nuclei and regular hydrogen bond networks among water molecules. Consequently, the freezing point of the electrolytes is reduced, and their low-temperature conductivity is improved.
3.2 Inorganic additive
To increase the application of ASIBs in cold climates, it is crucial to research novel electrolyte additions with extremely low freezing points. Nevertheless, the organic additives summarized above (such as DMSO, ADN, etc.) are combustible and toxic, which does not align with our demand for green energy and could potentially endanger public safety [47]. Simply adjusting the concentration of inorganic salt has been proven to be an effective method to improve the frost resistance of aqueous electrolytes [48].
The lowest solidification temperature was found by Jiao’s group in a new low-temperature electrolyte that contained 3.86 M CaCl2 and 1 M NaClO4 (Fig. 4a) [39]. A decrease in the amount of strong hydrogen bonding water (SHW) could considerably lower the freezing points of the electrolyte. The modified electrolyte exhibited a lower freezing point than other electrolytes when the concentration of CaCl2 was 3.86 M in 1 M NaClO4 because the SHW content was lowest at that point (Fig. 4b).
a Phase diagram of aqueous calcium chloride. b The component proportions of water with different hydrogen bonds for various electrolytes: CaCl2-based electrolytes. c Long cycle life at a current density of 10 C at different temperatures. d The cycle performance of the full batteries when tested under -50 °C at 10 C
The optimized electrolyte still exhibited an ideal high ionic conductivity (7.13 mS cm −1) even at -50 °C. The strong interaction between CaCl2 and water molecules could destroy the hydrogen bond network between the original water molecules and significantly reduce the freezing point of the solution. The full battery was tested after ten activation cycles at room temperature. At -30 °C, the specific discharge capacity could reach 74.5 mA h g−1 at 1 C. Even after 1000 cycles, the entire battery could still deliver a discharge capacity of 64.6 mA h g−1 at 1 C, with a capacity retention of 86.7 %. Interestingly, when cycled in challenging conditions, the battery system maintained high discharge capacity and long-term cycle stability. The capacity of the complete battery was recovered to its previous level after three difficult test cycles, as shown in Fig. 4c, further demonstrating the viability of the battery system in a low-temperature setting.
Similarly, this group also used Mg(ClO4)2 as an inert-support salt to establish a 3.5 M Mg(ClO4)2, with 0.5 M NaClO4 electrolyte system [40]. Mg2+ could destroy the original hydrogen bond network between water molecules significantly and increase the difficulty of ice formation, resulting in the electrolyte having a shallow freezing point (< 80 °C) and high ionic conductivity at -60 °C (4.86 mS cm−1). Even at -50 °C, the full batteries exhibited outstanding cycle performance at a current density of 10 C (Fig. 4d). The pouch cells in particular offered a viable method for the use of ultra-low-temperature batteries by powering motors at -60 °C and charging smartphones.
In conclusion, the metal salts in inorganic electrolyte additives can form strong interactions with water molecules, rather than forming hydrogen bonds, which break the original hydrogen bond networks among water molecules. The inorganic electrolyte additives have the advantage of being able to replace toxic and flammable organic additives while maintaining superior ionic conductivity and stability. However, the addition of high concentrations of salts leads to increased costs. To minimize costs and meet the requirement for environmental friendliness, localized high-concentration electrolytes and low-concentration inorganic salt electrolyte additives should have greater prospects.
3.3 Organic-Inorganic hybrid additives
Because of their exceptional solubility at low temperatures, some sodium salts containing fluoride anions, such as sodium triflate (NaOTF) and NaTFSI, are frequently utilized as sodium salts in SASIBs. However, organic sodium salts may cause environmental pollution due to their toxicity, and NaClO4 is unsuitable for industrial purposes due to its potent oxidizing abilities [49]. In the industry, electrolytes based on inorganic sodium sulfate, like sodium sulfate, are attractive due to their low cost. However, the limited solubility of the sodium sulfate-based aqueous electrolyte severely restricts its low-temperature tolerance, leading to a drop abruptly from 0 °C to -30 °C. The reduction in conductivity is a result of the decreased solubility of sodium sulfate [41]. In 2008, Chen et al. further revealed the positive effect of alcohol additives, such as methanol, on the conductivity of lead-acid batteries at low temperatures [50]. These additives effectively reduce the freezing points of water and the solubility of sodium sulfate due to their low polarity of the mixture solution.
Therefore, Liu et al. proposed a new Na2SO4-SiO2 hydrogel electrolyte with jet silica as a gel matrix and methanol as an antifreeze additive [41]. Figure 5a compared the freezing process while displaying the Na2SO4-SiO2 hydrogel composite structure at 25 °C and -30 °C. They found that a drop in temperature could trigger the production of sodium sulfate nuclei. Once the nucleus formed, the tendency of sodium sulfate crystal particles to detach occurred due to the reduction in surface free energy. The number of Na+ ions in the sodium sulfate aqueous electrolyte fell when sodium sulfate crystallized, which caused a sharp drop in conductivity. According to Fig. 5b, when ethanol was added to a 1 M Na2SO4 solution at room temperature, white deposits started to form in the solution as a result of the solvent’s decreased polarity, and the solution retained its high stability at low temperatures due to methanol being the strongest polarity among other monohydric alcohols, thus methanol was added to the hydrogel to acquire antifreeze function.
a Illustration of freezing process from 25 °C to -30 °C for aqueous Na2SO4 electrolytes and Na2SO4-SiO2 hydrogel electrolytes. b Pictures of Na2SO4 solutions. Purely 1 M Na2SO4 solution at 25 °C (Left); 40 ml 1 M Na2SO4 solution with the addictive of 10 mL ethanol at 25 °C (middle); 1 M Na2SO4 solution at -30 °C (right)
At present, the options for commercialized sodium salts are limited, and the solubility of sodium salts in the aqueous electrolyte is the key to determining the electrochemical performance of SASIBs. Organic–inorganic electrolyte additives can prevent the precipitation of sodium salts and reduce the freezing of water. In addition, seeking and developing appropriate suitable sodium salts remains an urgent issue in the field of electrolytes.
4 Summary and prospect
To expedite the commercial development and enhance the electrochemical performance of SASIB, various strategies have been employed by researchers. These strategies primarily focus on reducing the freezing points of the electrolytes and creating a complex solvent sheath structure. Current techniques such as the “Water in salt”, incorporating organic solvents as additives or co-solvents, and introducing inorganic additives as antifreeze agents have been utilized. In addition, the formation of unique solvent sheath structures plays a crucial role in confining water to the primary solvated shell of sodium ions and producing a stable SEI film that inhibits the ongoing consumption of electrolyte solvents. Consequently, these techniques destroy the hydrogen bond networks among water molecules, lower the freezing points of the electrolytes, enhance ionic conductivity, and broaden the electrochemical window of the electrolytes.
In this review, we provide a comprehensive summary of the electrolyte additives for SASIBs. These additives have a strong ability to coordinate with water molecules, reducing the content of free water and effectively inhibiting the activity of water. Those strategies of adding electrolyte additives improve the electrochemical performance of SASIBs. However, despite these remarkable achievements, the commercial development of SASIBs still confronts numerous unknown challenges. Therefore, several strategies have been proposed to address these challenges and further drive the commercialization of SASIBs, including:
-
1.
More advanced characterization methods and theoretical calculations are requested to further investigate the structure–activity relationship between the interface process and reaction kinetic retardation of SASIBs under low-temperature conditions.
-
2.
The future trend in enhancing battery safety and dependability involves researching novel electrolyte additives SASIBs, such as Methyl Acetate, Diethylene glycol monoethyl ether, ZnCl2, etc., and understanding their work principle in more extreme situations (< -80 °C).
-
3.
The presence of an inorganic-rich SEI layer greatly improves the performance of sodium storage. It not only facilitates the Na+ transfer between electrode materials and electrolytes but also contributes to the structural stability of the SEI interface. It is crucial for future research to further comprehend inorganic-rich SEI.
-
4.
Fast charging has garnered significant attention due to its ability to reduce the charging time and improve charging efficiency. The temperature, particularly at low temperatures, has a considerable impact on fast charging performance. A key step in the future commercial development of SASIBs is to enhance their fast charge capabilities, ultimately achieving highly efficient and cost-effective SASIBs.
Availability of data and materials
The date and materials are available upon reasonable request.
Abbreviations
- EEST:
-
Electrochemical energy storage technologies
- LIBs:
-
Lithium-ion batteries
- SIBs:
-
Sodium-ion batteries
- ESW:
-
Electrochemical stability window
- SEI:
-
Solid electrolyte interface
- ASIBs:
-
Aqueous sodium-ion batteries
- SASIBs:
-
Subzero-temperature aqueous sodium-ion batteries
- FT-IR:
-
Fourier transform infrared spectroscopy
- DFT:
-
Density functional theory
- PNZ:
-
Phenazine
- DMSO:
-
Dimethyl sulfoxide
- DMF:
-
N, N-Dimethylformamide
- WiSE:
-
Water-in-Salt electrolyte
- FA:
-
Formamide
- ADN:
-
Adiponitrile
- PEGMA:
-
Poly(ethylene glycol) methyl ether methacrylate
- BEMA:
-
Bisphenol A ethoxylate dimethacrylate
- CEI:
-
Cathode electrolyte interface
- SHW:
-
Strong hydrogen bonding water
- NaOTF:
-
Sodium triflate
- NaTFSI:
-
Sodium bis (trifluoromethane sulfonyl) imide
References
Yang P, Peng S, Benani N, Dong L, Li X, Liu R, Mao G (2022) An integrated evaluation on China’s provincial carbon peak and carbon neutrality. J Clean Prod 377:134497
Shen M, Kong F, Tong L et al (2022) Carbon capture and storage (CCS): development path based on carbon neutrality and economic policy. Carb Neutrality 1:37
Hu X, Sun J, Sun Z, Zhao Q, Chen Q, Chen J (2016) Rechargeable room-temperature na-CO2 batteries. Angew Chem Int Ed 55(22):6482–6486
Yu Y, Qin R, Shi X, Xie J, Lu T, Lu X (2023) Carbon-anchored Sb nanoparticles as high-capacity and stable anode for aqueous alkaline batteries. Battery Energy 2:20230016
Gabriel E, Ma C, Graff K, Conrado A, Hou D, Xing H (2023) Heterostructure engineering in electrode materials for sodium- ion batteries: recent progress and perspectives. Science 3(5):100139
Gao F, Yue X, Xu X et al (2023) A N/Co co-doped three-dimensional porous carbon as cathode host for advanced lithium–selenium batteries. Rare Met 42:2670–2678
Hu X, Joo P, Wang H, Matios E, Wang C, Luo J, Lu X, Yang K, Li W (2019) Nip the sodium dendrites in the bud on planar doped graphene in liquid/gel electrolytes. Adv Funct Mater 29(9):1807974
Liu R, Xu L, He X, Liu H, Ma X, Tao Z, Wan G, Ahmad N, Peng B, Shi L, Zhang G (2023) Constructing heterointerface of Bi/Bi2S3 with built-in electric field realizes superior sodium-ion storage capability. eScience 3(4):100138
Cheng Z, Zhao B, Guo Y et al (2022) Carbonyls: mitigating the large-volume phase transition of P2-type cathodes by synergetic effect of multiple ions for improved sodium-ion batteries. Adv Energy Mater 12:2103461
Cheng Z, Fan X, Yu L et al (2022) A rational biphasic tailoring strategy enabling high-performance layered cathodes for sodium-ion batteries. Angew Chem Int Ed 61:e202117728
Yu L, Cheng Z, Xu K et al (2022) Interlocking biphasic chemistry for high voltage P2/O3 sodium layered oxide cathode. Energy Storage Mater 50:730–739
Yu L, Chang Y, Liu M et al (2023) O3-Type Na0.95Ni0.40Fe0.15Mn0.3Ti0.15O2 cathode material with enhanced storage stability for high-energy Na-ion batteries. ACS Appl Mater Interf 15(19):23236–23245
Li Y, Mu L, Hu Y, Li H, Chen L, Huang X (2016) Pitch-derived amorphous carbon as high performance anode for sodium-ion batteries. Energy Storage Mater 2:139–145
Xie F, Zhang L, Ye C et al (2019) The application of hollow structured anodes for sodium-ion batteries: from simple to complex systems. Adv Mater 31:1800492
Gu S, Bai Y, Wu C et al (2022) Solvent effects on kinetics and electrochemical performances of rechargeable aluminum batteries. Energy Mater Adv 2022:9790472
Huang Z, Wang T, Li X, Cui H, Liang G, Yang Q, Chen Z, Chen A, Guo Y, Fan J, Zhi C (2022) Small-dipole-molecule-containing electrolytes for high-voltage aqueous rechargeable batteries. Adv Mater. 34(4):e2106180
Su C, He M, Amine R, Rojas T, Cheng L, Ngo A, Amine K (2019) Solvating power series of electrolyte solvents for lithium batteries. Energy Environ Sci 12(4):1249–1254
Li X, Chou J, Zhu Y, Wang W, Xin S, Guo Y (2023) Hydrogen isotope effects: A new path to high-energy aqueous rechargeable Li/Na-ion batteries. eScience 3(3):100121
Hou R, Wang Y, Sun Y, Lang J, Yang S, Yan X (2023) Hydrogen isotope effects: A new path to high-energy aqueous rechargeable Li/Na-ion batteries. eScience. 100121.
Liu X, Yu Z, Sarnello E, Qian K, Li T (2021) Microscopic understanding of the ionic networks of “water-in-salt” electrolytes. Energy Mater Adv 2021:7368420
Zhu N, Zhang K, Wu F, Bai Y, Wu C (2021) Ionic liquid-based electrolyte for aluminum/magnesium/sodium-ion batteries. Energy Mater Adv 2021:9204217
Yang Y, Yang W, Yang H, Zhou H (2023) Electrolyte design principles for low-temperature lithium-ion batteries. eScience 3(6):100170
Hu X, Matios E, Zhang Y, Wang C, Luo J, Li W (2021) Deeply cycled sodium metal anodes at low temperature and lean electrolyte conditions. Angew Chem Int Ed 60(11):5978–5983
Li Y, Wu F, Li Y, Liu M, Feng X, Bai Y, Wu C (2022) Ether-based electrolytes for sodium ion batteries. Chem Soc Rev 51:4484–4536
Wang S, Deng W, Geng Z, Li P, Hu N, Zhu L, Sun W, Li C (2023) Significantly raising tetracyanoquinodimethane electrode performance in zinc ion battery at low temperatures by eliminating impurities. Battery Energy 2:20220050
Hu M, Zhang H, Lv R (2020) Layered carbon-based pseudocapacitive materials for lithium/sodium-ion capacitor with high energy-power densities and long cycle life. Prog Nat Sci: Mater Int. 30:20–27
Zhang Q, Lu Y, Guo W, Shao Y, Hu Y (2021) Hunting sodium dendrites in nasicon-based solid-state electrolytes. Energy Material Advances 2021:9870879
Liu S, Wang L, Liu J, Zhou M, Nian Q, Feng Y, Tao Z, Shao L (2019) Na3V2(PO4)2F3-SWCNT: a high voltage cathode for non-aqueous and aqueous sodium-ion batteries. J Mater Chem A 7(1):248–256
Han J, Zarrabeitia M, Mariani A, Jusys Z, Hekmatfar M, Zhang H, Geiger D, Kaiser U, Behm R, Varzi A, Passerini S (2020) Halide-free water-in-salt electrolytes for stable aqueous sodium-ion batteries. Nano Energy 77:105176
Nian Q, Liu S, Liu J, Zhang Q, Shi J, Liu C, Wang R, Tao Z, Chen J (2019) All-climate aqueous dual-ion hybrid battery with ultrahigh rate and ultralong life performance. ACS Appl Energy Mater 2(6):4370–4378
Sun T, Liu C, Wang J, Nian Q, Feng Y, Zhang Y, Tao Z, Chen J (2020) A phenazine anode for high-performance aqueous rechargeable batteries in a wide temperature range. Nano Res 13(3):676–683
Wang X, Huang H, Zhou F, Das P, Wen P, Zheng S, Lu P, Yu Y, Wu Z (2021) High-voltage aqueous planar symmetric sodium ion micro-batteries with superior performance at low-temperature of -40 °C. Nano Energy 82:105688
Nian Q, Wang J, Liu S, Sun T, Zheng S, Zhang Y, Tao Z, Chen J (2019) Aqueous batteries operated at -50 °C. Angew Chem Int Ed 58(47):16994–16999
Ao H, Chen C, Hou Z, Cai W, Liu M, Jin Y, Zhang X, Zhu Y, Qian Y (2020) Electrolyte solvation structure manipulation enables safe and stable aqueous sodium ion batteries. J Mater Chem A 8(28):14190–14197
Zhu K, Sun Z, Li Z, Liu P, Chen X, Jiao L (2022) Aqueous sodium ion hybrid batteries with ultra-long cycle life at -50 °C. Energy Storage Mater 53:523–531
Sun Y, Zhang Y, Xu Z, Gou W, Han X, Liu M, Li C (2022) Dilute hybrid electrolyte for low-temperature aqueous sodium-ion batteries. Chemsuschem 15(23):e202201362
Wang H, Liu T, Du X, Wang J, Yang Y, Qiu H, Lu G, Li H, Chen Z, Zhao J, Cui G (2022) Hybrid electrolytes enabling in situ interphase protection and suppressed electrode dissolution for aqueous sodium-ion batteries. Batter Supercaps 5(10):e202200246
Rong J, Cai T, Bai Y, Zhao X, Wu T, Wu Y, Zhao W, Dong W, Xu S, Chen J, Huang F (2022) A free-sealed high-voltage aqueous polymeric sodium battery enabling operation at -25 °C. Cell Rep Phys Sci 3(3):100805
Zhu K, Li Z, Sun Z, Liu P, Jin T, Chen X, Li H, Lu W, Jiao L (2022) Inorganic electrolyte for low-temperature aqueous sodium ion batteries. Small 18(14):e2107662
Zhu K, Sun Z, Jin T, Chen X, Si Y, Li H, Jiao L (2022) Tailoring pure inorganic electrolyte for aqueous sodium-ion batteries operating at -60 °C. Batter Supercaps 5(12):1-8
Cheng Y, Chi X, Yang J, Liu Y (2021) Cost attractive hydrogel electrolyte for low- temperature aqueous sodium-temperature aqueous sodium-ion batteries. J Energy Storage 40:102701
Jaumaux P, Yang X, Zhang B, Safaei J, Tang X, Zhou D, Wang C, Wang G (2021) Localized water-in-salt electrolyte for aqueous lithium-ion batteries. Angew Chem Int Ed 60(36):19965–19973
Häupler B, Wild A, Schubert U (2015) Carbonyls: powerful organic materials for secondary batteries. Adv Energy Mater 5:1402034
Gao X, Yang J, Xu Z, Nuli Y, Wang J (2023) Recent progress of aqueous and organic/aqueous hybrid electrolytes for low-temperature rechargeable metal-ion batteries and supercapacitors. Energy Storage Mater 54:382–402
Yue F, Tie Z, Deng S, Wang S, Yang M, Niu Z (2021) An ultralow temperature aqueous battery with proton chemistry. Angew Chem Int Ed 60(25):13882–13886
Jiang H, Feng J, Zhao H, Li G, Yin G, Han Y, Yan F, Liu Z, Liu S (2018) Low temperature fabrication for high performance flexible CsPbI2Br Perovskite solar cells. Adv Sci 5:1801117
Zhu J, Xu Y, Fu Y, Xiao D, Li Y, Liu L, Wang Y, Zhang Q, Li J, Yan X (2020) Hybrid aqueous/nonaqueous water-in-bisalt electrolyte enables safe dual ion batteries. Small 16(17):e1905838
Thenuwara A, Shetty P, Kondekar N, Sandoval S, Cavallaro K, May R, Yang C, Marbella L, Qi Y, McDowell M (2020) Efficient low-temperature cycling of lithium metal anodes by tailoring the solid-electrolyte interphase. ACS Energy Lett 5(7):2411–2420
David G (2006) Churchill, chemical structure and accidental explosion risk in the research laboratory. J Chem Educ 83(12):1798–1803
Chen M, Chen H, Shu D, Li A, Finlow D (2008) Effects of preparation condition and particle size distribution on fumed silica gel valve-regulated lead-acid batteries performance. J Power Sources 181(1):161–171
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52201278) and the Young Talent Support Plan of Xi'an Jiaotong University (for X. F. H.). The authors acknowledge the Department of Science and Technology of Shaanxi Province (QCYRCXM-2022–126). The authors acknowledge Mr. Jinpo Jia and Youqi He for assistance with industry analysis.
Author information
Authors and Affiliations
Contributions
X.H. conceived this review. R.W. was the major contributor to writing the manuscript. S.Z. and S.P. reviewed and edited the manuscript. Y.T. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to the publication of this manuscript.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Zhang, S., Peng, S. et al. Research progress of electrolyte additives for subzero-temperature aqueous sodium-ion batteries. Carb Neutrality 3, 6 (2024). https://doi.org/10.1007/s43979-024-00081-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43979-024-00081-z