Abstract
This study aimed to assess the performance of hydroponics vegetated with Duranta erecta in removing nutrients from domestic wastewater in a pilot-scale study. Domestic wastewater was fed to four lines of hydroponic-constructed filter systems (HCFS). The lines 1 and 3 were filled with gravel, but the lines 2 and 4 were filled with polyester sponges. The experimental biofilters (lines 1 and 2) were planted with Duranta erecta, and the control biofilters (lines 3 and 4) were unplanted. This experiment was operated at hydraulic retention times (HRT) of 1, 3, 5, and 7 days which were conducted sequentially. Results indicated that the planted biofilters in gravel media removed nutrients better than the other planted biofilters in sponge media. Better removal efficiencies of 55 and 47% for total nitrogen (TN) and of 32 and 26% for total phosphorus (TP) removal by planted and control lines, respectively, were obtained at 7 days HRT. At the end of the experiment, TN and TP accumulation in the plant biomass sample increased from 132 to 216 g TN/Kg of dry weight, and from 53to 86 g TP/Kg of dry weight, respectively at 7 days HRT. Hence, it can be concluded that nature-based hydroponic filters planted with Duranta erecta might have a promising potential, mainly with gravel media. HCFS represents a very welcome technology to treat domestic wastewater at a decentralized level in developing countries, including Ethiopia.
Highlights
-
HCFS planted with Duranta erecta in gravel or polyester sponge media removed nutrients from domestic wastewater.
-
HCFS significantly (p<0.05) removed total nitrogen and total phosphorus from wastewater.
-
HCFS are sustainable and eco-friendly wastewater treatment solutions.
-
Gravel media is a better medium than polyester sponge to reduce nutrients in hydroponics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Untreated wastewater discharge into receiving water bodies poses threats to the public's health, the economy, and ecological integrity. In developing countries, including Ethiopia, only a small proportion of the wastewater is being treated. Poorly treated institutional effluents that leak into surface and groundwater sources are the main cause of poor water quality [1]. Domestic wastewater mainly composed of grey and black waters can be generated from homes, businesses, and institutions such as hospitals, schools, prisons, etc [2,3,4]. In Ethiopia, many institutions discharge untreated wastewater into lakes, wetlands, rivers, and streams, endangering the environment and posing a risk to downstream riverine communities [1, 5]. Kilinto prison camp discharges its wastewater into the adjoining little river called Akaki River, where animals drink and irrigation is carried out along its downstream and vicinity using this dirty river. Untreated domestic wastewater is also a cause of eutrophication problems (excess algal growth), which is caused by the presence of nutrients (nitrogen and phosphorus) in wastewater [6, 7].
Ammonia is the main form of nitrogen in wastewater, which increases BOD, decreases DO, and kills aquatic animals including fish [8]. Nutrients stimulate the growth of toxic phytoplankton species in water bodies [9, 10]. The consumption of fish and algae, which are contaminated by toxic phytoplankton, can cause serious health problems for both humans and animals. Skin irritation, nervous system impairment, liver damage, and gastrointestinal diseases are caused by toxic phytoplankton [11]. The other impact of the discharged wastewater is bad odor which is caused by the formation of hydrogen sulfide (H2S) due to the depletion of dissolved oxygen (DO). This in turn will cause toxicity and decrease the aesthetic value of the environment [12, 13].
Similar to other developing nations, there are experiences of wastewater treatment in Ethiopia though not satisfactory. There are promising practices in industrial parks for effective wastewater management, but studies in 14 towns revealed that there are no wastewater treatment facilities. Lack of investment cost, proper design and skilled labor, and lack of integration among relevant stakeholders might be major factors [1]. Wastewater treatment technologies should be properly applied and policies and strategies should support proper domestic and industrial wastewater disposal and utilization techniques [14]. Wastewater discharged from Addis Ababa municipality and the existing wastewater treatment plant does not meet environmental requirements [15].
There are technologies available for treating domestic wastewater to levels sufficient for reclaimed use of water, but they are expensive to construct and operate [16]. Therefore, the use of low-cost technology including constructed wetlands has increased [17]. Furthermore, an up-flow anaerobic sludge blanket followed by constructed wetlands is a promising technology to increase the suitability of reclaimed wastewater use [18]. Hydroponics is also similar to constructed wetlands technology in use for domestic wastewater treatment with its advantages as has been presented hereunder.
Hydroponics is a method of growing plants in a liquid nutrient solution without soil and with or without the use of artificial media [19,20,21]. The media can serve as structural support for the plant. It also serves as a filter media and environment for microbes [22]. Bioponics is using wastewater as a nutrient solution in hydroponic technology [23]. It has been reported that vertical hydroponics vegetated with Duranta erecta can remove heterotrophic bacteria, total coliform, and fecal coliform up to 98.7, 96.2, and 92.9%, respectively [4]. This indicates that it has the potential to be used as a decentralized wastewater treatment technology in developing countries [24]. Hydroponics has many advantages such as low operational and maintenance costs, high treatment capacity, low land requirement, and increased aesthetic value of the environment [21, 25].
There are many ornamental plants in Ethiopia such as Ephorbia Cotinifolia, Cordyline Terminalis, Nerium Oleander, Pinus Patula, and Duranta Erecta. However, none of these plants are specifically used to remove nutrients from wastewater. Duranta Erecta is one of the widely grown ornamental plants in Ethiopia. This plant is usually used to decorate homes and public parks [26]. Duranta Erecta is a multi-stemmed fast-growing plant that can tolerate different environmental factors such as drought and infertile soil [27, 28]. These properties of the plant make it preferable for hydroponics wastewater treatment.
It is noted that a few researches have been conducted regarding the use of hydroponics for the treatment of wastewater [4, 29]. However, no research has been carried out regarding nutrient removal using Duranta erecta planted HCFC. Moreover, no research has been conducted on using gravel and polyester sponges separately as media in a vertical filter arrangement to remove nutrients from wastewater. Therefore, this study aimed to assess the treatment performance of pilot-scale hydroponic systems with gravel or polyester sponge beds vegetated with Duranta Erecta for removing TN and TP from domestic wastewater.
2 Materials and methods
2.1 Experimental site
The experiment was conducted in the Akaki Kality sub-city, Addis Ababa, Ethiopia which is located at an altitude, annual, average temperature, rainfall, and humidity of 2300 m, 15.9 °C, 1089 mm, and 60.7%, respectively.
2.2 Experimental setup
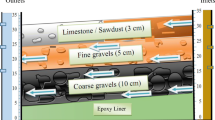
The pilot scale consisted of one reservoir tank (RT), two experimental and two control lines arranged outdoors in parallel rows, each containing three biofilters as shown in Fig. 1 [4]. Each treatment line holds 60 L of wastewater (20 L per biofilter). Thirty healthy plants were collected from the Afincho-ber area, Addis Ababa. The study followed a standard method during plant sample collection [30]. Then, they were planted in experimental line 1 (E1) filled with gravel media, and experimental line 2 (E2) filled with sponge media (five plants per biofilter) after carefully washing the plant’s roots and cutting them until the root length became 11 cm. Forestry tubes (Fig. 2), with 11 cm depth, 8 cm diameter, and 345 cm3 volume, were used to support the plant and the media by suspending on the biofilter’s lids. Five forestry tubes were used for each biofilter. A small hole was drilled in the bottom and wells of the forestry tubes so that the root could grow deep into the nutrient solution (wastewater) by passing through the holes of the forestry tube (Fig. 2). In each forestry tube of lines E1 and C1, sharp gravel (20 mm diameter), which has a mass of 387.28 g and porosity of 44%, was used as a medium. Similarly, in each forestry tube of lines E2 and C2, a polyester sponge, which has a mass of 29.28 g and a porosity of 68%, was used as a medium [4].
Acclimatization was conducted for ninety days. First, tap water was introduced, and then it was diluted gradually by 5, 15, 20, 30, 50, 80%, and finally 100% wastewater to avoid possible shock or stress to the plants due to high concentrations of pollutants in the wastewater [15].
Where: 1, 2 & 3 denote biofilters in experimental line 1 (E1), 4, 5 & 6 denote biofilters in experimental line 2 (E2), 7, 8 & 9 denote biofilters in control line 1 (C1), 10, 11 & 12 denotes biofilters in control line 2 (C2), RT denotes the reservoir tank and, A, B, C, D, E are sampling ports.
The pilot scale of the treatment system during the experiment period, the biofilter, and the forestry tube are depicted in Fig. 2.
2.3 Experimental operation
The raw wastewater, which is collected from the direct discharge point of Kilinto prison camp, was fed into the reservoir tank (RT), which holds 240 L of wastewater. It was distributed to the 2 experimental lines and 2 control lines. Since the biofilters have a vertical arrangement, the flow of wastewater from the main tank down to the biofilters was driven by gravity. The flow was adjusted by tap valves, which have an opening of 6 mm. The wastewater treatment was operated at HRT for 1, 3, 5, and 7 days in a continuous mode sequentially [31, 32]. The operating conditions of the treatment system are summarized in Table 1.
2.4 Wastewater sampling and analysis
The effluent of the Kilinto prison camp effluent was characterized for 4 months (one sample per month) using a composite sampling method (an equal amount of sample was taken in the morning, noon, and afternoon, then the samples were mixed). The samples were taken during 2 months of the Ethiopian wet season (August and September), followed by 2 months of the dry season (October and November). The temperature and pH were measured onsite while the total nitrogen (TN) and total phosphorus (TP) were analyzed in a laboratory using standard methods.
To assess the treatment potential of the hydroponics, per each HRT, a grab sampling method (one sample was taken from the treated effluent of experimental and control units) was used. A triplicate sample analysis was conducted and analyzed according to standard methods for the examination of water and wastewater [33]. The temperature and pH were measured by a portable pH/temperature meter (Model HANA 83141). The TN and TP were immediately analyzed by spectrophotometer (Model Hatch DR3900) using the 4500-TN persulfate digestion method, and 4500-TP Molybdovanadate with Acid Persulfate Digestion Method respectively.
2.5 Plant sampling and analysis
The taxonomy of Duranta erecta indicates that it is in the kingdom (Plantae), subkingdom (Viridiplantae), infrakingdom (Streptophyta), superdivision (Embryophyta), division (Tracheophyta), subdivision (Spermatophytina), class (Magnoliopsida), superorder (Asteranae), order (Lamiales), family (Verbenaceae), genus (Duranta), and species (Duranta erecta) [34].
The dry weight of the plant (DW), fresh weight of the plant (FW), expected dry weight of the plants per given biofilter (EDW), expected fresh weight of the plants per a given biofilter (EFW), and nutrient accumulation (nitrogen and phosphorus) of the plants were analyzed both at the start and the end of the experiment. At the start of the experiment, all 30 plant biomasses had relatively similar morphology, therefore one plant biomass was selected as representative of plant biomass to analyze the DW, FW, EDW, EFW, and nutrient concentration. However, at the end of the experiment, the plant morphology is affected by the availability of the nutrient. Therefore, one plant sample per biofilter of the experimental units was analyzed for DW, FW, EDW, EFW, and nutrient concentration. The dry mass and fresh mass of the plant were analyzed according to standard methods for plant sampling, measurement, and observation [35]. A random method was employed to select sample plants per biofilter and Eqs. 1–4 were used to calculate DW, FW, EDW, and EFW [36]:
where: DW denotes the dry weight of the plant, DWS denotes the dry weight of the sample plant, FW denotes the fresh weight of the plant, FWS denotes the fresh weight of the sample plant, A denotes the area under consideration, EDW denotes expected dry weight of the plant in a biofilter and EFW denotes the expected fresh weight of the plant in a biofilter.
The survival rate of the plant was calculated using Eq. 5:
where: SR denotes the survival rate of the plants per biofilter, I denotes the number of plants at the start of the experiment and F denotes the number of plants at the end of the experiment.
The TN and TP in the plants were analyzed using the Kjeldahl method and spectrophotometric vanadium phosphomolybdate method respectively [37]. Then the TN and TP in each biofilter were calculated using Eqs. 6 and 7 respectively [38].
2.6 Data analysis
The data were analyzed by Microsoft Excel 2019. One-way analysis of variance (ANOVA) was used with a 95% confidence interval to assess the treatment potential of the hydroponics. Regression analysis was used to determine the correlation between HRTs and the removal efficiency of the treatment system. The removal efficiency was calculated using Eq. 8 [39]:
where: \({\upeta }\) (%) denotes the removal efficiency, C1 (mg/l) denotes influent concentration and C2 (mg/l) denotes effluent concentration of the nutrients.
3 Result and discussion
3.1 Characteristics of the wastewater
The mean prison camp effluent temperature varied from 20.70 ± 2.43 to 23.60 ± 2.73 °C and the effluent pH varied from 5.57 ± 0.21 to 7.98 ± 0.59. The mean prison camp effluent concentration of TN and TP varied from 31.60 ± 11.51 to 84.30 mg/L and from 15.42 ± 6.36 to 19.80 ± 6.62 mg/L respectively, as shown in Table 2.
From Table 2, it can be noted that TN and TP concentrations of the prison camp effluent increased from August to November. This might be due to the decrement of rainfall intensity, which in turn decreased the dilution of the effluent by the rainfall [40]. The TN and TP concentration of the effluent is above the maximum allowable discharge standard, therefore treating this effluent before discharging into the environment is mandatory. The temperature and pH levels of the prison camp effluent are within allowable discharge standards.
3.2 Performance of the hydroponics
3.2.1 Temperature and pH
During the experiment, the mean influent temperature varied from 19.80 ± 0.61 to 23.00 ± 0.73 °C at HRT of 1, 3, 5, and 7 days. The mean effluent temperature of experimental and control lines (E-1, E-2, C-1 and C-2) varied from 17.50 ± 0.74 to 19.20 ± 0.56 °C, 18.20 ± 0.76 to 19.00 ± 0.62 °C, 18.80 ± 0.57 to 20.00 ± 0.83 °C and 19.30 ± 0.64 to 21.00 ± 0.61 °C, respectively at HRT of 1, 3, 5 and 7 days (Fig. 3A). A reduction of temperature in effluents was observed than that of influents in all experimental and control lines. E-2 showed a better performance in decreasing the temperature of the effluent followed by E-1, C-1, and C-2, respectively at HRTs of 1 and 3 days. However, E-1 showed a better performance in decreasing the temperature of the effluent followed by E-2, C-1, and C-2, respectively at HRTs of 5 and 7 days. During the experiment, the mean influent pH varied from 5.97 ± 0.32 to 7.93 ± 0.30 at HRT of 1, 3, 5, and 7 days. The mean effluent pH of E-1 (planted biofilters with gravel), E-2 (planted biofilters with polyester sponge), C-1 (unplanted biofilters with gravel), and C-2 (unplanted biofilters with polyester sponge) varied from 6.70 ± 0.43 to 7.80 ± 0.37, 7.50 ± 0.43 to 8.00 ± 0.32, 7.90 ± 0.31 to 8.20 ± 0.35, and 7.70 ± 0.64 to 8.20 ± 0.39 respectively at HRTs of 1, 3, 5 and 7 days (Fig. 3B).
A slight increment of pH in effluents was observed than that of influents in all experimental and control lines (Fig. 3B). This might be due to the consumption of organic acid by microorganisms in the remediation process [24].
3.2.2 Nutrient removal
The mean influent TN and TP concentration during the experiment varied from 122.74 ± 9.07 to 86.743 ± 10.38 mg/L and 33.46 ± 1.32 to 7.64 ± 2.91 mg/L at HRTs of 1, 3, 5, and 7 days respectively. The mean effluent TN and TP concentration during the experiment varied from 88.36 ± 7.71 to 55.72 ± 9.87 mg/L and 22.71 ± 3.67 to 6.26 ± 3.54 mg/L in E-1, 94.52 ± 7.02 to 69.11 ± 7.88 mg/L and 24.86 ± 2.61 to 6.53 ± 3.41 mg/L in E-2, 101.90 ± 8.70 to 77.24 ± 7.88 mg/L and 27.68 ± 2.42 to 7.03 ± 1.26 mg/ in C-1, and 122.56 ± 8.12 to 85.28 ± 8.18 mg/L and 29.97 ± 2.15 to 7.12 ± 3.64 mg/L in C-2 respectively (Table 3).
A reduction in the concentration of TN in effluents was observed than that of influents in all experimental and control lines. The removal of TN in E1 and E2 might be caused by ammonification, nitrification, assimilation by plants and microbes, denitrification, and adsorption [40, 41]. The removal of TN in C1 might be caused by adsorption, ammonification, nitrification, and denitrification. The removal of TN in C2 might be caused by ammonification, nitrification, denitrification, and adsorption. It is believed that volatilization and nitrate ammonification might not play a significant role in nitrogen removal since volatilization requires an environment whose pH > 11 and nitrate ammonification requires anoxic conditions [42, 43]. In this study, the mean pH of influents from the reservoir tank (RT) ranged from 5.97 to 7.93. The pH of Kilinto prison camp effluent is not in the range of pH that causes ammonia volatilization. Moreover, the diffusion of dissolved oxygen (DO) from the atmosphere to the vertical arrangement of the biofilters, and the diffusion of oxygen from the rhizosphere increase DO concentration and reduce the probability of anoxic conditions in the biofilters. This in turn prohibits nitrate ammonification.
A reduction in the concentration of TP in effluents was observed than that of influents in all experimental and control lines. The removal of TP in E1 and E2 might be caused by the adsorption of phosphate by the plant, sedimentation, and adsorption on the surface of the plant and media [44]. Microorganisms, specifically chemoautotrophs, play a vital role in decomposing organic phosphorus into phosphate so that the plants can absorb it. Chemohetrotrophs use organic matter as a source of carbon and energy. The removal of TP in C1 might be caused by sedimentation, microbial decomposition of organic phosphorus, and adsorption. The removal of TP in C2 might be caused by sedimentation, adsorption, and microbial decomposition of organic phosphorus. The plant root and the media might also play a significant role in removing TN and TP from the wastewater by providing surface area for the adsorption of nutrients, and habitat for microorganisms.
The removal efficiency of the treatment system in removing TN ranged from 19.95–54.60% for E1, 14.37–47.45% for E2, 7.68–27.82% for C1, and 2.65–5.21% for C2 (Fig. 4). The removal efficiency of C2 in removing TN decreased as HRT increased from 1 to 7 days. This might be because of the fact that even though polyester sponge is more porous than gravel, its voids are not connected (it has low water permeable properties). Therefore, it takes time for the effluent to pass through the voids of this media during the treatment process. This affects the treatment potential of C2. In addition, there are no plants in this experimental line, which enhances the pollutant removal potential. Moreover, at lower HRT, there are relatively higher organic loadings [45]. The organic loading decreases as HRT increases. This affects the capacity of microbes to remove nitrogen. Except for nitrifying bacteria, which are autotrophic, heterotrophic bacteria are responsible for most nitrogen cycles such as ammonification-ammonifying bacteria, nitrogen fixation-nitrogen fixing bacteria, denitrification-denitrifying bacteria, etc. [46]. Hence, the decrease in HRT will affect the removal of nitrogen.
The removal efficiency of the treatment system in removing TP ranged between 12.6–32.1% for E1, 10.73- 25.70% for E2, 6.52–17.09% for C1, and 3.20–10.43% for C2 (Fig. 5).
E1 showed a better TN and TP removal efficiency than E2 in all applied HRTs. This might be due to better provision of adsorption sites by gravel media than that by polyester sponge media, due to the close porosity of the last one [47]. In addition, gravel could provide a better environment for the attached microorganisms. Then E1 and E2 showed better TN and TP removal efficiency than their corresponding control lines did.
Regression analysis showed that there is a strong linear correlation between the applied HRTs and the removal efficiency of the treatment system in removing TN and TP. In the removal of TN, regression analysis showed R2 values of 0.96, 0.89, 0.94, and 0.24 for E1, E2, C1, and C2 respectively. In the removal of TP, regression analysis showed R2 values of 0.94, 0.89, 0.91, and 0.88 for E1, E2, C1, and C2 respectively. This indicates that the removal of nutrients depends on HRT, which in turn depends on the hydraulic loading rate (HLR). The ANOVA indicates that there is a significant difference (p < 0.05) between E1 and C1, between E2 and C2, in TN and TP removal. However, no significant difference (p > 0.05) was observed between E1 and E2, C1 and C2.
As shown in Table 4, the TN and TP removal efficiency of this study is compared with previous studies using other kinds of media and plant species.
From Table 4, it can be noted that the Duranta erecta planted hydroponics have relatively good performance in removing TN and TP from wastewater.
3.2.3 Plant nutrient accumulation
Changes in the plant survival rate (PSR) and the plant nutrient accumulation were observed in both experimental lines. The PSR in E-1 biofilter 1 (E1BF1) was 80%, in E-1 biofilter 2 (E1BF2) was 100% and in E-1 biofilter 3 (E1BF3) was 100%. The PSR in E-2 biofilter 1 (E2BF1) was 80%, in E-2 biofilter 2 (E2BF2) was 80% and in E-2 biofilter 3 (E2BF3) was 100%.
Then a reduction in the number of plants at the end of the experiment was observed in E1BF1, E2BF1, and E2BF2. It’s due to the reduction of PSR, which is caused by individual plant health conditions. In addition, the ability of the plants to adapt to high organic loading might also be the reason for the reduction of PSR [42]. Table 5 shows the EDW, EFW, TN, and TP accumulation by the plants in biofilters 1, 2, and 3 at the start and the end of the experiment.
An increase in EDW, EFW, and nutrient accumulation (TN and TP) was observed in both experimental units at the end of the experiment (Table 5). Plants in line E1 showed better biomass growth than plants in line E2. The maximum TN and TP accumulation of the plants was recorded in E1BF3 followed by E2BF3. At the end of the experiment, TN and TP accumulation in E1BF3 increased from 132.34 to 216 gN/Kg DW and 52.9 to 86 gP/Kg DW, respectively. TN and TP accumulation of plants in E2BF3 increased from 132.34 to 189 gN/Kg DW and 52.9 to 75.6 gP/Kg DW, respectively.
In both experimental lines (E1 and E2), the values of EDW, EFW, TN and TP accumulation in units of rank 1 at the end of the experiment (E1BF1 and E2BF1) were less than the values at the beginning of the experiment. This might be due to the reduction of the number of plants in biofilters of rank 1 to that of the number of plants in biofilters of rank 2 and 3. This might be due to the number of plants not decreasing in biofilters of rank 2 and 3 (except E2BF2). In addition, the plants in biofilters of rank 2 and 3 were less stressed due to the presence of relatively low nutrient content. This allowed the plants to develop better biomass, which in turn allowed them to accumulate more nutrients (Gebeyehu et al. [42]).
4 Conclusion
Our study has proved that the hydroponics planted with Duranta erecta removed nutrients (nitrogen and phosphorus) significantly (p < 0.05). In all applied HRT, the experimental line E1 (using gravel media) showed higher performance in removing both TN and TP followed by the experimental line E2 (using sponge media), and the control lines C1 and C2. At optimum HRT (7 days), a TN removal of 55 and 47% was achieved in the experimental lines E1 and E2, respectively. Similarly, at optimum HRT, a TP removal efficiency of 32 and 26% was achieved in the experimental lines E1 and E2, respectively. Regression analysis showed that there is a strong linear correlation between the applied HRTs and the removal efficiency of the treatment system. The maximum TN and TP accumulation by the plants was recorded in the experimental line E1 (biofilter 3) followed by the experimental line E2 (biofilter 3). During the experiment, TN and TP accumulation by plants in the experimental line E1 (biofilter 3) increased from 132 to 216 gN/KgDW, and from 53 to 86 gP/KgDW, respectively. TN and TP accumulation by plants in the experimental line E2 (biofilter 3) increased from 132 to 189 gN/KgDW, and from 53 to 76 gP/KgDW, respectively. Hence, hydroponics significantly (p < 0.05) removed TN and TP from domestic wastewater, mainly using gravel as media for plants. Then it has a promising potential to be used as an alternative decentralized wastewater treatment technology for developing countries such as Ethiopia.
Data availability
All data are available in this study.
References
Adugna D. Challenges of sanitation in developing counties-evidenced from a study of fourteen towns, Ethiopia. Heliyon. 2023. https://doi.org/10.1016/j.heliyon.2023.e12932.
Oladoja NA. Appropriate technology for domestic wastewater management in under-resourced regions of the world. Appl Water Sci. 2017;7(7):3391–406.
Agarwal S, Darbar S, Saha S. Challenges in management of domestic wastewater for sustainable development. In: McLeman Robert, editor. Current directions in water scarcity research. Amsterdam: Elsevier; 2022. p. 531–52.
Tibebu S, Worku A, Angassa K. Removal of pathogens from domestic wastewater using small-scale gradual hydroponics planted with duranta erecta, Addis Ababa, Ethiopia. J Environ Public Health. 2022;2022:3182996. https://doi.org/10.1155/2022/3182996.
Teshome FB. Municipal solid waste management in Ethiopia; the gaps and ways for improvement. J Mater Cycles Waste Manag. 2021;23:18–31.
Preisner M. Surface water pollution by untreated municipal wastewater discharge due to a sewer failure. Environ Process. 2020;7(3):767–80.
Menberu Z, Mogesse B, Reddythota D. Evaluation of water quality and eutrophication status of Hawassa Lake based on different water quality indices. Appl Water Sci. 2021;11:1–10.
Njiru JM, Aura CM, Okechi JK. Cage fish culture in lake victoria: a boon or a disaster in waiting? Fish Manag Ecol. 2019;26(5):426–34.
Li H, Xia Q, Wen S, Wang L, Lv L. Identifying factors affecting the sustainability of water environment treatment public-private partnership projects. Adv Civ Eng. 2019. https://doi.org/10.1155/2019/7907234.
Rose V, Rollwagen-Bollens G, Bollens SM, Zimmerman J. Effects of grazing and nutrients on phytoplankton blooms and microplankton assemblage structure in four temperate lakes spanning a eutrophication gradient. Water. 2021;13(8):1085.
Sini P, et al. Cyanobacteria, cyanotoxins, and neurodegenerative diseases: dangerous liaisons. Int J Mol Sci. 2021;22(16):8726.
Rubright SLM, Pearce LL, Peterson J. Environmental toxicology of hydrogen sulfide. Nitric oxide Biol Chem. 2017;71:1.
Adams JB, Taljaard S, Van Niekerk L, Lemley DA. Nutrient enrichment as a threat to the ecological resilience and health of South African microtidal estuaries. Afr J Aquat Sci. 2020;45(1–2):23–40.
Saravanan A, et al. Effective water/wastewater treatment methodologies for toxic pollutants removal: processes and applications towards sustainable development. Chemosphere. 2021;280:130595.
Angassa K, Leta S, Mulat W, Kloos H, Meers E. Organic matter and nutrient removal performance of horizontal subsurface flow constructed wetlands planted with phragmite karka and vetiveria zizanioide for treating municipal wastewater. Environ Process. 2018;5:115–30.
Abou-Elela SI, Hellal MS. Municipal wastewater treatment using vertical flow constructed wetlands planted with canna, phragmites and cyprus. Ecol Eng. 2012;47:209–13. https://doi.org/10.1016/j.ecoleng.2012.06.044.
Abou-Elela SI, El-Khateeb MA, Fawzy ME, Abdel-Halim W. Innovative sustainable anaerobic treatment for wastewater. Desalin Water Treat. 2013;51(40–42):7490–8.
El-Khateeb MA, El-Gohary FA. Combining UASB technology and constructed wetland for domestic wastewater reclamation and reuse. Water Sci Technol Water Supply. 2003;3(4):201–8.
Magwaza ST, Magwaza LS, Odindo AO, Mditshwa A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: a review. Sci Total Environ. 2020;698:134154.
Makopondo ROB, Rotich LK, Kamau CG. Potential use and challenges of constructed wetlands for wastewater treatment and conservation in game lodges and resorts in Kenya. Sci World J. 2020. https://doi.org/10.1155/2020/9184192.
Cifuentes-Torres L, Mendoza-Espinosa LG, Correa-Reyes G, Daesslé LW. Hydroponics with wastewater: a review of trends and opportunities. Water Environ J. 2021;35(1):166–80.
Recsetar MS, Fitzsimmons KM, Cuello JL, Hoppe-Jones C, Snyder SA. Evaluation of a recirculating hydroponic bed bioreactor for removal of contaminants of emerging concern from tertiary-treated wastewater effluent. Chemosphere. 2021;262:128121.
Wongkiew S, Koottatep T, Polprasert C, Prombutara P, Jinsart W, Khanal SK. Bioponic system for nitrogen and phosphorus recovery from chicken manure: evaluation of manure loading and microbial communities. Waste Manag. 2021;125:67–76.
Worku A, Tefera N, Kloos H, Benor S. Constructed wetlands for phytoremediation of industrial wastewater in Addis Ababa, Ethiopia. Nanotechnol Environ Eng. 2018;3(1):9.
Magwaza ST, Magwaza LS, Odindo AO, Mditshwa A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: a review. Sci Total Environ. 2019. https://doi.org/10.1016/j.scitotenv.2019.134154.
Regassa R. Useful plant species diversity in homegardens and its contribution to household food security in Hawassa city, Ethiopia. African J Plant Sci. 2016;10(10):211–33.
Francis JK. Duranta erecta L. golden dewdrop. In: Wildland Shrubs of the United States and its territories: thamnic descriptions, vol. 299, 2004. https://data.fs.usda.gov/research/pubs/iitf/iitf_gtr026.pdf#page=309.
Okunlola AI. The effects of cutting types and length on rooting of duranta repens in the nursery. Glob J Hum Soc Sci Geogr Geo-Sci Env Disaster Manag. 2013;13(3):1–5.
Haddad M, Mizyed N, Masoud M. Evaluation of gradual hydroponic system for decentralized wastewater treatment and reuse in rural areas of Palestine. Int J Agric Biol Eng. 2012;5(4):47–53.
J. Johnson and M. F. J. Morgan. Plant sampling guidelines. Sampl. Protoc. 2010; 1–2
Boonsong K, Chansiri M. Domestic wastewater treatment using vetiver grass cultivated with floating platform technique. AU J Technol. 2008;12(2):73–80.
Keeratiurai P. Efficiency of wastewater treatment with hydroponics. ARPN J Agr Biol Sci. 2013;8:800–5.
Rice EW, Baird RB, Eaton AD, Clesceri LS. Standard methods for the examination of water and wastewater, vol. 10. Washington DC: American Public Health Association; 2012.
Subsongsang R, Jiraungkoorskul W. An updated review on phytochemical properties of ‘golden dewdrop’ duranta erecta. Pharmacogn Rev. 2016;10(20):115.
R. A. F. M.A. Bell. Guide to plant and crop sampling, measurements and observations for agronomic and physiological reasearch in small grain cereals. Mexico; 1994.
Angassa K, Leta S, Mulat W, Kloos H, Meers E. Effect of hydraulic loading on bioremediation of municipal wastewater using constructed wetland planted with vetiver grass, Addis Ababa, Ethiopia. Nanotechnol Environ Eng. 2019;4:1–11.
Mostsara MR, Roy RN. Guide to laboratory establishment for plant nutrient analisys. Roma: FAO; 2008.
Angassa K, Leta S, Mulat W, Kloos H. Seasonal characterization of municipal wastewater and performance evaluation of a constructed wetland system in Addis Ababa, Ethiopia. Int J Energy Water Resour. 2020;4:127–38.
Kadlec RH, Wallace S. Treatment wetlands. Boca Raton: CRC Press; 2008.
Aniyikaiye TE, et al. Assessing environmental impacts of large centralized wastewater treatment plants with combined or separate sewer systems in dry/wet seasons by using LCA. Chemosphere. 2020;298(13):15674–90.
Vymazal J. Removal of nutrients in various types of constructed wetlands. Sci Total Environ. 2007;380(1–3):48–65.
Gebeyehu A, Shebeshe N, Kloos H, Belay S. Suitability of nutrients removal from brewery wastewater using a hydroponic technology with typha latifolia. BMC Biotechnol. 2018;18(1):74.
Vymazal J. Constructed wetlands for wastewater treatment. Water. 2010;2(3):530–49.
Bawiec A. Efficiency of nitrogen and phosphorus compounds removal in hydroponic wastewater treatment plant. Environ Technol. 2019;40(16):2062–72.
Alayu E, Leta S. Effectiveness of two-stage horizontal subsurface flow constructed wetland planted with cyperus alternifolius and typha latifolia in treating anaerobic reactor brewery effluent at different hydraulic residence times. Environ Syst Res. 2020;9(1):1–15.
Rose A, et al. The diversity of nitrogen-cycling microbial genes in a waste stabilization pond reveals changes over space and time that is uncoupled to changing nitrogen chemistry. Microb Ecol. 2021;81:1029–41.
Lennard WA, Leonard BV. A comparison of three different hydroponic sub-systems (gravel bed, floating and nutrient film technique) in an aquaponic test system. Aquac Int. 2006;14(6):539–50.
Haddad M, Mizyed N. Evaluation of various hydroponic techniques as decentralised wastewater treatment and reuse systems. Int J Environ Stud. 2011;68(4):461–76.
Worku A, Tefera N, Kloos H, Benor S. Bioremediation of brewery wastewater using hydroponics planted with vetiver grass in Addis Ababa, Ethiopia. Bioresour Bioprocess. 2018;5(1):39.
Su F, et al. Removal of total nitrogen and phosphorus using single or combinations of aquatic plants. Int J Environ Res Public Health. 2019;16(23):4663.
Abdel-Shafy HI, El-Khateeb MA. Integration of septic tank and constructed wetland for the treatment of wastewater in Egypt. Desalin Water Treat. 2013;51(16–18):3539–46.
Acknowledgements
The authors thank Addis Ababa Science and Technology University (AASTU) and Ethiopian Road Authority (ERA) for the material support for the research. The authors also thank the reviewers and editor of the journal for improving the quality of the manuscript by giving valuable comments and suggestions.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
The first author, Abebe Worku supervised the experiment and wrote, structured, read, edited, and approved the final manuscript. The second author, Solomon Tibebu wrote up the manuscript and conducted experiments in the field.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Worku, A., Tibebu, S. Performance of pilot-scale gravel and sponge bed hydroponic systems vegetated with Duranta erecta treating wastewater in a developing country, Ethiopia, Africa. Discov Water 4, 21 (2024). https://doi.org/10.1007/s43832-024-00075-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-024-00075-z