Abstract
Heavy metal contaminated waters are a significant environmental hazard, which require novel treatment methodologies. Further, the metals which might be recovered from these streams have significant intrinsic value, if they can be collected and concentrated. This work demonstrates, for the first time, the use of surfactin, an environmentally benign biosurfactant, for the flotation of metal ions (Cu2+, Ni2+, and Co2+). Surfactin, at a molar ratio of 1:3, has been shown to float 75.2%, 94.7%, and 98.2% of initial 100 \(\mu\) M solutions of copper nickel and cobalt, respectively. Increasing the gas flow rate modifies the float by increasing the carryover of water into the overflow. A pH of 5 was found to be ineffective (due to surfactin precipitation), while a pH of 10 was less effective than pH 7, likely due to the ionic speciation of the metals in solution tending to hydroxyl precipitates at a high pH. This work demonstrates an opportunity for the recovery of metals (at low concentrations) using biosurfactant-based froth flotation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the growth of industrial sectors to cater for the continuously growing global population, there is a proportional growth in waste produced by industry. Heavy metals are common contaminants in waste from mining, metal plating, fertilizer, paper, and dye industries, amongst many others [1,2,3,4]. As such, efficient methods to treat the growing output of heavy metal contaminated wastes are necessary.
While heavy metals can be toxic and dangerous, some are rare metals and hold some economic value if recovered. The valorisation of heavy metal contaminated waste could potentially encourage further treatment of wastewater from industries that may typically engage in discharge of contaminated wastes.
Several conventional treatment methods are typically employed for treatment of heavy metals from aqueous waste streams. Chemical precipitation is a simple and cost-effective process, resulting in its frequent use in the current treatment of heavy metal contaminated wastewater. However, chemical precipitation requires long settling times, large areas, and produces large volumes of sludge that requires problematic downstream treatment[5]. It is also limited in its efficiency and does not produce high purity water. Common alternative processes include ion exchange, membrane filtration, adsorption and reverse osmosis which produces potable water. Unfortunately, these methods often have issues of high costs, high energy requirements, secondary pollutant production, or limited efficiencies with large volumes of dilute solutions, which limit their ease of implementation [1, 6,7,8,9].The waste residues produced by these treatment methods are infrequently treated to recover metals, and are typically subject to end-of-pipe treatment, and disposed in landfills by accredited waste handling companies [10].
Some emerging alternatives to conventional selective extraction methods are under investigation in the literature, for example, adsorbent materials such as metal–organic frameworks (MOFs), molecularly imprinted polymers (MIPs) which show great ion selectivity and stability, and biofilters, which metabolise contaminants from the filtrate. However, common drawbacks of these novel methods include high production and operating costs, process complexity, or potential for secondary pollution, amongst others [9, 11,12,13,14].
Flotation, on the other hand, is a simple and low-cost process, which has been shown to be an effective means of ion removal from aqueous solutions. Many flotation techniques are available, including ion flotation, precipitative flotation, sorptive flotation, dissolved air flotation, and foam fractionation. The method applied, however, should be selected based on the quality of the up-stream solution, the target quality of the treated solution, and the chemistry of the solution [15, 16]. In cases of dilute concentration, precipitative flotation or sorptive flotation may be inefficient, and can produce large volumes of toxic sludge. Ion flotation however is effective at dilute concentrations and produces less sludge, while being simple and energy efficient [5, 6, 17].
Collectors are necessary in flotation to alter the hydrodynamic properties of the material being floated [16]. In ion flotation, oppositely charged collectors bind the desired metal ions (colligends) from solution and incorporate them in a hydrophobic complex that adheres to the surface of bubbles in the liquid [18, 19]. Surfactants typically play the role of both collector and frother in this process [5, 19]. The selection of an appropriate surfactant will depend on both the chemistry of the solution, and the properties of the froth required. The current state of the art uses synthetic surfactants such as sodium dodecyl sulphate (SDS) in ion flotation[5], however, there are environmental impacts associated with these chemicals which negatively impacts the process. There is therefore an opportunity for effective collecting and frothing surfactants that are environmentally benign to improve the environmental impacts of ion flotation.
Biosurfactants which are amphiphiles produced as secondary metabolites by a wide range of microorganisms are such a potential surfactant. Interest has been generated around these molecules due to their biodegradability and their ability to be produced from renewable carbon sources [20,21,22]. These properties are clear benefits over the synthetic chemical surfactants often used in conventional industrial processes. Biosurfactants also show good stability and activity at extreme pH, temperature, and ionic strength making them versatile [21].
Surfactin is a lipopeptide biosurfactant which shows remarkably powerful surface activity, with greater capacity for foaming and foam stability than that of SDS [23]. It has also been shown to be a chelating biosurfactant, meaning that it has the ability to bind metals into metal-surfactin complexes. The carboxylic acid groups present on the hydrophilic heptapeptide moiety of the surfactin have a pKa of around 5.4–5.8 [21, 23], and are anionic at pH greater than 5 [24]. The claw like structure formed by the carboxylate anions allows surfactin to bind metals in a precipitative reaction, producing stable complexes of the metal and surfactin.
Surfactin’s powerful surface activity also lends itself to foam formation which can be exploited for the fractionation of metal surfactin complexes out of aqueous solution. While surfactin has been shown to be effective as a frother in sorptive flotation [25], its chelating ability indicates that surfactin has potential as an effective collector for ion flotation, which is yet to be documented in the literature. This article strives to fill this space in the literature with a novel application of surfactin in ion flotation.This paper demonstrates the collecting (chelation) and foam fractionation capabilities of surfactin and demonstrates its potential application towards removal of metal ions from industrial wastewater. Surfactin is also demonstrated to specifically remove ions from dilute concentrations, potentially positioning this technology as one which can achieve near total removal of ions. With sufficient concentration of heavy metal ions out of the contaminated aqueous solution, there may be potential for the recovery of these heavy metals, which could allow industries to circumvent hazardous and complicated downstream disposal of extracted heavy metals. The impacts of air flow rate, molar ratio, and pH as operating conditions on foam fractionation are presented here to provide insights into key industrial operating criteria.
2 Materials and methods
2.1 Materials
635 mg/L Cu2+, 587 mg/L Ni2+, and a 589 mg/L Co2+ standard solutions were produced using 99% assay sulphate salts of each metal obtained from ScienceWorld and diluted in deionized water. These equate to approximately 10 mM standard solutions of Cu2+, Ni2+, and Co2+. All simulated solutions emulating heavy metal contaminated water used in the experiments were produced from dilutions of these standard solutions. Metal concentrations were modelled against metal concentrations found in industrial waste waters.
Sodium surfactin (90% purity) kindly donated by the Kanaka Corporation was used. Purity was proved by HPLC using a Phenomenex Luna C18 250 mm × 4.6 mm column with UV detection using acetonitrile and water mobile phases at 0.9 ml/min at 30 °C [21]. The surfactin was dissolved in deionized water to produce a 5.18 g/L, or approximately 5 mM standard solution of surfactin that was used in the experiments.
For the purpose of pH control, standard solutions of HNO3 and NaOH were used. A 0.1 M sodium hydroxide solution was produced from 97% assay solid NaOH salt. A 0.1 M HNO3 solution was produced by diluting a 55% nitric acid solution. Both NaOH and HNO3 were obtained from Kimix.
2.2 Ion flotation method
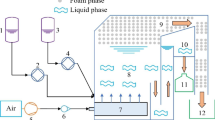
A custom micro-flotation rig after that by Bradshaw and O’Connor [26] was used. The rig was modified to include a sparging stone to provide a greater dispersion and density of bubbles in the cell for ion flotation as opposed to single mineral flotation. Synthetic air (0.21% O2 and 0.79% N2) was used as the flotation gas with the flowrate controlled using a needle valve. The aqueous solution within the column was continuously cycled using a peristaltic pump operating at 300 mL/min, as a method of maintaining homogeneity. A sampling port was present at the base of the column to allow sample collection.
100 M\(\mu\) metal ion solutions with the appropriate ratio of surfactin were produced by diluting 1.5 ml of 10 mM metal standard solution and the appropriate volume of 5 mM surfactin standard solution in deionised water up to 150 ml for flotation. pH was measured using a HANNA Edge pH meter with a general purpose HI11310 double junction electrode and was adjusted dropwise to the required pH using 0.1 M solutions of NaOH and HNO3. The solution was mixed for 30 min using a magnetic stirrer to allow the precipitation reaction to reach equilibrium. The air flowrate was set and allowed to stabilise before adding the solution to the flotation cell. The operating conditions for the ion flotation experimental runs are listed in Table 1. Flotation experiments were run at ambient temperature (22 °C).
The flotation process was allowed to proceed for 50 min, or until the surfactin concentration remaining in the residual solution was insufficient to maintain foam formation. 1 ml samples were taken from the residual solution at 0, 2, 5, 10, 20, 30, 40, and 50 min. A further sample was taken from the final foam overflow and the volume of overflow was also measured after completion of the flotation.Concentration factor (CF) and percentage ion removal (R) from feed were calculated using Eqs. 1 and 2 respectively, where \({C}_{0}\), \({C}_{fl,t}\), and \({C}_{re,t}\) are the ion concentration in the initial solution, the ion concentration in the floated fraction at time \(t\), and the ion concentration in the residual fraction at time \(t\). Concentrations can be calculated in either molarity, or g/L. Molarity was used here because this more effectively compares the reaction chemistry between surfactin and each individual metal ion tested. As a result all concentrations will be reported in \(\mu\) M in the results. The factors mentioned were used as metrics to evaluate the effectiveness of the ion flotation process.
2.3 Characterization
Samples taken from the residual solution and from the foam overflow were adjusted to pH < 1 using a 0.1 M HNO3 solution to break the complex and precipitate the surfactin. This was done to ensure that no complex was trapped during filtration of samples. The samples were filtered using 0.22 μm nylon syringe filters and the samples were sent for metal analysis. The concentration of heavy metal ions was determined by inductively coupled plasma mass spectrometry (ICP-MS) using an Avio 500 ICP from Perkin Elmer.
3 Results
3.1 Comparison of metals’ behaviour
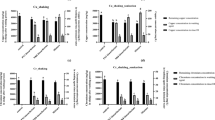
The flotation of each metal was compared at the experimental condition of 0.08 L/min flowrate, pH 7, and a 1:3 ratio of metal ions to surfactin. Results are presented in both Fig. 1 and Table 2.
Results from the experimental ion flotation run with conditions of \({\mathrm{Q}}_{\mathrm{air}}\) of 0.08 L/min, pH 7, and 1:3 initial ion to surfactin ratio. Results include the extent of ion removal (primary axis), the distribution of water recovery in the overflowing foam phase and in the residual solution after flotation (primary axis), and the concentration factor of ions into the overflowed foam fraction (secondary axis)
For all three metals, significant ion recovery is observed with surfactin flotation, with 75% of Cu2+, 95% of Ni2+, and 98% of Co2+ recovered into the overflow after 50 min of flotation, concentrating the ions up to 225 M\(\mu\), 267 M\(\mu\), and 259 M\(\mu\) respectively. This compares well to other surfactants reported in the literature: Polat and Erdogan used SDS as a collector in an ion flotation process to extract copper from solution. At a molar ratio of 1:3.2 Cu2+ to SDS, an ion removal of 74% was achieved. In work by Yuan et al. [27], a tea-derived biosurfactant, saponin, was utilised as a collector in treating copper, cadmium, and lead solutions, where ion removal reached up to 81%, 71%, and 90% respectively at an ion to saponin ratio of 1:3. The Cu2+ ion removal of 75% in this work mirrors that achieved in the literature, and the near complete extraction of Ni2+ and Co2+ at the same conditions shows the promise of this simple process which makes use of an environmentally benign collector.
The effective treatment of the model metal ion contaminated solutions is made clear by the results in Table 2, notably in the cases of Ni2+ and Co2+ where the heavy metal ion concentrations were reduced by 92% and 97% respectively, down to concentrations of only 8 M\(\mu\) and 3 M\(\mu\), less than 0.5 ppm in both cases.Cu2+ was the ion that had the least extraction. In previous experiments investigating the precipitative reaction between heavy metal ions and surfactin [28], it was noted that Cu-surfactin complex precipitate appears to agglomerate more readily than the Ni- and Co-surfactin complexes. The agglomeration may have caused greater backflux of the Cu-surfactin complexes out of the rising froth phase. The possibly greater hydrophobicity of the Cu-surfactin complex may also have promoted bubble coalescence in the froth and reduced froth stability[29]. Figure 2 provides insight into the rate of ion concentration out of the solution into the foam overflow. It is clear that the rate of removal of Cu2+ metal ions is slower than that of Ni2+ and Co2+, which both share a similar rate of removal. It can also be noted that the slope of the Ni2+ and Co2+ curves flatten as time approaches 50 min, meaning the rate of removal for those ions is slowing and removal is approaching completion. However, the rate of Cu2+ ion removal does not appear to be slowing, as the Cu2+ curve maintains a steady slope across the entire 50-min flotation. This indicates that a longer flotation residence time may allow for greater removal of Cu2+ ions, and that the lower removal efficiency for Cu2+ is a result of a slower flotation process.
The fraction of water entrained in the foam and partitioned into the froth overflow, or water recovery, was 32%, 35%, and 39% respectively for the Cu2+, Ni2+, and Co2+ solutions. The lower entrainment of water in the Cu2+ ion flotation supports the hypothesis that Cu-surfactin complex hydrophobicity may be reducing the froth stability. Yuan et al. noted that a saponin biosurfactant collector produces a fine, wet foam[27]. A defrother was suggested, and it was noted that there was little impact on ion removal (within 5%). The described wet foam similarly resulted from the surfactin collector, and as such a defrother may be an option to reduce water recovery. Alternatively, a longer column that would allow greater water drainage from the rising foam due to increased foam residence time may be required [30].
3.2 Effect of air flowrate
Investigation into the impact of air flowrate was performed, with the extent of ion removal, concentration factor, and water distribution between the overflow and residual solution shown in Fig. 3. The ion concentrations in the foam overflow and residual solution are given in Table 3. It is expected that increasing flowrate of air through the flotation cell would lead to an increase in metal ion removal [6], and this appears to be the case initially when increasing \({Q}_{air}\) from 0.06 L/min to 0.08 L/min, as removal of Cu2+ increases from 43 to 75%, removal of Ni2+ increases from 80 to 95%, and Co2+ removal increases from 89 to 98%.
The extent of (i) Cu2+, (ii) Ni2+, and (iii) Co2+ ion removal from aqueous solution (primary axis), the fraction of water present in both the residual unfloated water phase and overflow phase (primary axis), and the concentration factor of metal ions into the foam overflow fraction after ion flotation with surfactin at Qair of 0.06, 0.08, and 0.1 L/min. pH was maintained at 7, and ion:surfactin ratio was initially 1:3
The increase in \({Q}_{air}\) leads to an increased quantity of bubbles, and hence a greater bubble surface area ascending through the flotation cell at any given time, assuming flowrate has little effect on bubble size. This greater bubble surface area explains the increase in ion removal seen when \({Q}_{air}\) increases from 0.06 to 0.08 L/min. The increase in water recovery seen when \({Q}_{air}\) is increased to 0.08 L/min is also likely a result of increasing bubble velocity through the flotation cell, and a similar effect was noted by Jia et al. [5].
A further increase of \({Q}_{air}\) to 0.10 L/min has little impact on the extent of ion removal for both Ni2+ and Co2+, however this is likely as a result of the ion removal being near complete. As such, the % of Ni2+ removed only increased by 1% to 96%, and the removal of Co2+ was still within 1% of the removal at 0.08 L/min. This could indicate that as extraction of ions by ion flotation approaches completeness, the impact of increasing air flowrate decreases.
Observing Fig. 4ii and iii gives insight into the effect of changing air flowrate on the rate of extraction during the flotation process. The flotation rate appears to have little dependence on the air flowrate, with the extraction at 0.08 L/min and 0.10 L/min occurring at similar rates for Ni2+ and Co2+, and the rate of ion extraction only being slightly slower for both ions at 0.06 L/min.
The concentration of metal ions remaining in the residual solution during the ion flotation of a (i) Cu2+ solution, (ii) Ni2+ solution, and (iii) Co2+ solution. The ion flotation experimental runs were done at \({\mathrm{Q}}_{\mathrm{air}}\) of 0.06 L/min, 0.08 L/min, and 0.10 L/min. The pH was controlled at 7, and the initial ratio of metal ions to surfactin was 1:3 in all cases
The notable decrease in ion removal seen for Cu2+ ions can be explained by Fig. 4i, which shows that the flotation of Cu2+ ions at \({Q}_{air}\) = 0.10 L/min effectively stopped after 30 min. At this point the froth was no longer stable and foam overflow also ceased. Whilst the reason for this is unclear, it suggests that all froth-forming surfactin had overflowed at this point. This did not occur with the other metal ion solutions, and the extent of extraction was not as great as that achieved at flowrates of 0.06 L/min and 0.08 L/min, suggesting extraction of Cu-surfactin complexes was not complete.
The concentration factor is dependent not only on the extent of partitioning of ions into the foam fraction, but also the entrainment of water in the foam phase. With greater volumes of water entrained in the foam overflow, the ion concentration becomes more dilute. This is demonstrated in Fig. 3 by the lower concentration factor seen in cases where the percentage of water in the foam overflow is higher. For this reason, the greatest concentration factor for all three metal ion solutions was achieved at the lowest air flowrate of 0.06 L/min, where the least water was recovered in the overflow, despite the ion removal being greater at higher air flowrates.
From these results it appears that the ideal \({Q}_{air}\) would be dependent on the desired outcome of the flotation. Lower air flowrate appears to reliably result in a greater concentration factor (and therefore a more concentrated metal product), at the cost of a slower flotation process and perhaps lower extent of ion removal. If more extensive ion removal is required, it appears that larger air flowrates may be more effective, although this comes at the cost of increased volumes of water becoming entrained in the foam fraction and overflowing, resulting in a reduced concentration factor. In cases where water is being treated to reuse or recycle, or if entrainment of other components in aqueous solution into the foam is undesirable, the flowrate would need to be optimised to allow enough ion extraction without undesirably partitioning large volumes of water into the metal ion rich foam overflow phase.
3.3 Effect of solution pH
The chemistry and activity of surfactin in solution is greatly affected by solution pH. Below around pH 5, the surfactin is protonated and becomes neutral, thereby simultaneously losing its water solubility and metal ion chelating ability. The loss of water solubility and chelating ability is clearly shown in, Fig. 5, where at pH 5 there is no extraction of metal ions from the solution following flotation with any of the heavy metals. In fact, there was little foam formation observed due to the precipitation of the surface active surfactin from the solution, resulting in the entire water volume being present in the residual phase after sparging, as there was insufficient foam formation to generate a foam overflow. The partitioning of some fraction of the metal ions out of the residual solution into the little foam that was formed can be seen in Table 4, however with no overflow, there is no ion removal. This is in line with the literature which states that lower pH and higher concentration of H+ ions reduces recovery in ion flotation due to displacement of the colligend from complexes by H+ [31]. This is therefore not a symptom of the use of a biosurfactant but rather typical of ion flotation using anionic collectors. The protonation of the collector imposes a lower limit on metal recovery using this flotation agent.
The extent of (i) Cu2+, (ii) Ni2+, and (iii) Co2+ ion removal from aqueous solution (primary axis) the fraction of water present in both the residual unfloated water phase and overflow phase (primary axis), and the concentration factor of metal ions into the foam overflow fraction after ion flotation with surfactin at pH 5, 7, and 10. \({\mathrm{Q}}_{\mathrm{air}}\) was maintained at 0.08 L/min, and ion to surfactin ratio was initially 1:3
The greatest extent of ion removal was achieved at pH 7 for all three tested heavy metal ions. Thereafter, as pH increased to 10, the ion removal decreased notably for both Ni2+ and Co2+, while it increased slightly for Cu2+. The lower ions removal is due to the hydrolysis of metal ions at basic conditions. In Fig. 6, it can be seen that as pH approaches 10, all metals begin to form neutral hydroxide species that are insoluble in aqueous solution. As these hydroxide species are neutral, it is expected that little interaction with the anionic surfactin will occur. The extraction seen at pH 10 may be due to the entrainment of their hydroxide species in the foam, however the concentration factors of the metals being between 1.75 and 2.25 suggests that some selective flotation of the hydroxide species may be occurring. Basak and Charewicz [32, 33] speculated that an anionic collector can adsorb to the surface of a neutral Cu(OH)2 and Co(OH)2 species, floating and concentrating that species. This suggests that the flotation process may be effective as a precipitate flotation process at basic pH, however the removal and recovery is not as great as that seen at pH 7, where the process is ion flotation.
Based on these observations, the operating pH of ion flotation with surfactin appears to be limited to near neutral pH, as acidic pH sees the precipitation of surfactin from solution without binding colligends and basic pH leads to formation of neutral metal species that do not interact with the surfactin collector and thereby reduce the ion removal achievable by ion flotation. This means that in cases where aqueous solutions treated by ion flotation are at acidic or basic pH, some pH adjustment may be necessary in order to achieve maximum ion removal or concentration into the froth overflow.
3.4 Effect of metal ion to surfactin ratio
The volume of water entrained in the froth, or water recovery, appears to be strongly dependent on the concentration of foaming surfactant in the solution. The influence of collector structure and frothing ability on water recovery in ion flotation has been shown [34], and increased frother dosage in conventional froth flotation leads to increased water recovery by increasing froth stability [35]. Therefore, the increases in excess surfactin not involved in collecting available as a frother has a strong influence on froth stability and water recovery, where increasing the concentration of the surfactin available as a frother should increase water recovery.
This influence of changing concentrations of excess surfactin can be seen in Fig. 7. In the case, where metal ion to surfactin ratio was initially 1:3, the water recovery for each metal ion solution was between 30 and 40%. When the initial metal ion to surfactin ratio was 1:1, the collector and colligend are present in the theoretical stoichiometric proportions, and as such there was little to no surfactin in excess that could act as a frother and form a stable foam. Without the formation of a stable froth, the flotation process was impossible and so there was no concentration of metal ions out of the solution.
The extent of (i) Cu2+, (ii) Ni2+, and (iii) Co2+ ion removal from aqueous solution (primary axis) the fraction of water present in both the residual unfloated water phase and overflow phase (primary axis), and the concentration factor of metal ions into the foam overflow fraction after ion flotation with surfactin at initial metal ion to surfactin ratios of 1:1, 1:3, and 1:10. \({\mathrm{Q}}_{\mathrm{air}}\) was maintained at 0.08 L/min, and pH was maintained at pH 7
When the ratio of metal ions to surfactin was increased to 1:10, the significant increase in excess surfactin present in the solution had little influence on the dynamics of the metal ion extraction, as can be seen in Fig. 8. The increased removal of Cu2+ ions seen in both Figs. 7i and 8 in addition to surfactin concentrating the copper can potentially be attributed to the increased water recovery at 1:10 ion to surfactin, which leads to greater ion extraction as a result of ion entrainment in overflow water. It may also be a result of the excess surfactin providing a more stable foam, as previous experiments indicated that Cu-surfactin complexes may be more hydrophobic and agglomerate more strongly than those of Ni- and Co-surfactin [28]. As such, Cu-surfactin complexes may destabilise foam more than the complexes of other metals, as hydrophobic complexes lead to bubble coalescence [29].
While the extraction of metal ions at the ratio of 1:10 is closer to complete, with extraction of Cu2+ reaching 94% and that of both Ni2+ and Co2+ reaching nearly 100%, the increase in water recovery results in a far lower concentration factor as the concentrate overflow is diluted. The lower concentration of metal ions in the overflow can also be seen in Table 5, in comparison to the 1:3 metal ion to surfactin ratio case. This means that in applications where nearly complete heavy metal extraction is necessary, a greater excess of surfactin may be desirable. However, in cases where a concentrated overflow fraction is desired, such as if the metal ions were to be recovered after flotation, it would likely be best to optimise the ratio of ions to surfactin to a point where a sufficient fraction of the metal ions are concentrated without encouraging a large water recovery. Alternatively, optimisation of the flotation cell to have a taller column allowing for greater residence time of froth and hence water drainage is an option. Some optimisation of the process is required to achieve the desired recovery and concentration, and these conditions may further be a function of metal species present as well as their concentrations.
Polat and Erdogan [36] used SDS in the ion flotation of Cu2+, and similarly noted that at lower concentrations of the collector, near a 1:1 molar ratio of colligend to collector, there was little froth formation as a result of limited excess collector. At a molar ratio of 1:3.2 Cu2+ to SDS, an ion removal of 74% was achieved. Interestingly, at a higher concentration of SDS, with a molar ratio of near 1:6.4 Cu2+ to SDS, a lower removal of 70% was achieved [36]. Thus, surfactin demonstrated significantly improved metal recovery than other tested surfactants from literature. Yuan et al. [27] also observed that increasing bio-collector to colligend ratio resulted in greatly improved ion removal, as was observed in this work.
4 Conclusions
In order to determine the potential of surfactin as a collector in ion flotation, surfactin was used in experimental ion flotation runs on solutions of 100 mM Cu2+, Ni2+, and Co2+. At a pH of 7, air flowrate of 0.08 L/min, and an initial molar ratio of 1:3 metal ion colligends to surfactin collector, 75% of Cu2+, 95% of Ni2+, and 98% of Co2+ ions were recovered in the foam overflow. A lower air flowrate resulted in reduced ion recovery, but greater concentration in the foam overflow as a result of lower water recovery.
It was determined that the ion flotation process with a surfactin collector has a strong dependence on pH. At low pH, surfactin is protonated and unavailable for ion binding or froth formation. Basic pH resulted in the formation of hydroxide species of metals, however with metal species continuing to be concentrated in the overflow even at basic conditions. It is concluded that the surfactin collector may adsorb to neutral hydroxide species and allow flotation.
Decreasing the concentration of surfactin to an equimolar concentration to the metal ions resulted in limited froth formation, as a result of all surfactin being involved in metal binding and precipitation. This highlights the necessity for an excess of surfactin to act as frother, or the need for an additional frothing or foaming agent to create and stabilise the foam. Increasing the concentration of surfactin to 10 × that of the metal ions resulted in near complete removal of the Ni2+ and Co2+ ions, and greatly improved Cu2+ recovery, however water recovery approximately doubled as a result of the excess surfactin.
These results indicated that greater air flowrates and higher concentrations of surfactin may lead to excellent recovery of metal ions from solution. Optimisation of the flotation cell to increase foam residence time or introduction of a defrother may reduce water recovery and allow greater concentration of metal ions in the overflow.
The successful concentration of heavy metals contaminants from aqueous streams may potentially allow for the collection, and valorisation of these pollutants. Further investigation is necessary to confirm the reversibility of the surfactin-metal complexation and isolation of heavy metals from the mixture, however, recovery of these heavy metals could represent an alternative income for industries employing this methodology, and may circumvent the necessity for disposal of the concentrate.
This investigation demonstrates that surfactin, an environmentally benign, biodegradable surfactant, is a promising collector for ion flotation.
Data availability
Not applicable.
Code availability
Not applicable.
References
Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review. J Environ Manag. 2011;92(3):407–18. https://doi.org/10.1016/j.jenvman.2010.11.011.
Khraisheh MAM, Al-degs YS, Mcminn WAM. Remediation of wastewater containing heavy metals using raw and modified diatomite. Chem Eng J. 2004;99(2):177–84. https://doi.org/10.1016/J.CEJ.2003.11.029.
Bailey SE, Olin TJ, Bricka RM, Adrian DD. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999;33(11):2469–79. https://doi.org/10.1016/S0043-1354(98)00475-8.
Nguyen HTH, et al. Pilot-scale removal of arsenic and heavy metals from mining wastewater using adsorption combined with constructed wetland. Minerals. 2019. https://doi.org/10.3390/MIN9060379.
Jia K, et al. Ion flotation of heavy metal ions by using biodegradable biosurfactant as collector: application and removal mechanism. Miner Eng. 2022. https://doi.org/10.1016/j.mineng.2021.107338.
Liu Z, Doyle FM. A thermodynamic approach to ion flotation. I. Kinetics of cupric ion flotation with alkylsulfates. Colloids Surf A Physicochem Eng Asp. 2001;178(1–3):79–92. https://doi.org/10.1016/S0927-7757(00)00555-0.
Mishra S, Lin Z, Pang S, Zhang Y, Bhatt P, Chen S. Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J Hazard Mater. 2021;418:126253. https://doi.org/10.1016/j.jhazmat.2021.126253.
Dias JM, Alvim-Ferraz MCM, Almeida MF, Rivera-Utrilla J, Sánchez-Polo M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: a review. J Environ Manag. 2007;85(4):833–46. https://doi.org/10.1016/j.jenvman.2007.07.031.
Chai WS, et al. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod. 2021;296:126589. https://doi.org/10.1016/J.JCLEPRO.2021.126589.
Heuss-Aßbichler S, John M, Huber AL. A new procedure for recovering heavy metals in industrial wastewater. Waste Manag Environ VIII. 2016;1:85–96. https://doi.org/10.2495/wm160091.
Rouhani F, Morsali A. Fast and selective heavy metal removal by a novel metal-organic framework designed with in-situ ligand building block fabrication bearing free nitrogen. Chemistry. 2018;24(21):5529–37. https://doi.org/10.1002/CHEM.201706016.
Srivastava NK, Majumder CB. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater. 2008;151(1):1–8. https://doi.org/10.1016/j.jhazmat.2007.09.101.
Alipour S, Azar PA, Husain SW, Rajabi HR. Synthesis, characterization and application of spherical and uniform molecularly imprinted polymeric nanobeads as efficient sorbent for selective extraction of rosmarinic acid from plant matrix. J Market Res. 2021;12:2298–306. https://doi.org/10.1016/J.JMRT.2021.04.021.
Aryan Z, Rajabi HR, Khajehsharifi H, Sheydaei O. Highly selective determination of alanine in urine sample using a modified electrochemical sensor based on silica nanoparticles-imprinted polymer. J Iran Chem Soc. 2022;19(10):4139–48. https://doi.org/10.1007/S13738-022-02589-6/TABLES/2.
Edzwald JK. Dissolved air flotation and me. Water Res. 2010;44(7):2077–106. https://doi.org/10.1016/j.watres.2009.12.040.
Mondal S, Acharjee A, Mandal U, Saha B. Froth flotation process and its application. Vietnam J Chem. 2021;59(4):417–25. https://doi.org/10.1002/vjch.202100010.
Saleem H, Pal P, Haija MA, Banat F. Regeneration and reuse of bio-surfactant to produce colloidal gas aphrons for heavy metal ions removal using single and multistage cascade flotation. J Clean Prod. 2019;217:493–502. https://doi.org/10.1016/j.jclepro.2019.01.216.
Tavera FJ, Escudero R. Flotation of colloidal lead carbonate using series of spargered flotation cells. In: 2006 TMS Fall Extraction and Processing Division: Sohn International Symposium, vol. 3, no. 3, 2006; pp. 397–407.
Grieves RB, Bhattacharyya D, Ghosal JK. Surfactant-colligend particle size effects on ion flotation: influences of mixing time, temperature, and surfactant chain length. Colloid Polym Sci. 1976. https://doi.org/10.1007/BF01410918.
Fiechter A. Biosurfactants: moving towards industrial application. Trends Biotechnol. 1992;10:208–17.
Augustyn AR, Pott RWM, Tadie M. The interactions of the biosurfactant surfactin in coal flotation. Colloids Surf A Physicochem Eng Asp. 2021. https://doi.org/10.1016/J.COLSURFA.2021.127122.
Czinkóczky R, Németh Á. Techno-economic assessment of Bacillus fermentation to produce surfactin and lichenysin. Biochem Eng J. 2020;163:107719. https://doi.org/10.1016/j.bej.2020.107719.
Arutchelvi J, Sangeetha J, Philip J, Doble M. Self-assembly of surfactin in aqueous solution: Role of divalent counterions. Colloids Surf B Biointerfaces. 2014;116:396–402. https://doi.org/10.1016/j.colsurfb.2013.12.034.
Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NAH. Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl Biochem Biotechnol. 2008;150(3):289–303. https://doi.org/10.1007/s12010-008-8153-z.
Zouboulis AI, Matis KA, Lazaridis NK, Golyshin PN. The use of biosurfactants in flotation: application for the removal of metal ions. Miner Eng. 2003;16(11):1231–6. https://doi.org/10.1016/j.mineng.2003.06.013.
Bradshaw DJ, O’connor CT. Measurement of the sub-process of bubble loading in flotation. Miner Eng. 1996;9(4):443–8.
Yuan XZ, Meng YT, Zeng GM, Fang YY, Shi JG. Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf A Physicochem Eng Asp. 2008;317(1–3):256–61. https://doi.org/10.1016/J.COLSURFA.2007.10.024.
Pott RWM, Tadie M, Schlebusch LD. A method of removing metal ions from solution. UK Intellectual Property Office. 2022; Patent No. 2214049.5
Hunter TN, Pugh RJ, Franks GV, Jameson GJ. The role of particles in stabilising foams and emulsions. Adv Colloid Interface Sci. 2008;137(2):57–81. https://doi.org/10.1016/J.CIS.2007.07.007.
Wang L, Runge K, Peng Y. The observed effect of flotation operating conditions and particle properties on water recovery at laboratory scale. Miner Eng. 2016;94:83–93. https://doi.org/10.1016/J.MINENG.2016.05.003.
Rubin AJ, Donald Johnson J. Effect of pH on ion and precipitate flotation systems. Anal Chem. 1967;39(3):298–302.
Basak S, Charewicz WA. Flotation of metal hydroxide precipitates. 11. Flotation of zinc and cobalt hydroxides. J Chem Tech Biotechnol. 1986;36:557–61.
Basak S, Charewicz WA. Flotation of metal hydroxide precipitates. I. Flotation of copper hydroxide. J Chem Tech Biotechnol. 1986;36:74–8.
Hoseinian FS, Rezai B, Kowsari E, Safari M. The effect of water recovery on the ion flotation process efficiency. Physicochem Probl Miner Process. 2020;56(5):919–27. https://doi.org/10.37190/PPMP/126990.
Wiese J, Harris P. The effect of frother type and dosage on flotation performance in the presence of high depressant concentrations. Miner Eng. 2012;36–38:204–10. https://doi.org/10.1016/J.MINENG.2012.03.028.
Polat H, Erdogan D. Heavy metal removal from waste waters by ion flotation. J Hazard Mater. 2007;148(1–2):267–73. https://doi.org/10.1016/J.JHAZMAT.2007.02.013.
Funding
The authors would like to acknowledge funding support from the Wilhelm Frank Trust South Africa.
Author information
Authors and Affiliations
Contributions
IS: Methodology, Investigation, Writing—Original Draft. MT: Conceptualization, Writing—Review & Editing, Supervision, Project administration, Funding acquisition. RP: Conceptualization, Writing—Review & Editing, Supervision, Project administration, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlebusch, I., Pott, R.W.M. & Tadie, M. The ion flotation of copper, nickel, and cobalt using the biosurfactant surfactin. Discov Chem Eng 3, 7 (2023). https://doi.org/10.1007/s43938-023-00023-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-023-00023-8