Abstract
Metformin (MET), an antidiabetic compound, has received increasing attention, as it cannot be effectively removed during conventional wastewater treatment processes and may act as an endocrine disruptor. Electron beam irradiation (EBI) is an eco-friendly process able to degrade and neutralize biohazardous pollution almost instantly. In this context, this study applied EBI to MET degradation and detoxification in aqueous solutions. A 98% MET degradation rate and TOC removal of 19.04 ± 1.20% at a 1.0 kGy EBI dose was obtained, with up to 65% mineralization reached at 5.0 kGy. Toxicity assays were performed with Vibrio fischeri, Saccharomyces cerevisiae, and Daphnia similis, and the findings indicate that generated byproducts were only more toxic to D. similis. This reveals the need to assess organisms belonging to different trophic levels. A cytotoxic assessment employing Allium cepa roots demonstrated no toxic effects concerning untreated and irradiated samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Active pharmaceutical ingredients have received increasing attention, as they are designed to alter specific physiological functions, making them biologically active against non-target species [1]. Metformin (MET), an antidiabetic compound, has become a pharmaceutical of emerging concern, as it is one of the most widely prescribed anti-hyperglycemic agents worldwide and remains the first-choice for the treatment of type 2 diabetes [2]. The International Diabetes Federation estimated a global diabetes prevalence of 9.3% (463 million people) in 2019, with a projected increase to 10.2% (578 million) by 2030 and to 10.9% (700 million) by 2045 [3]. Brazil holds the fifth position among the countries with the highest prevalence of diabetes mellitus [4]. This comprises both a public health and environmental issue, as Brazil presents several water-related sanitary problems.
Metformin (MET), an antidiabetic compound, is a small molecule with various biological activities, distinguished by unique attributes, such as five N atoms. In its hydrochloride form, electron deficiency is delocalized over these atoms, making MET an emerging cancer treatment for several cancer types, including colorectal, breast, pancreas, and prostate cancers [5]. This compound exhibits an oral bioavailability between 50 and 60% and is not fully metabolized, being excreted virtually unchanged (90%) in urine [6, 7]. MET can be transformed into guanyl urea through wastewater treatment biological processes [8]. Its routine detection in several environmental matrices indicates, however, that MET is not completely removed by conventional treatment technologies [9,10,11,12,13,14]. Although environmental impact studies concerning MET are still relatively recent and scarce [14, 15], this antidiabetic has been detected in wastewater treatment plant influents (14 to 95 µg L−1) and effluents (0.7 to 6.5 µg L−1), surface water (up to 234 ng L−1), and tap water (34 ng L−1) in Germany [10]. Some assessments indicate that MET can affect the vertebrate endocrine systems at environmentally relevant concentrations (1–100 µg L−1) [14, 16,17,18], also inducing aggressive behavior in some fish species (40 µg L−1) [19].
The increasing environmental awareness taking place in recent decades has boosted an ongoing search for innovative and more effective chemical and physicochemical technologies to facilitate the degradation of pharmaceutical residues in water [20]. Advanced oxidation/reduction processes (AORPs) have emerged in this sense as promising water and wastewater treatment technologies considering their ability to completely eliminate pollutants, as well as their high efficiency rates and wide applicability [21]. AORPs are particularly represented by electron beam irradiation (EBI) and gamma irradiation [22].
Ionizing radiation comprises an emerging advanced oxidation/reduction technology trend. This technique displays the potential to degrade a wide variety of water pollutants due to the simultaneous generation of oxidizing and reducing species, consisting in an environmentally friendly method, due to its chemical-free nature [21]. Both electron beam and γ-ray ionization result in water radiolysis, generating reactive species such as ⋅OH, e−aq, and ⋅H [23,24,25]. The hydroxyl radical (⋅OH) is the main pollutant degradation initiator [26].
Electron beam irradiation is adequate for practical long-term applications [27,28,29]. Several studies have, in fact, demonstrated the application potential of this technology in the detoxification of different organic compounds, such as pharmaceuticals, dyes, and surfactants from complex matrices, including textile effluents and sewage wastewater [30,31,32,33,34,35,36,37,38]. For instance, Garcia et al. reported a 95% color reduction alongside a 70% toxicity reduction towards D. similis and V. fischeri when a 5 kGy dose was applied to a textile effluent [34]. In another study, Silva et al. reported that a 5.0 kGy dose applied to a 50% v/v fluoxetine solution in raw domestic sewage resulted in 80% and 22% acute toxicity reductions towards D. similis and V. fischeri, respectively [39].
More efficient technologies have become increasingly necessary due to high contaminant concentrations in and the complexity of industrial and urban wastewater. Previous studies have demonstrated low to moderate MET rejections (< 60%) when applying different processes, such as ozonation, UVC photolysis, photocatalysis (TiO2/UVC) and chlorination [40, 41]. Thus, alternative processes aiming at improving MET removal rates in order to reduce biota impacts are paramount. In this regard, ionizing radiation can be combined with other techniques or compounds to increase pollutant removal efficiencies [42], such as persulfate [43,44,45], peroxymonosulfate [46], titanium dioxide [47], hydrogen peroxide [48], or ozonation [48, 49].
Persulfate-based reactions are very powerful for the treatment of a broad range of impurities [50], enhancing contaminant degradation and mineralization [44, 45, 51]. In this regard, Zhang et al. demonstrated the complete degradation of 8 mg L−1 triclosan in the presence of 1.5 mM persulfate at a 300 Gy dose, much lower than the dose required for control group degradation without the addition of persulfate (600 Gy) [44].
In this context, this study aimed to investigate EBI as an alternative for MET degradation, mineralization, and detoxification. Degradation and mineralization processes were monitored by LC–MS/MS and TOC removal rates, respectively. Ecotoxicological assays were performed to assess the toxicity of the resulting degradation products as well as the efficiency of a combined EBI and persulfate process.
2 Material and methods
2.1 Chemicals
Metformin [C4H11N5, MM = 165.62 g mol−1; 1,1-Dimethylbiguanide; CAS 1115-70-4] was purchased from Sigma Aldrich (> 97%). All aqueous solutions prepared for the irradiation experiments were diluted in ultra-pure water (Millipore Milli-Q). Acetonitrile and formic acid (chromatographic grade) were purchased from Supelco/Millipore. Sodium persulfate (Na2S2O8; ≥ 98%) was purchased from Merck. The MET solutions were prepared at 8.2 ± 0.5 mg L−1 in order to simulated real conditions based on previous studies [40, 52].
2.2 Irradiation procedure
Aqueous sample irradiations were performed in batches employing a Dynamitron® Industrial Electron Accelerator at 37.5 kW and energy set at 1.4 MeV. A 246 mL sample volume was added to a rectangular container (Pyrex®), to ensure a 4 mm thickness and guarantee maximum penetrability [30, 34, 38, 39]. The vessels were irradiated by the electron beam twice placed on an automated conveyor at 6.72 m min−1. Irradiations took place at room temperature with absorbed doses ranging from 1.0 to 5.0 kGy. All experiments were performed in triplicate.
Concerning the combined process of EBI alongside persulfate addition, 0.5 mmol of persulfate were added to the aqueous samples prior to the 1.0 and 5.0 kGy irradiation procedures.
2.3 Analytical determinations
UV–vis spectra were obtained employing a UV-1800Shimadzu UV Spectrophotometer. Total Organic Carbon (TOC) values were determined on a Shimadzu TOC-L apparatus to obtain organic carbon removal rates following irradiation. Chemical MET characterizations were performed on an Agilent HPLC 1290 equipment coupled to a Sciex 3200 QTrap apparatus. A Restek Ultra Aqueous column (150 × 2.1 mm × 3.0 µm) was used maintained at room temperature. Isocratic analyses were performed using (A) H2O + 0.1% formic acid, (B) acetonitrile + 0.1% formic acid (35:65 v/v) at a 350 µL min−1 flow rate and 5.0 µL injection volume. The MET retention time was of 1.34 min. A mass spectrometry assessment was performed in the + ESI mode and quantifications were calculated based on the multiple reaction monitoring (MRM) of m/z 130.138 → 71.1 transitions.
2.4 Toxicity assays
Acute toxicity assays with treated and untreated MET solutions employing the microcrustacean D. similis and bacteria V. fischeri were performed according to Brazilian ABNT Standards.
Daphnia similis toxicity assays were performed according to ABNT NBR 12713/2016 [53] guidelines. Four replicates consisting of five neonates (6–24 h) were placed in for each MET concentration (twenty organisms in total). After 48 h, immobility rates were evaluated. All tests were performed in a dark room at 20 ± 1 °C. The Median Effective Concentration (EC50%) was calculated from the estimated endpoint by the Trimmed Spearman-Karber method [54]. Acute toxicity results were expressed as toxicity units (TU = 100/EC50). An analysis of variance (ANOVA) was performed to evaluate the significance of the verified differences between average values for the experimental treatments and the control group at a significance threshold level of 5%. A post hoc Tukey test was conducted when the ANOVA revealed significant treatment differences.
Acute V. fischeri toxicity assays followed ABNT NBR 15411/2019 [55] guidelines. V. fischeri bioluminescence was detected using a Microbics 500® photometer. The tests consisted in exposing the bacteria for 15 min to a sample or a series of sample dilutions, verifying bioluminescence emission inhibition. The results were based on the value of the gamma effect (relation between lost and remaining light) for a given sample concentration, reported as toxicity factors (TF). This is equivalent to the highest sample dilution, where no test organisms exhibit bioluminescence inhibitions of over 20%. All toxicity experiments were performed in duplicate.
Saccharomyces cerevisiae toxicity assays were performed according previous works [56], based on yeast suspension conductivity monitoring after 30 min of exposure due to fermentation inhibition under toxic conditions. The statistical analyses consisted of F- and t-tests. The results were expressed as toxicity factors (TF), where the highest concentration of the sample in which no inhibition greater than 10% of the test organisms is observed. Five replicates were performed for all toxicity experiments.
In silico methods were also used to compare toxicity findings. The ECOSAR program predicts toxicity values for multiple chemical classes based on compound structure/classes (e.g., neutral organics, aliphatic amines, esters, among others). The toxicity of MET and that of its degradation products are based on aliphatic amine-acids, due to this compound’s chemical structure. U.S. EPA’s ECOSAR (version 2.0) models were used to predict toxicities against daphnids, fish, and green algae [Median Lethal Concentration (LC50) or Median Effective Concentration (EC50)].
2.5 Cytotoxic determinations
Cytotoxicit MET potential was assessed using meristematic Allium cepa cells according to an adapted methodology [57,58,59]. Initially, a set of onion bulbs obtained from a local market were grown in distilled water. After 72 h, the grown roots were exposed to untreated MET samples and samples irradiated at 5.0 kGy along with a negative control (distilled water) for 48 h. Roots with a cap size of approximately 2 cm were collected for each sample. The root meristems were then placed in microtubes, and the meristematic cell hydrolysis was performed in 1.0 mol L−1 HCl at 60 °C for 10 min. The root tips were then cut and placed on three slides per sample (triplicate) for a mitotic division analysis. The meristematic cells were dyed using the Panótico Rápido® kit and assessments were conducted by optical microscopy. Cytogenetic analyses were performed employing the mitotic index (MI), concerning the ratio between the total number of cells in the division process and the total number of observed cells. An ANOVA test was performed at a 5% significance threshold level to detect significant differences.
3 Results and discussion
3.1 Electron beam irradiation metformin aqueous solutions removal and mineralization
The effects of EBI on MET were monitored by UV–Vis as depicted in Fig. 1. The characteristic MET absorption band at 233 nm was noted in all the samples [60]. After treatment, an absorbance decrease at 233 nm was verified, indicating MET removal. Moreover, absorbance increases at 207 and 258 nm were verified in the irradiated samples, indicating the formation of intermediates. According to Collin et al. [61], an absorption peak at 258 nm may be associated with the formation of 4,2,1-AIMT (4-amino-2-imino-1-methyl-1,2-dihydro-1,3,5-triazine), considering this is a characteristic aromatic compound wavelength [60, 62].
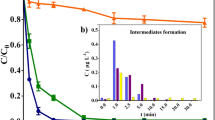
Chromatographic analyses are paramount in pharmaceutical degradation studies, due to their ability to identify both the investigated compounds and their radiolysis products. The electrospray ionization mode of the LC/MS–MS technique prevents the identification of radiolysis products, due to their low energy. Degradability can be calculated by comparing the peak area of the investigated compound before and after the irradiation process. Herein, the MET removal efficiency was calculated employing the peak area (cps, counts per second). A 98.08 ± 0.05% removal was achieved at the 1.0 kGy dose. Degradation increased by increasing the absorbed dose, reaching 99.30 ± 0.06 and 99.41 ± 0.10% at 2.5 and 5.0 kGy, respectively.
Organic pollutant decomposition can be controlled through the absorbed dose to achieve partial or complete decomposition [23, 63]. Although the findings indicate increased TOC removal with increasing doses (Fig. 2), up to 5.0 kGy, the EBI process was unable to complete pharmaceutical mineralization, resulting in the several intermediate byproducts. TOC removal rates of 19.04 ± 1.20%, 39.69 ± 2.28%, and 65.41 ± 0.71% were achieved at 1.0, 2.5, and 5.0 kGy, respectively. Furthermore, lower MET solution pH values were verified with increasing absorbed doses, from 6.93 ± 1.5 to 4.9 ± 0.2 at 5.0 kGy (Fig. 2).
The findings indicate that MET degradation by EBI was achieved even at low doses. Although some authors have demonstrated efficient removal by AOPs [64, 65], low to moderate MET removal rates have been reported for different processes. For instance, Gartiser et al. removed only 35% of MET employing an ozone-based treatment (0.5 mg L−1 MET initial concentration) [40], while Quintão et al. reported the removal efficiencies for 10 mg L−1 treated MET samples of 60% after 30 min of ozonation, 9.2% after 30 min UVC photolysis; 31% after 30 min of photocatalysis (TiO2/UVC), and 60% after 5 min of chlorination [41].
Electron beam irradiation is based on water radiolysis, in which water molecule excitation and ionization effects produce highly reactive radicals, such as hydrated electron (e − aq), hydroxyl radicals (⋅OH) and the hydrogen atom (H⋅. These can, in turn, react and result in rapid organic pollutant degradation [24].
Notably, toxicity decreases and harmful organic pollutant mineralization are not necessarily correlated with their degradation. Therefore, toxicity assessments of intermediate degradation products becomes crucial, as more harmful degradation products may emerge compared to the original contaminant [66].
3.2 Toxicity of untreated and irradiated aqueous metformin solutions
Although incomplete pollutant oxidation by AOPs may produce more hazardous byproducts, toxicity changes following treatment are still unknown [67] and should, therefore, be monitored.
Possible degradation products reported in the literature are listed in Table 1. In this sense, oxidation products may vary with experimental conditions prior to irradiation. For instance, MET hydroperoxide was obtained under aerated conditions, while MET dimers were obtained under non-aerated conditions, and 1-methylbiguanide and 4,2,1-AIMT, under both aerated and non-aerated conditions [61, 62].
Initially, the LC50, and EC50 were predicted by ECOSAR for fish, green algae, and daphnids to investigate the toxic effects of the generated MET intermediates. Only two intermediates (P4 and P10) were more toxic than the parental compound, indicating the need for critical assessments concerning MET disposal in aquatic environments.
Experimental and in silico MET toxicity data were also compared. Previous studies reported EC5072h > 77.2 mg L−1 and > 320 mg L−1 for Desmodesmus subspicatus and Raphidocelis subcapitata algae, respectively [68, 69]. Cleuvers estimated an EC5048h of 64 mg L−1 for D. magna [69], while Godoy et al. and Tominaga et al. estimated EC5048h values of 14.3 mg L−1 and 20.4 mg L−1 for D. similis [70, 71]. In other assessments, EC5096h > 86.0 mg L−1 and LC5096h of 1315.5 mg L−1 were reported for Danio rerio fish [68, 70]. Concerning the in silico and experimental data, Table 1 indicates that the results obtained for daphnids and fish are not accurate for MET toxicity predictions, as lower experimental data values are noted.
Producing correct predictions across a wide range of chemical compounds is still a challenge [72], as not all endpoints of a specific chemical are available and the predictive ability of (Quantitative) Structure Activity Relationship (QSAR) for the combined toxicities of mixed organic pollutants still requires further improvements [66]. In this regard, Sanderson and Thomsen evaluated the acute toxicity data of 275 pharmaceuticals and noted that > 92% of the determined acute toxicities were predictable using a generic QSAR [73]. Previous studies also verified that ECOSAR was not accurate to predict the toxicity of certain pharmaceuticals [38]. Therefore, experimental toxicological assessments were also carried out herein to evaluate the toxicity of MET and its intermediates.
The toxicity of the non-irradiated and irradiated MET samples towards D. similis, V. fischeri, and S. cerevisiae, depicted in Fig. 3, indicate that D. similis is more sensitive to MET than V. fischeri. Jacob et al. reported an EC1030min of 870.79 mg L−1 for V. fischeri [74], indicating lower MET sensibility when compared to our study, as no toxic effects were noted at 8.2 ± 0.5 mg L−1.
Acute D. similis (in toxic units, TU = 100/EC50%), V. fischeri, and S. cerevisiae (in toxic factor, TF) data concerning MET samples treated by electron beam irradiation at different doses. Initial conditions: [MET]0 = 8.2 ± 0.5 mg L−1, pH = 6.93 ± 1.5. Different letters (a–c) indicate significant differences (Tukey’s test, p < 0.05)
Regarding the treated samples, the V. fischeri and S. cerevisiae toxicity assay results do not indicate increased toxicity following EBI. In contrast, Daphnia similis was more sensitive to the formed byproducts, with increased toxicity followed by decreased toxicity following EBI. An increase from 1.35 ± 0.0 TU to 4.42 ± 0.59 TU was noted at the 1.0 kGy dose. Conversely, decreases to 2.98 ± 0.07 TU and 3.09 ± 0.23 TU were achieved at higher doses (2.5 kGy and 5.0 kGy, respectively).
Heightened toxicity has been reported following irradiation treatment by several studies. Zhang et al. reported that MET chlorination byproducts in drinking water are toxic to worms, human cells, and mice [75]. These byproducts were not genotoxic, although toxicities to living worms and human HepG2 cells at millimolar doses were noted. Furthermore, the evaluated byproducts were harmful to mice at 250 ng/L, destroying small intestine integrity. In addition, distinct situations have been reported following treatment by different AOPs. Maćerak et al. for example, reported increased bioluminescence inhibition in V. fischeri following UV and UV/H2O2 treatment [65]. Quintão et al. on the other hand, verified no cytotoxicity increases for HepG2 human hepatoma cells for both ozonation, UVC photolysis, photocatalysis (TiO2/UVC), and chlorination untreated and treated MET samples [41]. In another study, Carbuloni et al. (2020) demonstrated decreased toxicity, in the form of increased Lactuca sativa germination, following TiO2 photodegradation [60]. Toxicity assessments should, therefore, be conducted with different trophic level representatives to achieve safe treated wastewater disposal.
3.3 Cytotoxic assessments
Cytotoxicity assays employing A. cepa were performed for untreated and irradiated MET samples irradiated at 5.0 kGy. No significant effects were noted for either compared to the control (Fig. 4).
Previous studies have demonstrated genotoxic MET potential [76,77,78]. For instance, Yuzbasioglu et al. reported that MET increased the frequency of chromosome aberrations and sister chromatid exchanges in human lymphocytes, especially at the 48 h exposure time point [78]. Moreover, it has been demonstrated that MET affects stem cell differentiation and enhances immunomodulatory stem cell properties, while also exerting anti-aging, anti-oxidative, and anti-inflammatory effects [79,80,81,82]. In contrast, other assessments have demonstrated that MET inhibits cancer cell growth [83, 84]. Al-Zaidan et al. for example, reported that MET specifically targets cancerous cells, with more significant effects compared to normal cells [85]. In another assessment, the lifespan of Caenorhabditis elegans was increased and cancer cell growth inhibition was induced following MET treatment [86]. These contrasting data indicate that further studies should be performed to investigate potential genotoxic MET biota effects.
Electron beam irradiation is an eco-friendly and high-tech process in the field of health product sterilization, and its application to water treatment has been increasingly discussed. Ionizing radiation displays many advantages, such as the ability to treat large water flows, the lack of excess redox agents in the treated water and the use of a minimal amount of chemicals. Because of this, important automation advances and increased water treatment control, as well as the ability to neutralize biohazardous pollutants almost instantly, have been performed with EBI [87].
3.4 Effects of electron beam irradiation and persulfate on metformin toxicity
The effects of EBI combined with persulfate were monitored by UV–Vis spectrophotometry (Fig. 5). Decreases in absorbance values at 233 nm were noted following irradiation at 1.0 kGy (Fig. 5a). A slight improvement in MET degradation employing EBI/PS was noted when compared to EBI. Moreover, increased in absorbance values at 207 and 258 nm were noted in both irradiated samples, indicating the formation of intermediates. However, increased absorbance values were verified only at 207 nm for EBI/PS-treated samples (Fig. 5b).
The persulfate-based process involves the in situ generation of highly reactive and short-lived sulfate radicals (SO4−⋅) [88]. The observed MET removal improvement is related to the higher production of oxidative sulfate radicals formed from the reaction between persulfate and solvated electrons (Eq. 1) [43, 89]. In addition, persulfate can also react with H⋅ to produce sulfate, though H⋅ production is not as significant as the production of the solvated electron (G (H⋅) = 0.06 and G(e−aq) = 0.27) and H⋅ is much less reactive (k = 2.5 × 107 M−1 s−) [43]. In addition, persulfate also reacts with OH⋅ radicals to generate S2O8−⋅ radicals.
Previous studies have demonstrated that the addition of persulfate can improve organic contaminant removal [44, 45, 51]. Nevertheless, this did not significantly affect degradation rates in the present study, with the low removal efficiencies observed herein potentially associated to the presence of dissolved oxygen in the aqueous solutions. Criquet and Leitner noted that dissolved oxygen concentrations comprise a limiting parameter for the degradation of p-hydroxybenzoic acid, in which excess dissolved oxygen levels did not significantly improve p-hydroxybenzoic acid degradation rates. The authors [90]. Thus, suggest that oxygen participates in many radical mechanisms. Herein, the applied persulfate solution radiolysis led to the generation of dissolved oxygen, which, in the presence of persulfate, partly balanced oxygen consumption resulting from the oxidation reaction.
Regarding toxicological performance, the toxicity of the non-treated and irradiated samples in the presence and absence of persulfate for D. similis and V. fischeri are depicted in Fig. 6. Toxicities increased for both organisms following the EBI/PS treatment, indicating the generation of more toxic byproducts.
Wastewater toxicity can be affected by oxidants remaining at the end of the reaction and, in some cases, by oxidants that recombine with reactive species during the AOP [66]. However, the effects of remaining oxidants on solution toxicity have not been widely addressed to date. Therefore, further studies should be conducted to improve the toxicological performance of the EBI/PS process.
4 Conclusion
The results reported herein demonstrate the potential of electron accelerators to be effectively used for the removal of the antidiabetic MET as an environmental pollutant. Electron beam irradiation was effective in removing MET in aqueous solutions up to 99% degradation and 65.41% of mineralization at 5.0 kGy. The findings also indicate the need to assess biological effects following EBI at distinct trophic levels in order to reduce biota impacts, as increased toxicity was observed only for D. similis following exposure to an irradiated MET solution. Finally, the addition of persulfate did not enhance MET removal and increased toxicity against D. similis and V. fischeri. Further studies employing combined processes should be conducted to optimize MET removal and detoxification rates.
Data availability
The data set generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Godoy AA, Kummrow F. What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit Rev Environ Sci Technol. 2017;47(16):1453–96. https://doi.org/10.1080/10643389.2017.1370991.
Baker C, Retzik-Stahr C, Singh V, Plomondon R, Anderson V, Rasouli N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther Adv Endocrinol Metabol. 2021;12:2042018820980225. https://doi.org/10.1177/2042018820980225.
Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2020;162: 108072. https://doi.org/10.1016/j.diabres.2020.108072.
Correr CJ, Coura-Vital W, Frade JC, Nascimento RC, Nascimento LG, Pinheiro EB, Ferreira WM, Reis JS, Melo KFS, Pontarolo R, Lenzi MSA, Almeida J, Pedrosa HC, João WS. Prevalence of people at risk of developing type 2 diabetes mellitus and the involvement of community pharmacies in a national screening campaign: a pioneer action in Brazil. Diabetol Metab Syndr. 2020;12:1–11. https://doi.org/10.1186/s13098-020-00593-5.
Mondal S, Samajdar RN, Mukherjee S, Bhattacharyya AJ, Bagchi B. Unique features of metformin: a combined experimental, theoretical, and simulation study of its structure, dynamics, and interaction energetics with DNA grooves. J Phys Chem B. 2018;122(8):2227–42. https://doi.org/10.1021/acs.jpcb.7b11928.
Tucker GT, Wesolowski CA. Metformin disposition—a 40-year-old mystery. Br J Clin Pharmacol. 2020;86(8):1452. https://doi.org/10.1111/bcp.14320.
Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996. https://doi.org/10.5005/jp/books/11384_11.
Trautwein C, Kümmerer K. Incomplete aerobic degradation of the antidiabetic drug Metformin and identification of the bacterial dead-end transformation product Guanylurea. Chemosphere. 2011;85(5):765–73. https://doi.org/10.1016/j.chemosphere.2011.06.057.
Trautwein C, Berset JD, Wolschke H, Kümmerer K. Occurrence of the antidiabetic drug Metformin and its ultimate transformation product Guanylurea in several compartments of the aquatic cycle. Environ Int. 2014;70:203–12. https://doi.org/10.1016/j.envint.2014.05.008.
Tisler S, Zwiener C. Formation and occurrence of transformation products of metformin in wastewater and surface water. Sci Total Environ. 2018;628:1121–9. https://doi.org/10.1016/j.scitotenv.2018.02.105.
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere. 2017;174:437–46. https://doi.org/10.1016/j.chemosphere.2017.01.101.
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol. 2002;36(6):1202–11. https://doi.org/10.1021/es011055j.
Papageorgiou M, Zioris I, Danis T, Bikiaris D, Lambropoulou D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci Total Environ. 2019;694:133565. https://doi.org/10.1016/j.scitotenv.2019.07.371.
Ambrosio-Albuquerque EP, Cusioli LF, Bergamasco R, Gigliolli AAS, Lupepsa L, Paupitz BR, Barbieri PA, Borin-Carvalho LA, de Brito P-C. Metformin environmental exposure: a systematic review. Environ Toxicol Pharmacol. 2021;83: 103588. https://doi.org/10.1016/j.etap.2021.103588.
Elizalde-Velázquez GA, Gómez-Oliván LM. Occurrence, toxic effects and removal of metformin in the aquatic environments in the world: recent trends and perspectives. Sci Total Environ. 2020;702:134924. https://doi.org/10.1016/j.scitotenv.2019.134924.
Crago J, Bui C, Grewal S, Schlenk D. Age-dependent effects in fathead minnows from the anti-diabetic drug metformin. Gen Comp Endocrinol. 2016;232:185–90. https://doi.org/10.1016/j.ygcen.2015.12.030.
Niemuth NJ, Jordan R, Crago J, Blanksma C, Johnson R, Klaper RD. Metformin exposure at environmentally relevant concentrations causes potential endocrine disruption in adult male fish. Environ Toxicol Chem. 2015;34(2):291–6. https://doi.org/10.1002/etc.2793.
Niemuth NJ, Klaper RD. Low-dose metformin exposure causes changes in expression of endocrine disruption-associated genes. Aquat Toxicol. 2018;195:33–40. https://doi.org/10.1016/j.aquatox.2017.12.003.
MacLaren RD, Wisniewski K, MacLaren C. Environmental concentrations of metformin exposure affect aggressive behavior in the Siamese fighting fish, Betta splendens. PLoS ONE. 2018;13(5):e0197259. https://doi.org/10.1371/journal.pone.0197259.
Trojanowicz M. Removal of persistent organic pollutants (POPs) from waters and wastewaters by the use of ionizing radiation. Sci Total Environ. 2020;718:134425. https://doi.org/10.1016/j.scitotenv.2019.134425.
Khan S, Sayed M, Sohail M, Shah LA, Raja MA. Advanced oxidation and reduction processes. In: Ahuja S, editor. Advances in water purification techniques. Amsterdam: Elsevier; 2019. p. 135–64. https://doi.org/10.1016/B978-0-12-814790-0.00006-5.
Batchelor B. Advanced reduction and oxidation-reduction processes for water treatment. In: Maurice P, editor. Encyclopedia of water: science, technology, and society. Hoboken: Wiley; 2019. p. 1–13. https://doi.org/10.1002/9781119300762.wsts0020.
Capodaglio AG. High-energy oxidation process: an efficient alternative for wastewater organic contaminants removal. Clean Technol Environ Policy. 2017;19:1995–2006. https://doi.org/10.1007/s10098-017-1410-5.
Buxton GV. An overview of the radiation chemistry of liquids. Les Ulis: EDP Sciences; 2008.
Trojanowicz M, Bojanowska-Czajka A, Bartosiewicz I, Kulisa K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)–a review of recent advances. Chem Eng J. 2018;336:170–99. https://doi.org/10.1016/j.cej.2017.10.153.
Csay T, Homlok R, Illes E, Takacs E, Wojnarovits L. The chemical background of advanced oxidation processes. Isr J Chem. 2014;54(3):233–41. https://doi.org/10.1002/ijch.201300077.
Wang S, Wang J, Chen C, He S, Hu J, Zhang Y. First full-scale application of electron beam technology for treating dyeing wastewater (30,000 m3/d) in China. Radiat Phys Chem. 2022;196:110136. https://doi.org/10.1016/j.radphyschem.2022.110136.
Wang S, Wang J. Electron beam technology coupled to Fenton oxidation for advanced treatment of dyeing wastewater: from laboratory to full application. ACS EST Water. 2022;2(5):852–62. https://doi.org/10.1021/acsestwater.2c00040.
Wang J, Wang S, Chen C, Hu J, He S, Zhou Y, Zhu H, Wang X, Hu D, Lin J. Treatment of hospital wastewater by electron beam technology: removal of COD, pathogenic bacteria and viruses. Chemosphere. 2022;308:136265. https://doi.org/10.1016/j.chemosphere.2022.136265.
Tominaga FK, Silva TT, Boiani NF, de Jesus JMS, Teixeira ACSC, Borrely SI. Is ionizing radiation effective in removing pharmaceuticals from wastewater? Environ Sci Pollut Res. 2021;28:23975–83. https://doi.org/10.1007/s11356-020-11718-8.
Tominaga FK, dos Santos Batista A P, Teixeira ACSC, Borrely SI. Degradation of diclofenac by electron beam irradiaton: toxicitiy removal, by-products identification and effect of another pharmaceutical compound. J Environ Chem Eng. 2018;6(4):4605–11. https://doi.org/10.1016/j.jece.2018.06.065.
Borrely SI, de Andrade Silva LG, Del Sole SV, Garcia VSG, Boiani NF, Rosa JM. Electron beam irradiation of textile effluents and non-ionic ethoxylated surfactant for toxicity and color removal. Braz J Rad Sci. 2019. https://doi.org/10.15392/bjrs.v7i2A.702.
Boiani NF, Silva VHO, Garcia VSG, Del Sole SV, Borrely SI. Electron beam irradiation of pharmaceuticals aiming at toxicity reduction: a binary mixture of fluoxetine and propranolol. Ecotoxicol Environ Contam. 2019;14(1):53–8. https://doi.org/10.5132/eec.2019.01.06.
Garcia VS, Rosa JM, Borrely SI. Toxicity and color reduction of a textile effluent containing reactive red 239 dye by electron beam irradiation. Radiat Phys Chem. 2020;172:108765. https://doi.org/10.1016/j.radphyschem.2020.108765.
Borrely SI, Duarte CL, Sampa MHO. Ionizing radiation as a tool for detoxification of whole effluents. In: Méndez-Vilas A, editor. Recent advances in multidisciplinary applied physics. Amsterdam: Elsevier; 2005. p. 259–64. https://doi.org/10.1016/B978-008044648-6.50042-2.
Borrely SI, Morais AV, Rosa JM, Badaró-Pedroso C, da Conceição PM, Higa MC. Decoloration and detoxification of effluents by ionizing radiation. Radiat Phys Chem. 2016;124:198–202. https://doi.org/10.1016/j.radphyschem.2015.11.001.
Borrely SI, Gonçalves AA, Oikawa H, Duarte CL, Rocha FR. Electron beam accelerator for detoxification of effluents: when radiation processing can enhance the acute toxicity? Radiat Phys Chem. 2004;71(1–2):455–8. https://doi.org/10.1016/j.radphyschem.2004.03.087.
Tominaga FK, Boiani NF, Silva TT, dos Santos JG, Lebre DT, Leo P, Borrely SI. Electron beam irradiation applied for the detoxification and degradation of single ciprofloxacin aqueous solution and multiclass pharmaceutical quaternary mixture. Sep Purif Technol. 2023;307:122818. https://doi.org/10.1016/j.seppur.2022.122818.
Silva VHO, dos Santos Batista AP, Silva Costa Teixeira AC, Borrely SI. Degradation and acute toxicity removal of the antidepressant Fluoxetine (Prozac®) in aqueous systems by electron beam irradiation. Environ Sci Pollut Res. 2016;23:11927–36. https://doi.org/10.1007/s11356-016-6410-1.
Gartiser S, Hafner C, Kronenberger-Schäfer K, Happel O, Trautwein C, Kümmerer K. Approach for detecting mutagenicity of biodegraded and ozonated pharmaceuticals, metabolites and transformation products from a drinking water perspective. Environ Sci Pollut Res. 2012;19:3597–609. https://doi.org/10.1007/s11356-012-0925-x.
Quintão FJO, Freitas JRL, de Fátima Machado C, Aquino SF, de Queiroz Silva S, de Cássia Franco Afonso R J. Characterization of metformin by-products under photolysis, photocatalysis, ozonation and chlorination by high-performance liquid chromatography coupled to high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2016;30(21):2360–8. https://doi.org/10.1002/rcm.7724.
Sampa MH, Tkács E, Gehringer P, Rela PR, Ramirez T, Amro H, Trojanowicz M, Botelho ML, Han B, Solpan D, Cooper WJ, Emmi SS, Wojnarovits L. Remediation of polluted waters and wastewater by radiation processing. Nukleonika. 2007;52(4):137–44.
Criquet J, Leitner NKV. Electron beam irradiation of aqueous solution of persulfate ions. Chem Eng J. 2011;169(1–3):258–62. https://doi.org/10.1016/j.cej.2011.02.025.
Zhang Z, Hu D, Chen H, Chen C, Zhang Y, He S, Wang J. Enhanced degradation of triclosan by gamma radiation with addition of persulfate. Radiat Phys Chem. 2021;180:109273. https://doi.org/10.1016/j.radphyschem.2020.109273.
Zhang Z, Chen H, Wang J, Zhang Y. Degradation of carbamazepine by combined radiation and persulfate oxidation process. Radiat Phys Chem. 2020;170:108639. https://doi.org/10.1016/j.radphyschem.2019.108639.
Wang S, Wang J. Oxidative removal of carbamazepine by peroxymonosulfate (PMS) combined to ionizing radiation: degradation, mineralization and biological toxicity. Sci Total Environ. 2019;658:1367–74. https://doi.org/10.1016/j.scitotenv.2018.12.304.
Chitose N, Ueta S, Seino S, Yamamoto TA. Radiolysis of aqueous phenol solutions with nanoparticles. 1. Phenol degradation and TOC removal in solutions containing TiO2 induced by UV, γ-ray and electron beams. Chemosphere. 2003;50(8):1007–13. https://doi.org/10.1016/S0045-6535(02)00642-2.
Torun M, Abbasova D, Şolpan D, Güven O. Caffeine degradation in water by gamma irradiation, ozonation and ozonation/gamma irradiation. Nukleonika. 2014;59(1):25–35.
Chen H, Wang J. Degradation of sulfamethoxazole by ozonation combined with ionizing radiation. J Hazard Mater. 2021;407:124377. https://doi.org/10.1016/j.jhazmat.2020.124377.
Wacławek S, Lutze HV, Grübel K, Padil VV, Černík M, Dionysiou DD. Chemistry of persulfates in water and wastewater treatment: a review. Chem Eng J. 2017;330:44–62. https://doi.org/10.1016/j.cej.2017.07.132.
Liu N, Lei ZD, Wang T, Wang JJ, Zhang XD, Xu G, Tang L. Radiolysis of carbamazepine aqueous solution using electron beam irradiation combining with hydrogen peroxide: efficiency and mechanism. Chem Eng J. 2016;295:484–93. https://doi.org/10.1016/j.cej.2016.03.040.
Hussain S, Naeem M, Chaudhry MN. Estimation of residual antibiotics in pharmaceutical effluents and their fate in affected areas. Pol J Environ Stud. 2016;25(2):607. https://doi.org/10.15244/pjoes/61229.
ABNT Brazilian Association of Technique Standards. Aquatic ecotoxicology: acute toxicity—test with Daphnia spp (Cladocera, Crustacea). 2016. p. 12713.
Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol. 1977;11(7):714–9. https://doi.org/10.1021/es60130a004.
ABNT Brazilian Association of Technique Standards. Ecotoxicology aquatic: inhibitory effect on Vibrio fischeri bioluminescence—Part 3: method using freeze-dried bacterias. 2019. pp. 15411–3.
Dolezalova J, Rumlova L. A new biological test of water toxicity–yeast Saccharomyces cerevisiae conductometric test. Environ Toxicol Pharmacol. 2014;38(3):977–81. https://doi.org/10.1016/j.etap.2014.10.009.
Düsman E, Luzza M, Savegnago L, Lauxen D, Vicentini VEP, Tonial IB, Sauer TP. Allium cepa L. as a bioindicator to measure cytotoxicity of surface water of the Quatorze River, located in Francisco Beltrão, Paraná, Brazil. Environ Monit Assess. 2014;186:1793–800. https://doi.org/10.1007/s10661-013-3493-8.
Frumi Camargo A, Venturin B, Bordin ER, Scapini T, Spitza Stefanski F, Klanovicz N, Dalastra C, Kubeneck S, Preczeski KP, Rossetto V, Weirich S, Carezia C, Ulkovski C, Júnior FWR, Müller C, Fongaro G, Mossi AJ, Treichel H. A low-genotoxicity bioherbicide obtained from trichoderma koningiopsis fermentation in a stirred-tank bioreactor. Ind Biotechnol. 2020;16(3):176–81. https://doi.org/10.1089/ind.2019.0024.
Fiskesjö G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102(1):99–112. https://doi.org/10.1111/j.1601-5223.1985.tb00471.x.
Carbuloni CF, Savoia JE, Santos JS, Pereira CA, Marques RG, Ribeiro VA, Ferrari AM. Degradation of metformin in water by TiO2–ZrO2 photocatalysis. J Environ Manage. 2020;262:110347. https://doi.org/10.1016/j.jenvman.2020.110347.
Collin F, Khoury H, Bonnefont-Rousselot D, Therond P, Legrand A, Jore D, Gardès-Albert M. Liquid chromatographic/electrospray ionization mass spectrometric identification of the oxidation end-products of metformin in aqueous solutions. J Mass Spectrom. 2004;39(8):890–902. https://doi.org/10.1002/jms.656.
Khouri H, Collin F, Bonnefont-Rousselot D, Legrand A, Jore D, Gardès-Albert M. Radical-induced oxidation of metformin. Eur J Biochem. 2004;271(23–24):4745–52. https://doi.org/10.1111/j.1432-1033.2004.04438.x.
Capodaglio AG. Contaminants of emerging concern removal by high-energy oxidation-reduction processes: state of the art. Appl Sci. 2019;9(21):4562. https://doi.org/10.3390/app9214562.
Dolatabadi M, Ahmadzadeh S. A rapid and efficient removal approach for degradation of metformin in pharmaceutical wastewater using electro-Fenton process: optimization by response surface methodology. Water Sci Technol. 2019;80(4):685–94. https://doi.org/10.2166/wst.2019.312.
Maćerak AL, Kerkez Đ, Bečelić-Tomin M, Pilipović DT, Kulić A, Jokić J, Dalmacija B. Removal of diclofenac and metformin from water in laboratory photo reactor. Proceedings. 2018. https://doi.org/10.3390/proceedings2201288.
Wang J, Wang S. Toxicity changes of wastewater during various advanced oxidation processes treatment: an overview. J Clean Prod. 2021;315:128202. https://doi.org/10.1016/j.jclepro.2021.128202.
Babu DS, Srivastava V, Nidheesh PV, Kumar MS. Detoxification of water and wastewater by advanced oxidation processes. Sci Total Environ. 2019;696:133961. https://doi.org/10.1016/j.scitotenv.2019.133961.
Moermond CT, Smit CE. Derivation of water quality standards for carbamazepine, metoprolol, and metformin and comparison with monitoring data. Environ Toxicol Chem. 2016;35(4):882–8. https://doi.org/10.1002/etc.3178.
Cleuvers M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett. 2003;142(3):185–94. https://doi.org/10.1016/S0378-4274(03)00068-7.
Godoy AA, Domingues I, Nogueira AJA, Kummrow F. Ecotoxicological effects, water quality standards and risk assessment for the anti-diabetic metformin. Environ Pollut. 2018;243:534–42. https://doi.org/10.1016/j.envpol.2018.09.031.
Tominaga FK, Boiani NF, Silva TT, Garcia VSG, Borrely SI. Acute and chronic ecotoxicological effects of pharmaceuticals and their mixtures in Daphnia similis. Chemosphere. 2022;309:136671. https://doi.org/10.1016/j.chemosphere.2022.136671.
Melnikov F, Kostal J, Voutchkova-Kostal A, Zimmerman JB, Anastas PT. Assessment of predictive models for estimating the acute aquatic toxicity of organic chemicals. Green Chem. 2016;18(16):4432–45. https://doi.org/10.1039/C6GC00720A.
Sanderson H, Thomsen M. Comparative analysis of pharmaceuticals versus industrial chemicals acute aquatic toxicity classification according to the United Nations classification system for chemicals: assessment of the (Q) SAR predictability of pharmaceuticals acute aquatic toxicity and their predominant acute toxic mode-of-action. Toxicol Lett. 2009;187(2):84–93. https://doi.org/10.1016/j.toxlet.2009.02.003.
Jacob RS, de Souza SLV, d’Auriol M, Lebron YAR, Moreira VR, Lange LC. Diazepam, metformin, omeprazole and simvastatin: a full discussion of individual and mixture acute toxicity. Ecotoxicology. 2020;29(7):1062–71. https://doi.org/10.1007/s10646-020-02239-8.
Zhang R, He Y, Yao L, Chen J, Zhu S, Rao X, Tang P, You J, Hua G, Zhang L, Ju F, Wu L. Metformin chlorination byproducts in drinking water exhibit marked toxicities of a potential health concern. Environ Int. 2021;146:106244. https://doi.org/10.1016/j.envint.2020.106244.
Harishankar MK, Logeshwaran S, Sujeevan S, Aruljothi KN, Dannie MA, Devi A. Genotoxicity evaluation of metformin and glimepiride by micronucleus assay in exfoliated urothelial cells of type 2 diabetes mellitus patients. Food Chem Toxicol. 2015;83:146–50. https://doi.org/10.1016/j.fct.2015.06.013.
Amador RR, Longo JPF, Lacava ZG, Dórea JG, Santos MDFMA. Metformin (dimethyl-biguanide) induced DNA damage in mammalian cells. Genet Mol Biol. 2012;35:153–8. https://doi.org/10.1590/S1415-47572011005000060.
Yuzbasioglu D, Mahmoud JH, Mamur S, Unal F. Cytogenetic effects of antidiabetic drug metformin. Drug Chem Toxicol. 2022;45(2):955–62. https://doi.org/10.1080/01480545.2020.1844226.
Zhang R, Liang Q, Kang W, Ge S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biol Int. 2020;44(1):70–9. https://doi.org/10.1002/cbin.11202.
Mu W, Wang Z, Ma C, Jiang Y, Zhang N, Hu K, Li L, Wang Z. Metformin promotes the proliferation and differentiation of murine preosteoblast by regulating the expression of sirt6 and oct4. Pharmacol Res. 2018;129:462–74. https://doi.org/10.1016/j.phrs.2017.11.020.
Chung MM, Nicol CJ, Cheng YC, Lin KH, Chen YL, Pei D, Lin CH, Shih YN, Yen CH, Chen SJ, Huang RN, Chiang MC. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp Cell Res. 2017;352(1):75–83. https://doi.org/10.1016/j.yexcr.2017.01.017.
Jiang LL, Liu L. Effect of metformin on stem cells: molecular mechanism and clinical prospect. World J Stem Cells. 2020;12(12):1455. https://doi.org/10.4252/wjsc.v12.i12.1455.
Romero R, Erez O, Hüttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, Pacora P, Yoon BH, Grossman LI. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol. 2017;217:282–302. https://doi.org/10.1016/j.ajog.2017.06.003.
Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci. 2012;122(6):253–70. https://doi.org/10.1042/CS20110386.
Al-Zaidan L, El Ruz RA, Malki AM. Screening novel molecular targets of metformin in breast cancer by proteomic approach. Front Public Health. 2017;5:277. https://doi.org/10.3389/fpubh.2017.00277.
Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, Mou F, Kacergis MC, Talkowski ME, Carr CE, Gygi SP, Zheng B, Soukas AA. An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell. 2016;167(7):1705–18. https://doi.org/10.1016/j.cell.2016.11.055.
Ponomarev AV, Ershov BG. The green method in water management: electron beam treatment. Environ Sci Technol. 2020;54(9):5331–44. https://doi.org/10.1021/acs.est.0c00545.
Lee J, Von Gunten U, Kim JH. Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ Sci Technol. 2020;54(6):3064–81. https://doi.org/10.1021/acs.est.9b07082.
Neta P, Huie RE, Ross AB. Rate constant for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data. 1988;17(3):1027–284. https://doi.org/10.1063/1.555808.
Criquet J, Leitner NKV. Reaction pathway of the degradation of the p-hydroxybenzoic acid by sulfate radical generated by ionizing radiations. Radiat Phys Chem. 2015;106:307–14. https://doi.org/10.1016/j.radphyschem.2014.07.016.
Acknowledgements
The authors thank the International Atomic Energy Agency (IAEA), the Brazilian National Council for Scientific and Technological Development (CNPq), the Foundation of the Institute of Technological Research (FIPT), and the Institute of Technological Research (IPT).
Funding
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico, 141947/2018-7.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FKT, JMSdJ, NK, MMR, DTL, TTS. The first draft of the manuscript was written by FKT and all authors commented on previous versions of the manuscript. SIB, ACSCT and PL revised it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declared that there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tominaga, F.K., de Jesus, J.M.S., Klanovicz, N. et al. Ecotoxicological assessment of metformin as an antidiabetic water residue treated by electron beam accelerator irradiation. Discov Water 4, 5 (2024). https://doi.org/10.1007/s43832-023-00053-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00053-x