Abstract
Lead (Pb) contamination in water poses a significant threat to public health across the globe which requires effective remediation strategies. The main objective of this study is to achieve a removal efficiency of Pb(II) ions from aqueous media using activated Syzygium cumini (java plum) stem biochar. It was prepared by slow pyrolysis at 400 °C after treating the biomass with potassium hydroxide for activation. These biochars were characterized thoroughly (SEM, SEM–EDX, TEM, FTIR, XRD, elemental analyses, and SBET) to conduct a set of batch experiments. The effect of several parameters such as pH, temperature, contact time, adsorbent dosage, initial lead concentrations, and co-existing ions were explored. The decrease in starting lead concentrations from 50 to 10 mg L−1 resulted in improved removal efficiency. The removal effectiveness of activated biochar was 97%, but non-activated biochar was just 19%. Lead adsorption increased considerably as pH increased from 3 to 5. Further, the activated biochar was optimized through various isotherms models, kinetic equations, and thermodynamic analysis. It was observed that the pseudo-second-order kinetic model and Temkin isotherms were the best-fitted models that identify the mechanism of chemisorption and monolayer sorption. According to this study, activated biochar is a promising biosorbent for removing lead from an aqueous solution.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water pollution is a global environmental problem where more than 2.1 billion people do not have access to clean drinking water [1]. The water is getting polluted due to an increase in various pollutants in the surface and subsurface systems [2]. Among various contaminants, heavy metals are one of the major contaminants generated from either natural or anthropogenic activities causing contaminants to soil and existing water bodies [3, 4]. Among heavy metals, lead ion [Pb(II)] is one of the priority pollutants as it is considered a contaminant of emerging concern and ranked second (after arsenic) according to the Agency for Toxic Substances and Disease Registry (ATSDR). The Bureau of Indian Standards (BIS), World Health Organisation (WHO), and American Water Works Association (AWWA) have established 0.05 mg L−1 of lead as the ideal limit for drinking water [5]. Lead smelting, battery recycling, and coal combustion are prominent sources of lead pollution [6]. It damages the liver, kidney, bones, and reproductive system once it enters the human body through the food chain [7]. Lead can cause severe health problems for children such as delaying their mental or physical development and impairing their ability to pay attention and learn [8]. Therefore, over the last few decades, the effective removal of lead from polluted water has become an important research subject.
Lead removal technologies include ultrafiltration, ion exchange, membrane filtration, electrocoagulation, membrane separation, adsorption, and flocculation [9,10,11,12]. Among them, adsorption is one of the cost-effective techniques for the removal of lead or other contaminants from an aqueous solution [13,14,15]. Further, the adsorption process is simple, flexible, environment friendly, easy, capable of regeneration, and lacks toxic by-products [14, 16, 17]. Various adsorbents such as activated carbon, graphene oxide, bentonite, zeolite, and biochar have been applied for the removal of lead from aqueous solution [18,19,20,21]. The studies highlight that the removal efficiencies of Pb(II) are dependent on adsorbent dosages, initial Pb(II) concentration, pH, and contact time (Table 1). Among these adsorbents, biochar has been used as a feasible and alternative solution for the elimination of lead [22,23,24,25].
The development of biochar for the purification of water is a comparatively new practice [5, 26,27,28,29]. Biochar, a solid material enriched in carbon, is obtained upon the pyrolysis of biomass through slow pyrolysis, fast pyrolysis or gasification methods [30,31,32]. It possesses unique physiochemical features, such as high pore volume, big surface area, and rich functional surface groups [33]. This material is an effective, durable, and eco-friendly solution for the removal of contaminants from aqueous solution [34,35,36]. In pure form, biochar may have a lower adsorption capacity compared to traditional adsorbents [37]. Recently, researchers have enhanced the biochar properties, adsorption capacity, and metal remediation efficiency through modifications, metal impregnation, magnetization, and/or activation [24, 35, 38,39,40,41]. The surface area of the biochar, pore structure, and functional groups associated with biochar enhanced after the modifications [42]. The adsorption of lead from aqueous solution by activated carbon [21, 43] and by agricultural waste-derived biochar have been reported by various researchers as well [25]. However, research on biochar modifications through a variety of techniques is still an emerging area of study [37]. Researchers are increasingly recognising the benefits of biochar modifications through the activation process. Activation can be done either through physical activation where biochar can be activated at a high temperature (> 900 °C) in the presence of steam or CO2 [44], or by chemical activation under which biochar can be activated through various agents such as potassium hydroxide [45], potassium carbonate (K2CO3), zinc chloride (ZnCl2), and phosphoric acid (H3PO4) [46]. Specifically, chemical activation with KOH has been widely developed for the synthesis of activated biochar by various researchers [47,48,49]. Biochar developed from tobacco stems, celery stalks, and canola straws has high efficiency in removing lead from aqueous solution [50, 51]. Unfortunately, there is currently a lack of information on the effect of biochar washing on the elimination of released compounds from biochar after chemical treatment.
This study aims to evaluate the efficiency of the adsorption of lead onto activated biochar obtained from Syzygium cumini stem (java plum) upon a slow pyrolysis process. The present study aims to assess the adsorption of Pb(II) onto low-cost activated biochar which was synthesized in a single-step process. The primary goal of this research is to (a) analyse the physiochemical properties of both raw and activated biochar, and (b) use batch experiments to evaluate Pb(II) removal efficiency under various conditions such as pH, initial concentration, biochar dose, contact time, temperature, and co-existing anions. Furthermore, adsorption isotherms, kinetics, and thermodynamic behaviour are used to estimate the Pb(II) removal mechanisms using activated biochar in this study.
2 Materials and methods
2.1 Reagents and equipment
Analytical or guaranteed reagents are used during the experiments which were purchased from either Merck or unless otherwise specified. Pb(II) stock solution was prepared by dissolving [Pb(NO2)2] into de-ionized water. The pH 7 was achieved using either 0.1 M HNO3 or 0.1 N NaOH using a pH meter (Hanna instruments). A static water bath (BUCHI, B-461) was used for adsorption studies. A vortex mixer (Scientific Industries, T3-1280) was used to stir the samples.
2.2 Feedstock and biochar preparation
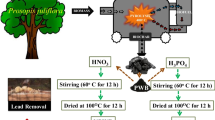
The Syzygium cumini (java plum) stem part was collected from the local market of Rajgir, India (25° 00′ 44.4″ N 85° 24′ 37.1″ E). A small piece of dried stem was cut and washed with ultra-pure water 4–5 times, and soaked in ultra-pure water for 72 h to remove the color and impurities from the biomass. The prepared biomass was oven-dried for 72 h at 75 °C to remove moisture as much as possible and was kept in a container after cooling. Further, the pyrolysis experiments were conducted in a fixed-bed reactor (muffle furnace). The Syzygium cumini stem biomass was pyrolyzed in the muffle furnace at 400 °C for 2 h. The Syzygium cumini stem biochar (SCB) was soaked in ultra-pure water for 24 h and then washed properly to leach color, and then oven-dried at 75 °C for further adsorption studies.
2.3 Preparation of activated Syzygium cumini stem biochar (ACB)
In this study, potassium hydroxide chemical was selected for the activation of biochar as a result of a large improvement in the metal sorption [52]. The schematic diagram for the development of activated biochar is shown in Fig. 1. The activated Syzygium cumini stem biochar (ACB) was prepared through single-step activation process according to the method described by Basta et al. [53]. Briefly, 2 g of oven dried Syzygium cumini stem was soaked into 250 mL of 2 M KOH solution and stirred for 12 h at 120 revolutions per minute (rpm). The resulting biomass was repeatedly washed with ultra-pure water and oven dried at 75 °C for 24 h. The activated Syzygium cumini stem biomass was pyrolyzed in the muffle furnace at 400 °C for 2 h. The ACB was soaked in ultra-pure water for 24 h, washed properly to leach color, and oven-dried at 75 °C for further adsorption studies.
2.4 Proximate and ultimate analysis
Total C, N, and H contents were measured with EUROM EA3000 elemental analyzer. The moisture content, volatile matter, and ash content were determined according to the D1762-84 method. Feedstock and biochar moisture content were estimated by oven drying for 2 h at 105 °C. The samples were heated to 950 °C for 11 min (covered crucibles) to determine the volatile matter and at 750 °C for 2 h (uncovered crucible) for the ash content [54]. The results are reported in weight percent on an ash basis.
2.5 Characterization of raw and activated biochar
The morphology of raw and activated biochar was investigated by Scanning electron microscopy (SEM) (Make: deMicro, Orchid, India), at a 20,000-V accelerating voltage. All samples were Au-coated before mounting on a copper stab with double-stick carbon tape. Elemental sample compositions were analyzed to a ~ 2–3 μm depth by SEM/EDX (EDX, EDAX Inc., USA). Similarly, the structural and chemical characterization of raw and activated biochar was carried out using Fourier transform infrared (FTIR) spectra (Agilent Technologies, USA) and X-ray diffraction (Rigaku Corporation, Japan), respectively. BET surface area for SCB and ACB was determined by a micrometric ASAP 2020 surface area analyzer. The samples (0.15 g) were degased at 250 °C for 12 h at > 10−3 Torr.
2.6 Preparation of stock solution and analytical method for Pb(II)
Pb(II) (1000 mg L−1) stock solution was obtained by dissolving lead nitrate salt [Pb (NO3)2] into ultra-pure water. 0.1 M HNO3 and 0.1 N NaOH were used to adjust the pH. The concentration of Pb(II) was measured by UV–VIS spectrophotometer at 212 nm (Agilent Technology, ALIGENT CARY100) [55, 56]. The proposed wavelength for Pb(II) analysis using Spectrophotometer is based on calibration and validation processes using preliminary experiments.
3 Results and discussion
3.1 Biochar pyrolysis
Based on the weight of the initial biomass, slow pyrolysis produced 27 weight percent of stem biochar from Syzygium cumini. Table 2 provides proximate analyses of feedstock, SCB, and ACB. ACB consists of higher carbon content and hydrogen content than SCB [C (70.27 vs 45.70%) and H (1.99 vs 1.92%)]. Biochar properties are generally dependent on the nature of feedstock, temperature, residence time, and reactor type. For example, the tea residue and paddy straw biochar were prepared at 650 °C and 550 °C, respectively, contributing the highest carbon content in tea residue biochar. The molar H/C ratio describes the degree of char carbonization [57, 58]. The H/C molar ratio for Syzygium cumini stem was 0.12, whereas those for SCB and ACB were 0.02 and 0.03, respectively (Table 2). Biochar’s reduced H/C molar ratio contained low organic wastes (like cellulose) and a high carbonization value [57]. Because it indicates the polar-group content produced from carbohydrates, the molar ratio of oxygen to carbon (O/C) is employed as an indication for surface hydrophilicity [57]. The molar O/C ratio was 1.11 for Syzygium cumini stem and 0.20 and 0.32 for SCB and ACB, respectively. SCB and ACB surface areas were found as 145.11 and 168.98 m2 g−1, respectively (Table 2). Pyrolysis at a lower temperature (400 °C) resulted in SCB with a lower surface area and higher ash content. Biochar surface area normally increases with a higher pyrolysis temperature [59, 60].
3.2 Biochar characterization
The SEM micrographs of the SCB and ACB showed their irregularly shaped structures (Fig. 2). The SEM images of SCB showed microscopic sizes of the particles with irregular shapes and sharp edges (Fig. 2A, B). The ACB were seen as larger particles and appeared as an agglomerated form of pristine biochar. (Fig. 2C, D). The XRD spectra of SCB and ACB are presented in Fig. 3. The spectra showed a broad hump region between 2θ of 18–28° corresponds to the presence of amorphous carbon [61]. This represents crystal plane index C(002) which is formed due to azimuthal and parallel orientation of partially carbonized aromatic clusters [62]. Another small hump around 2θ of 43° represents the C(100) plane of carbonized aromatic carbon planes [62]. Further, SCB showed sharp peaks at 2θ of 14.9 and 24.13 corresponds to whewellite, and 2θ of 29.65 and 37.7 corresponds to the presence of calcite (Fig. 3). ACB has lowered the strength of the whewellite and calcite peaks. Submerging the biochar in KOH activates it, causing calcite and whewellite to dissolve and potassium-containing minerals such as sylvite to emerge. This tendency is also consistent with the EDX data (Fig. 4), which revealed the presence of calcium in SCB and potassium in ACB.
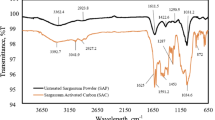
Broad peaks of –OH stretching bands from organic or inorganic components observed from 4000 to 3000 cm−1 are seen in the FTIR spectra of SCB and ACB (Fig. 5). Small peaks in SCB and ACB at 2854 cm−1 and 2927 cm−1, respectively, are attributed to the C–H symmetric stretching vibration in organic carbon. The presence of carbon dioxide is the cause of sharp peaks in SCB and ACB at 2360 cm−1. The normal range of 2000–2400 cm−1 is associated with triple bond stretching of O=C=O, –C≡C– and –C≡N. According to Reig et al. [63], there are multiple asymmetric peaks in the wide range of 1926–1314 cm−1, with the peak maxima at 1685 cm−1 in SCB and ACB. These comprise contributions of complex conjugated C=C systems in the samples as well as C=O stretching of different functional groups in ketones, carboxylic acids, esters, and anhydrides. The aromatic ring's C=C bond stretching is responsible for the peak observed at 1560 cm−1 in both SCB and ACB [62]. Reig et al. [63] ascribed the –C–O bond to the SCB peak at 1099 cm−1. The carbonate –C=O stretching found in calcite is the cause of the band at 881 cm−1 in ACB biochar [63]. According to Li et al. [64], the ‘asymmetric bending vibrations’ of Si–O–Si and the ‘symmetric stretching vibrations’ of Si–O are responsible for the peaks in the ranges 798 cm−1 to 669 cm−1 and 459 cm−1, respectively.
3.3 Effect of contact time on Pb(II) sorption
Removal efficiency (%) of Pb(II) using raw and activated biochar was studied as a function of contact time (360 min). The sorption experiments were performed for SCB and ACB at pH 7.0 ± 0.1 with an initial Pb(II) concentration of 50 mg L−1 and biochar dosage of 0.5 g L−1 at temperature 302 K (Table 3). It was inspected that the removal efficiency (%) of Pb(II) is high and increased with increase in contact time in an aqueous solution. The sorption of Pb(II) with a concentration of 50 mg L−1 on ACB is more than SCB (Fig. 6). The rapid increase in removal efficiency for ACB at an early stage occurs because of the existence of vigorous sites. The removal efficiency also enhances the sorption capacity due to instant mass transfer. Pb(II) removal efficiency increased from 54 to ~ 85% for ACB whereas, SCB shows negligible change in removal efficiency (~ 20%) after 360 min (using equation S1). The sorption capacity of Pb(II) is 1.97 mg g−1 over the raw biochar and 8.47 mg g−1 for activated biochar (using equation S2). The increase in removal efficiency for activated biochar might be ascribed to the electrostatic force of attraction between Pb(II) ions and active site availability on biochar surfaces and large surface area [24, 65].
3.4 Effect of pH on sorption and Pb(II) removal mechanism
The effect of Pb(II) sorption on SCB and ACB is shown in Fig. 7. The pH effect of Pb(II) on SCB and ACB was studied at a pH range of 3–7. To understand the effect of pH on the removal efficiency of Pb(II), the sorption experiments were performed over SCB and ACB at varying pH of 3, 5, and 7 with an ‘initial lead concentration’ of 50 mg L−1, biochar dosage of 0.5 g L−1, and 302 K temperature in an aqueous solution. The maximum sorption appeared at pH 5. Sorption of Pb(II) increased consequently as the pH increased from 3 to 5 and became constant from pH 5 to 7. However, the maximum removal of Pb(II) occurred at pH 3 (~ 46%) for SCB and at pH 5 (~ 86%) for ACB, respectively (using equation S1). The sorption capacity of Pb(II) over SCB at pH 3 was 4.64 mg g−1 and 5.74 mg g−1 over ACB (using equation S2) at a metal concentration of 50 mg L−1 and an adsorbent dose of 0.5 g L−1. At high pH values, Pb(II) removal over ACB sorption has been inhibited as a result of competition between hydrogen and metal ions, due to which the metal cations had been restricted as an outcome of repulsive force. This indicates that an ion-exchange mechanism is dominant for the sorption process. At pH 7.0, Pb(II) ions can be solvated, hydrolyzed, and polymerized into insoluble hydroxide/oxide species, where precipitation can succeed as shown in Eqs. (1–3) [66], given below:
With an increase in pH, the adsorbent surfaces are negatively charged, so it loses protons from carboxylic acids, enols, and acidic alcohol groups which attract and bind Pb(II). At low pH (< 7), metal ion gets precipitated and form lead hydroxide/oxide. Hence, sorption and absorption are affected by the competition of precipitation; therefore, they cannot be independently studied [5]. The same observation has been documented in the literature with various biomass under similar conditions [67, 68]. As a result, equilibrium and kinetic studies were carried out at an initial pH of 5.
3.5 Effect of initial Pb(II) concentrations
The effect of initial Pb(II) concentration on removal efficiency using activated biochar was studied at pH 5 and a biochar dose of 0.5 g L−1. As shown in Fig. 8, the removal efficiency for Pb(II) concentrations of 10 mg L−1 onto activated biochar was 94% (using equation S1), and the sorption capacity was 9.87 mg L−1 (using equation S2). The increase in removal efficiency (%) of lower concentrations of Pb(II) attributed to the active sites generated at the surface of ACB to the increase in removal efficiency (%) at lower concentrations of Pb(II) [69,70,71].
3.6 Effect of ACB dose on Pb(II) removal
Figure 9 illustrates the effect of ACB dosage on Pb(II) removal at pH 5 ± 0.1, ‘initial lead concentration’ of 50 mg L−1, biochar dosage of 0.5–3.0 g L−1, and temperature of 302 K. The removal effectiveness of Pb(II) increased from ~ 86 to ~ 97% with different biochar dosages at a concentration of 50 mg L−1. Biochar doses increased but Pb(II) ion concentrations in an aqueous solution remained constant due to the abundance of active sites available for Pb(II) sorption. The maximum sorption (~ 97%) was achieved (using equation S1) and the sorption capacity was 9.74 mg g−1 (using equation S2) at 1.5 g L−1 as compared to 0.5 and 1 g L−1 doses of ACB because of a huge number of active sites available over the biochar surfaces. However, the removal efficiency decreased as the dose increased from 1.5 to 3 g L−1 due to less mass transfer at the adsorbate and adsorbent phase in an aqueous solution. Similar findings have been documented in the literature for Pb(II) removal with KOH-activated camphor leaves [72].
3.7 Effect of temperature on Pb(II) removal
Figure 10 depicts the effect of temperature on the efficiency of Pb(II) removal. Pb(II) sorption decreased slightly as temperature increased at pH 5. The removal efficiency (%) of Pb(II) at 302 and 310 K was 86%, while at 320 K it was 77% (using equation S1), and the sorption capacity at 302 and 310 K was 8.58 mg g−1, while at 320 K, it was 7.74 mg g−1 (using equation S2). Therefore, a lower temperature was found to be more advantageous in Pb(II) sorption from an aqueous solution. Pb(II) ions have an exothermic nature, which influences their proclivity to desorb over the biochar. A similar experiment was conducted for the sorption of Pb(II) ions using pecan shell biochar, and the results showed that increase in temperature can reduce the sorption of Pb(II) ions [68].
3.8 Effect of interfering ions
The effect of interfering ions, for example, \({\text{Cl}}^{-}, {\text{NO}}_{3}^{-}, {\text{ SO}}_{4}^{2-}, \; \text{and} \;{\text{PO}}_{4}^{3-}\) was investigated by mixing 50 mg L−1 concentration from different salts, such as NaCl, NaNO3, Na2SO4, and Na3PO4, with 50 mg L−1 of ‘initial lead concentration’ at pH 5 and a biochar dose of 0.5 mg L−1. The co-existing ions negligibly influenced the removal of Pb(II) using ACB in an aqueous solution (Fig. 11). Chloride and nitrate are spherically sorbing ions and have a removal efficiency of 85%, and the sorption capacity was 8.55 mg g−1. As a result, the sorption of Pb(II) has observed the least effect. At the same time, phosphate and sulphate are spherically sorbing ions with removal efficiencies of 76% and 84%, respectively, and sorption capacities of 7.65 mg g−1 and 8.42 mg g−1, which were able to form complexes on the active sites of the functional groups of biochar surfaces as compared with the lead ions.
3.9 Sorption isotherms
Pb(II) sorption equilibrium studies were conducted on ACB at an initial pH of 5 and 302 K temperature. Then all the data were analysed with the help of Langmuir, Freundlich, and Temkin isotherm model along with their correlation coefficient obtained for Pb(II) removal onto activated biochar as given in Table 4. Out of these three sorption models, the Temkin isotherm model best fitted the data (R2 = 0.96). The justification of the Temkin isotherm model as the best-fitted model is indicated by the value of R2 (using Eqs. 4–6).
3.9.1 Sorption kinetics
Pseudo-1st-order and pseudo-2nd-order kinetic models were used to identify the rate parameters required for Pb(II) metal sorption. Pseudo-1st and pseudo-2nd-order kinetic models were employed to simulate Pb(II) sorption on ACB. Table 5 displays the values for k1, qe, and the regression coefficients. The pseudo-1st-order regression coefficient was 0.64 only (using Eq. 7), i.e., poorly correlated between log (qe-qt) vs t. At the same time, the adsorbate amount (on the adsorbent) and the equilibrium amount have a significant impact on pseudo-2nd-order energy. 2nd-order regression coefficients predominate the 1st-order coefficients (0.999) (using Eq. 8).
The qe values and regression coefficients for both kinetics are summarized in Table 5. Where, \({q}_{e}\; \text{and} \;{q}_{t}\) are the sorption capacity of the adsorbent (mg g−1), t is the time (minutes), and K1 and K2 are constant for pseudo-1st-order and pseudo-2nd-order kinetics, respectively.
3.9.2 Sorption thermodynamics studies
The sorption thermodynamics studies show the nature or accuracy of the sorption mechanism, whether endothermic or exothermic. The data was plotted between lnK vs 1/T for the study of Pb(II) sorption over ACB (using Eqs. 9–11).
where R is the “universal gas constant” and T is the absolute temperature. The sorption capacity at 302 and 310 K was 8.58 mg g−1 and 7.74 mg g−1 for 320 K (using equation S2). Sorption on ACB is shown by the Thermodynamic Parameters (ΔH°, ΔS°, and ΔG°) for Pb(II) (Table 6). The negative value of ∆H° (− 25,982.7 kJ mol−1) indicated that the Pb(II) sorption over ACB follows exothermic mechanisms under the experimental conditions, and the negative value of ∆S° (− 70.5649 kJ mol−1) showed a random decrease in the surface area of ACB sorption process. The fact that the ∆G° value is negative which indicates that Pb(II) sorption is spontaneous. When Pb(II) ions were removed utilizing an activated date seed and banana stem, a similar outcome was documented [73, 74].
4 Conclusions and future recommendations
The sorption results demonstrated that Pb(II)sorption was influenced by pH of the solution. From pH 3 to 5, Pb(II) sorption increased dramatically before stabilizing between pH 5 to 7. The electrostatic attraction and repulsion that exist between the Pb(II) and biochar could adequately explain this behavior. The removal efficiency of Pb(II) ions over ACB and equilibrium state of the system were determined by examining the impact of the initial Pb(II) concentration and contact time, respectively. It was discovered that as initial Pb(II) concentrations decreased, the removal efficiency of Pb(II) over the surface of ACB was increased. During the batch sorption experiments, the effect of adsorbent dosage on sorption was investigated, and it was discovered that sorption of Pb(II) across the surface of adsorbents increased with an increase in adsorbent dosage. The improved surface area and more active sites for interactions lead to improved removal efficiency when the dosage of adsorbent increased. At pH 5, the sorption of Pb(II) reduced somewhat with increasing temperature. The removal efficiency (%) of Pb(II) of concentration 50 mg L−1 at 302 and 310 K temperature was found ~ 86% only, whereas the removal efficiency was observed at ~ 77% at 320 K, however, the sorption capacity at 302 and 310 K temperature was 8.58 mg g−1 and it was 7.74 mg g−1 at 320 K temperature.
The sorption equilibrium for Temkin, Langmuir, and Freundlich isotherms has been analyzed and the data fits perfectly well with the Temkin isothermal model confirming the capacities of the multi-layered sorption. A pseudo-1st-order and pseudo-2nd-order kinetics were used to determine the sorption kinetic process. Sorption of pseudo-2nd-order kinetics over an activated biochar has been discovered, which means that Pb(II) sorption over the active biochar surface constitutes a multisectoral process and involves an external sorption and Pb(II) ion diffusion in the interior of the absorbent.
The thermodynamics analysis with the negative value of ∆H° (− 25,982.7 kJ mol−1) indicated that the Pb(II) sorption over activated biochar follows exothermic mechanisms under the experimental conditions, and the negative value of ∆S° (− 70.5649 kJ mol−1) showed a random decrease in the surface area of activated biochar during the sorption process. The negative ∆G° value specifies that the sorption of Pb(II) was feasible and spontaneous. The removal efficiency of Pb(II) was compared between raw biochar and activated biochar. When compared to standard biochar production, biochar modification necessitates more energy and materials, i.e., raising the production cost. As a result, future aims include achieving a balance between optimizing performance by lowering production costs, and creating new technologies and methodologies. Based on this study, the following recommendations are proposed for future studies.
-
As a novel sorbent, nanoparticles tend to agglomerate in aqueous solutions, reducing particle reactivity and making recycling more challenging. Biochar-based compounds can help nanoparticles to operate in a better way by acting as support [75]. Metal/metal oxide-biochar nanocomposites have gained popularity in recent years due to their ability to execute the combined adsorption benefits of biochar and nanomaterials, making them appealing and useful for environmental applications.
-
Because the use of a modifying agent and a different process method increases production costs, procedures must be addressed before scaling up the production and putting it into practice to resolve the cost-adsorption capacity conflict. To obtain better quality products of interest, pyrolysis parameters must be tuned to correspond to different modification processes, making biochar both economically and environmentally beneficial. Furthermore, future advances should aim to improve the modified biochar’s adsorption capacity in the face of interference and competition from single metals and other contaminants in natural wastewater.
-
It’s also important to think about the stability of the modified biochar composite. This necessitates long-term tests to determine whether the composite's bonding strength can withstand scouring and soaking by water while maintaining outstanding adsorption capabilities which may be verified by adsorbent analysis after each cycle of batch experiment.
-
If metals/metal oxides, organics, and nanoparticles are present in water systems, the toxicity of modified biochar should be assessed. Future research should also look into pollutant recovery and the reuse of saturated biochar which may be confirmed by characterization of adsorbent after completion of batch experiment.
Biochar is not only effectively removes Pb(II) from water but also mitigates greenhouse gas emissions by sequestering carbon. Its low-cost production and abundance make it an attractive option for water treatment in resource-constrained regions. By utilizing biochar as an adsorbent, these countries can address water pollution issues affordably while simultaneously reducing their carbon footprint and promoting sustainable development. Therefore, developing low-cost biochar with high adsorption capacity would be beneficial for pollutant remediation, especially in the low-income countries, where water pollution severely impacts the population. Developing biochar capable of adsorbing several heavy metals/water pollutants would be helpful for remediating water with multiple pollutants. Also, such multipurpose materials would be useful for pollution remediation of different locations affected by different pollutants. All these works need to be supported with proper risk analysis prior to large scale and widespread implementation in natural environment.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Morgan CE, Bowling JM, Bartram J, Kayser GL. Attributes of drinking water, sanitation, and hygiene associated with microbiological water quality of stored drinking water in rural schools in Mozambique and Uganda. Int J Hyg Environ Health. 2021;236: 113804.
Nazaripour M, Reshadi MAM, Mirbagheri SA, Nazaripour M, Bazargan A. Research trends of heavy metal removal from aqueous environments. J Environ Manag. 2021;287: 112322.
Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review. J Environ Manag. 2011;92:407–18.
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F, Zamora-Ledezma E, Ni M, Alexis F, et al. Heavy metal water pollution: a fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov. 2021;22: 101504.
Mohan D, Pittman CU, Bricka M, Smith F, Yancey B, Mohammad J, et al. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci. 2007;310:57–73.
Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5:47.
Lu X, Ning X, Lee P-H, Shih K, Wang F, Zeng EY. Transformation of hazardous lead into lead ferrite ceramics: crystal structures and their role in lead leaching. J Hazard Mater. 2017;336:139–45.
Lanphear BP, Roghmann KJ. Pathways of lead exposure in urban children. Environ Res. 1997;74:67–73.
Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng. 2017;5:2782–99.
Zhao M, Xu Y, Zhang C, Rong H, Zeng G. New trends in removing heavy metals from wastewater. Appl Microbiol Biotechnol. 2016;100:6509–18.
Chang Q, Zhang M, Wang J. Removal of Cu2+ and turbidity from wastewater by mercaptoacetyl chitosan. J Hazard Mater. 2009;169:621–5.
Mohan D, Pittman CU Jr. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J Hazard Mater. 2006;137:762–811.
Karunanayake AG, Todd OA, Crowley M, Ricchetti L, Pittman CU, Anderson R, et al. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem Eng J. 2018;331:480–91.
Pawar RR, Kim M, Kim J-G, Hong S-M, Sawant SY, Lee SM. Efficient removal of hazardous lead, cadmium, and arsenic from aqueous environment by iron oxide modified clay-activated carbon composite beads. Appl Clay Sci. 2018;162:339–50.
Yogeshwaran V, Priya AK. Adsorption of lead ion concentration from the aqueous solution using tobacco leaves. Mater Today Proc. 2021;37:489–96.
Chai WS, Cheun JY, Kumar PS, Mubashir M, Majeed Z, Banat F, et al. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod. 2021;296: 126589.
Huang Q, Liu M, Deng F, Wang K, Huang H, Xu D, et al. Mussel inspired preparation of amine-functionalized Kaolin for effective removal of heavy metal ions. Mater Chem Phys. 2016;181:116–25.
Wu J, Wang T, Wang J, Zhang Y, Pan W-P. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: enhanced the ion exchange and precipitation capacity. Sci Total Environ. 2021;754: 142150.
Gul Zaman H, Baloo L, Pendyala R. Application in the optimization of Pb(II) adsorption by chitosan from produced water by using response surface methodology. Int J Environ Sci Technol. 2023;20:197–208.
Xing P, Wang C, Ma B, Chen Y. Removal of Pb(II) from aqueous solution using a new zeolite-type absorbent: potassium ore leaching residue. J Environ Chem Eng. 2018;6:7138–43.
Dong L, Pan S, Liu J, Wang Z, Hou L, Chen G. Performance and mechanism of Pb(II) removal from water by the spent biological activated carbon (SBAC) with different using-time. J Water Process Eng. 2020;36: 101255.
Mohanty SK, Valenca R, Berger AW, Iris KM, Xiong X, Saunders TM, et al. Plenty of room for carbon on the ground: potential applications of biochar for stormwater treatment. Sci Total Environ. 2018;625:1644–58.
Boni MR, Chiavola A, Marzeddu S. Remediation of lead-contaminated water by virgin coniferous wood biochar adsorbent: batch and column application. Water Air Soil Pollut. 2020;231:1–16.
Mohan D, Singh P, Sarswat A, Steele PH, Pittman CU. Lead sorptive removal using magnetic and nonmagnetic fast pyrolysis energy cane biochars. J Colloid Interface Sci. 2015;448:238–50.
Tian S, Gong X, Yu Q, Yao F, Li W, Guo Z, et al. Efficient removal of Cd(II) and Pb(II) from aqueous solution using biochars derived from food waste. Environ Sci Pollut Res. 2023;30:122364–80.
Kundu D, Sharma P, Bhattacharya S, Gupta K, Sengupta S, Shang J. Study of methylene blue dye removal using biochar derived from leaf and stem of Lantana camara L. Carbon Res. 2024;3:22.
Kumar R, Sharma P, Sharma PK, Rose PK, Singh RK, Kumar N, et al. Rice husk biochar—a novel engineered bio-based material for transforming groundwater-mediated fluoride cycling in natural environments. J Environ Manag. 2023;343: 118222.
Kumar R, Sharma P, Rose PK, Sahoo PK, Bhattacharya P, Pandey A, et al. Co-transport and deposition of fluoride using rice husk-derived biochar in saturated porous media: effect of solution chemistry and surface properties. Environ Technol Innov. 2023;30: 103056.
Sinha R, Kumar R, Sharma P, Kant N, Shang J, Aminabhavi TM. Removal of hexavalent chromium via biochar-based adsorbents: state-of-the-art, challenges, and future perspectives. J Environ Manag. 2022;317: 115356.
Brewer CE, Schmidt-Rohr K, Satrio JA, Brown RC. Characterization of biochar from fast pyrolysis and gasification systems. Environ Progr Sustain Energy. 2009;28:386–96.
Mohan D, Pittman CU Jr, Steele PH. Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels. 2006;20:848–89.
Abhishek K, Shrivastava A, Vimal V, Gupta AK, Bhujbal SK, Biswas JK, et al. Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: a state-of-the-art review. Sci Total Environ. 2022;853: 158562.
Sharma P. Biochar application for sustainable soil erosion control: a review of current research and future perspectives. Front Environ Sci. 2024;12:1373287.
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, et al. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19–33.
Hussin F, Aroua MK, Szlachta M. Biochar derived from fruit by-products using pyrolysis process for the elimination of Pb (II) ion: an updated review. Chemosphere. 2022;287: 132250.
Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, et al. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem Eng J. 2019;366:608–21.
Mahdi Z, El Hanandeh A, Yu QJ. Preparation, characterization and application of surface modified biochar from date seed for improved lead, copper, and nickel removal from aqueous solutions. J Environ Chem Eng. 2019;7: 103379.
Kamali M, Appels L, Kwon EE, Aminabhavi TM, Dewil R. Biochar in water and wastewater treatment-a sustainability assessment. Chem Eng J. 2021;420: 129946.
Liu T, Lawluvy Y, Shi Y, Ighalo JO, He Y, Zhang Y, et al. Adsorption of cadmium and lead from aqueous solution using modified biochar: a review. J Environ Chem Eng. 2022;10: 106502.
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, et al. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere. 2015;125:70–85.
Sinha R, Kumar R, Abhishek K, Shang J, Bhattacharya S, Sengupta S, et al. Single-step synthesis of activated magnetic biochar derived from rice husk for hexavalent chromium adsorption: equilibrium mechanism, kinetics, and thermodynamics analysis. Groundw Sustain Dev. 2022;18: 100796.
Sakhiya AK, Anand A, Kaushal P. Production, activation, and applications of biochar in recent times. Biochar. 2020;2:253–85.
Cechinel MAP, Ulson de Souza SMAG, Ulson de Souza AA. Study of lead (II) adsorption onto activated carbon originating from cow bone. J Clean Prod. 2014;65:342–9.
Alvarez J, Lopez G, Amutio M, Bilbao J, Olazar M. Physical activation of rice husk pyrolysis char for the production of high surface area activated carbons. Ind Eng Chem Res. 2015;54:7241–50.
Chae G, Yun S, Mayer B, Kim K, Kim S, Kwon J, et al. Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ. 2007;385:272–83.
Yahya MA, Al-Qodah Z, Ngah CWZ. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev. 2015;46:218–35.
Singh G, Kim IY, Lakhi KS, Srivastava P, Naidu R, Vinu A. Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2 adsorption capacity. Carbon N Y. 2017;116:448–55.
Yang K, Zhu L, Yang J, Lin D. Adsorption and correlations of selected aromatic compounds on a KOH-activated carbon with large surface area. Sci Total Environ. 2018;618:1677–84.
Laksaci H, Khelifi A, Trari M, Addoun A. Synthesis and characterization of microporous activated carbon from coffee grounds using potassium hydroxides. J Clean Prod. 2017;147:254–62.
Zhou Z, Xu Z, Feng Q, Yao D, Yu J, Wang D, et al. Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar. J Clean Prod. 2018;187:996–1005.
Nzediegwu C, Naeth MA, Chang SX. Lead(II) adsorption on microwave-pyrolyzed biochars and hydrochars depends on feedstock type and production temperature. J Hazard Mater. 2021;412: 125255.
Rajapaksha AU, Chen SS, Tsang DCW, Zhang M, Vithanage M, Mandal S, et al. Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere. 2016;148:276–91.
Basta AH, Fierro V, El-Saied H, Celzard A. 2-Steps KOH activation of rice straw: an efficient method for preparing high-performance activated carbons. Bioresour Technol. 2009;100:3941–7.
Imam T, Capareda S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J Anal Appl Pyrolysis. 2012;93:170–7.
Li Z, Zhu Z, Chen Y, Hsu C-G, Pan J. Spectrophotometric determination of lead in biological samples with dibromo-p-methyl-methylsulfonazo. Talanta. 1999;48:511–6.
Khan H, Ahmed MJ, Bhanger MI. A simple spectrophotometric method for the determination of trace level lead in biological samples in the presence of aqueous micellar solutions. Spectroscopy. 2006;20:285–97.
Chun Y, Sheng G, Chiou CT, Xing B. Compositions and sorptive properties of crop residue-derived chars. Environ Sci Technol. 2004;38:4649–55.
Kuhlbusch TAJ. Method for determining black carbon in residues of vegetation fires. Environ Sci Technol. 1995;29:2695–702.
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem. 2011;59:2501–10.
Ahmad M, Lee SS, Dou X, Mohan D, Sung J-K, Yang JE, et al. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol. 2012;118:536–44.
Patel M, Kumar R, Pittman CU, Mohan D. Ciprofloxacin and acetaminophen sorption onto banana peel biochars: environmental and process parameter influences. Environ Res. 2021;201: 111218.
Mohan D, Abhishek K, Sarswat A, Patel M, Singh P, Pittman CU. Biochar production and applications in soil fertility and carbon sequestration—a sustainable solution to crop-residue burning in India. RSC Adv. 2018;8:508–20.
Reig F. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta. 2002;58:811–21.
Li B, Yang L, Wang C, Zhang Q, Liu Q, Li Y, et al. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere. 2017;175:332–40.
Mishra S, Srivastava S, Tripathi RD, Kumar R, Seth CS, Gupta DK. Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere. 2006;65:1027–39.
Burgess J. Metal ions in solution. Ellis Horwood series in chemical science. New York: Wiley; 1978.
Herath C, Gunasekara C, Law DW, Setunge S. Performance of high volume fly ash concrete incorporating additives: a systematic literature review. Constr Build Mater. 2020;258: 120606.
Crisler GB, Burk GA, Simmons P, Quigley M, Mlsna T. Lead removal using biochars obtained from slow pyrolysis of dry and water-soaked pecan shell biomass. Sep Sci Technol. 2020;55:1947–56.
Al-Homaidan AA, Al-Houri HJ, Al-Hazzani AA, Elgaaly G, Moubayed NMS. Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arab J Chem. 2014;7:57–62.
Amin MT, Alazba AA, Shafiq M. Adsorption of copper (Cu2+ ) from aqueous solution using date palm trunk fibre: isotherms and kinetics. Desalin Water Treat. 2016;57:22454–66.
Pranata Putra W, Kamari A, Najiah Mohd Yusoff S, Fauziah Ishak C, Mohamed A, Hashim N, et al. Biosorption of Cu(II), Pb(II) and Zn(II) ions from aqueous solutions using selected waste materials: adsorption and characterisation studies. J Encapsul Adsorpt Sci. 2014;4:25–35.
Li J, Michalkiewicz B, Min J, Ma C, Chen X, Gong J, et al. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture. Chem Eng J. 2019;360:250–9.
Salman JM, Hameed BH. Effect of preparation conditions of oil palm fronds activated carbon on adsorption of bentazon from aqueous solutions. J Hazard Mater. 2010;175:133–7.
Liu G, Li J, Fu J, Jiang G, Lui G, Luo D, et al. An oxygen-vacancy-rich semiconductor-supported bifunctional catalyst for efficient and stable zinc-air batteries. Adv Mater. 2019;31:1806761.
Xu T, Zhou L, He Y, An S, Peng C, Hu J, et al. Covalent organic framework with triazine and hydroxyl bifunctional groups for efficient removal of Lead(II) ions. Ind Eng Chem Res. 2019;58:19642–8.
Wang Y, Zeng X, Feng T, Chen H, Liu G. Efficient removal of Pb(II) from aqueous solution by using an exothermic padding waste derived magnetic adsorbent. Bioresour Technol Rep. 2022;18: 101057.
Zhang H, Fan X, Liu X, He Q, Zhao W, Han L, et al. Green and cost-efficient functionalized clay adsorbent enables one-step ultraefficient removal of Pb(II) at very low and high concentrations. Appl Clay Sci. 2023;238: 106934.
Lakkaboyana SK, Khantong S, Asmel NK, Obaidullah S, Kumar V, Kannan K, et al. Indonesian kaolin supported nZVI (IK-nZVI) used for the an efficient removal of Pb(II) from aqueous solutions: kinetics, thermodynamics and mechanism. J Environ Chem Eng. 2021;9: 106483.
Keskin ZS. Efficient adsorption of Pb(II) ions using novel adsorbent polyacrylamide/coffee ground composite: isotherm, kinetic and thermodynamic studies. Polym Bull. 2023. https://doi.org/10.1007/s00289-023-05111-x.
Acknowledgements
The authors would like to acknowledge the Department of Science and Humanities, Vignan’s Foundation for Science, Technology and Research (Deemed to be University), Vadlamudi 522213, Guntur, Andhra Pradesh, India for providing SEM images of biochar.
Permissions to collect the plants/plant parts
The collection of Syzygium cumini stem used in this study complied with local or national guidelines.
Funding
No funding has been received from any source in this paper.
Author information
Authors and Affiliations
Contributions
Prabhakar Sharma conceived and planned the study, wrote and reviewed the manuscript, and supervised the research. Abhilasha performed the experiments, prepared the figures, and wrote the initial draft of the paper. Kumar Abhishek wrote and reviewed the paper. Sayan Bhattacharya wrote and reviewed the paper. Shubhalakshmi Sengupta prepared SEM images, wrote and reviewed the paper. Chandra Shekhar Seth wrote and reviewed the paper.
Corresponding author
Ethics declarations
The author has read, understood, and complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and is aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Ethics approval and consent to participate
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, P., Abhilasha, Abhishek, K. et al. Removal of lead in water by potassium hydroxide-activated biochar developed from Syzygium cumini stem. Discov Chem Eng 4, 17 (2024). https://doi.org/10.1007/s43938-024-00056-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-024-00056-7