Abstract
This study assessed the physicochemical and microbiological quality of sachet drinking water samples in Kumasi, a major city in Ghana. Samples were collected from various sources within the city. Physicochemical properties, including pH, total dissolved solids, and concentrations of calcium, sodium, potassium, and magnesium ions, were analyzed following established protocols. Additionally, fluoride concentration was determined. The assessment criteria for water quality were based on the World Health Organization’s and the Ghana Standards Authority’s recommended standards for drinking water. The samples were also subjected to microbial analysis to detect the presence of E. coli and coliforms, and to evaluate microbial quality. The findings indicated that most physicochemical properties of the samples met the World Health Organization’s standards for safe drinking water, except for the slightly acidic pH. Total dissolved solids and the concentrations of calcium, sodium, potassium, fluoride and magnesium ions were within acceptable ranges. Strong positive correlations were observed among various physicochemical parameters of sachet water. However, microbial analysis revealed that 67% of the samples were contaminated with pathogenic microorganisms, including E. coli and coliforms, indicating poor microbiological quality. While sachet water samples generally meet physicochemical safety standards, addressing microbial quality is essential to ensure the safety of drinking water in Kumasi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
An essential human right, access to clean water sources is denied to millions of people in low- and middle-income nations daily [1, 2]. Approximately 785 million people still rely on untreated water sources worldwide [3]. About 2.2 billion people globally did not have access to safe drinking water as of 2019 [4, 5].
Approximately 79% of Ghanaians reported having access to safe drinking water by 2018 and about 50% of people in northern Ghana lacked access to safe drinking water [6, 7].
According to the United Nations Children’s Fund (UNICEF), 76% of households in Ghana are at risk of drinking water that is contaminated with fecal matter. Moreover, only 4% of households treat water suitably before drinking, and 93% of households do not treat water at all [8].
Over the past few decades, improvements have been made regarding the availability of clean drinking water. Between 2000 and 2017, the proportion of the world’s population with drinking water access increased from 81 to 89% [9, 10].
Despite this development, there are still significant differences in who has access to clean drinking water, depending on where they live and who they are. For instance, compared to 9% in Latin America and the Caribbean, an estimated 63% of the population in sub-Saharan Africa did not have access to drinking water services in 2017 [11]. Inadequate access to safe drinking water negatively impacts marginalized and disadvantaged populations, such as rural communities and poor people [12].
Access to clean and safe drinking water is a concern that calls for ongoing investment and cooperation between different sectors and stakeholders. The infrastructure for water delivery can be improved, regulation and monitoring can be strengthened, and customer behavior change can be encouraged. There are still issues with maintaining drinking water quality, especially in rural regions, despite government and private sector investments in water infrastructure in Ghana to increase access to safe water [13,14,15].
Diseases like cholera, typhoid, and hepatitis A can all be contracted by drinking contaminated water [16,17,18,19]. These illnesses, especially in young children and the elderly, can result in severe dehydration, malnutrition, and in some cases, death. Exposure to hazardous compounds and chemicals in drinking water can have negative health impacts in addition to waterborne infections. For instance, high fluoride levels in water could lead to dental fluorosis, skeletal fluorosis and other harmful effects [20,21,22].
The popularity of sachet drinking water in Ghana has soared as a low-cost, practical substitute for bottled water, especially for those who cannot afford expensive water treatment systems or do not trust tap water [23,24,25,26]. However, contamination claims and insufficient treatment procedures have raised questions regarding the quality of the sachet drinking water [23,24,25,26].
Sachet water production is essential in Ghana, creating thousands of job opportunities and making a sizable economic contribution. Sachet water manufacturing makes up almost 70% of all packaged water produced in Ghana, with an estimated annual turnover of four hundred million Ghana cedis [27]. With small-scale business owners operating in various regions, the industry has also been observed to support the informal sector [27]. Additionally, the development of other allied businesses, like packaging and labeling, transportation and logistics, and distribution, has benefited from the production of sachet water, stimulating economic growth across the nation.
This study aimed to evaluate the quality of sachet drinking water sold in Ghana, focusing on assessing the microbiological safety and compliance with national and international water quality standards. This study focused exclusively on sachet water samples from Kumasi, Ghana. Therefore, the findings may not be fully representative of sachet water quality in other regions or countries.
2 Materials and methods
2.1 Study area
With a population of almost two million, Kumasi, the capital of Ghana’s Ashanti Region, is the second-largest city in the nation [28]. The city covers an area of 57 square kilometers, with some mountainous sections and a topography that is gently undulating to hilly [29]. There are two main seasons in Kumasi each year: the wet and the dry. The year averages 1300 mm of precipitation, with the main wet season between March and July and a minor rainy season between September and November [30]. The dry season, also known as harmattan, is characterized by dry, cold, hazy and dusty winds and typically lasts from the end of November to the middle of March [31]. The map of the study site is shown in Fig. 1.
2.2 Sampling
Three samples of thirty sachet drinking water brands were collected from various places, including houses, supermarkets, and street vendors across the Kumasi Metropolis, into sterilized plastic containers. The samples were gathered and brought to the laboratory at 4 °C in an ice chest.
2.3 Physicochemical properties
Standard techniques were used to analyze the sachet drinking water samples’ pH, electrical conductivity (EC), and total dissolved solids (TDS) [32]. The JENWAY pH meter was used to determine the parameters. The JENWAY pH meter/multimeter was calibrated with buffer solutions (pH 4.00, pH 7.00, and 10.00) prepared according to the manufacturer’s instructions. The buffer solutions were confirmed to be within their expiration dates. The JENWAY pH meter/multimeter was turned on and stabilized for a few minutes to reach the operating temperature. The electrode of the JENWAY pH meter was rinsed with distilled water and then immersed in the pH 7.00 buffer solution. The electrode was fully submerged, ensuring proper contact with the solution. After allowing sufficient time for stabilization, the adjustment controls on the JENWAY pH meter/multimeter were used to adjust the reading to pH 7.00. The adjustment was made following the manufacturer’s instructions. The electrode was rinsed with distilled water and immersed in the pH 4.00 buffer solution. The JENWAY pH meter/multimeter reading was adjusted using the adjustment controls. The electrode was rinsed with distilled water and immersed in the pH 10.00 buffer solution. The JENWAY 3510 pH meter/multimeter reading was adjusted per the manufacturer’s instructions. The calibration was verified by measuring the pH 7.00 buffer solution once again. The meter reading was checked to ensure consistency and closeness to pH 7.00. After calibration, the electrode was rinsed with distilled water and gently blotted with a clean tissue or cloth. The electrode was then used to determine the water samples’ pH, EC and TDS.
2.4 Determination of anions and cations
Ion chromatography (Metrohm Compact IC) was used to measure the concentration of anions (chloride, sulfate, and nitrate), and a Flame photometer (Jenway PFP7) was used to measure the cations (sodium and potassium) in the sachet drinking water samples. The titrimetric method was used to determine the alkalinity, total hardness, calcium and magnesium ion concentrations.
2.5 Determination of alkalinity
A representative water sample was collected in a clean container, ensuring its freedom from any contaminants. The sample was filtered through a Whatman No. 41 ashless filter paper to remove any suspended particles. A measured volume of 50 mL, was placed in a clean 250 mL Erlenmeyer flask. A few drops of phenolphthalein indicator were added to the sample and the sample was titrated with 0.1 M sulfuric acid solution until the solution changed from pink to colorless. The alkalinity concentration was calculated based expressed as CaCO3 equivalent.
2.6 Determination of total hardness
A representative water sample was collected in a clean container, ensuring its freedom from any contaminants. The sample was preserved as needed to prevent changes in hardness during transportation. The samples were filtered through a Whatman No. 41 ashless filter paper. A measured volume of 50 mL was placed in a clean 250 mL Erlenmeyer flask. A few drops of Eriochrome Black T indicator were added to the sample. The sample underwent titration with 0.1 M EDTA (ethylene–diamine–tetraacetic acid) solution. The EDTA solution was added dropwise to the sample while gently swirling the flask. The endpoint was signaled by a color change from wine-red to blue. The total hardness concentration was calculated based on the volume and concentration of the EDTA and expressed as CaCO3 equivalent.
2.7 Microbiological analysis
The sachet drinking water samples were analyzed microbiologically to detect the presence of total coliforms, faecal coliforms, and E. coli. The bacteriological analysis was performed using the multiple-tube fermentation technique. Nutrient agar and MacConkey agar were used as the microbial analysis culture media. Sterile conditions were maintained throughout the analysis. The microbial growth count was reported in colony-forming units per milliliter (CFU/mL) [33, 34].
2.7.1 Water Quality Index
The Weighted Arithmetic Water Quality Index Method was used to estimate the water quality index (WQI) [35, 36] for further ranking (Table 1) on the potability of sachet water.
The quality parameters are calculated using Eq. (1).
where Ap is the mean value of the parameter, S is the WHO drinking water guideline value for each parameter, and Ip is the ideal value for every parameter. All the parameters were assumed to have an ideal value of zero except pH, which is 7. The relative weight (Wp) was calculated using Eq. (2)
where K is a constant, which is estimated with Eq. (3).
The water quality index is calculated as the summation of (Wp × Qp) divided by the summation of Wp, as shown in Eq. (4).
2.8 Statistical analysis
The descriptive statistics and Pearson’s correlation were estimated using Microsoft Excel 2019 software. All the parameters were determined at a 95% confidence level.
3 Results and discussion
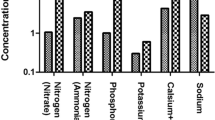
Table 2 presents the descriptive statistics of the physicochemical properties of sachet water. The pH values of the samples ranged from 5.38 to 8.03, with a skewness of 1.02. The median pH value was 6.15, which is lower than the WHO-recommended value for drinking water.
The results showed that most of the physicochemical properties of the sachet drinking water samples fell within the WHO-recommended levels for drinking water. However, the median pH value of 6.15 was lower than the recommended value of 6.5–8.5, indicating that some samples may be slightly acidic. This is a cause for concern as low pH levels can cause the release of harmful metals such as lead into the water. The main source of water for sachet water production is groundwater. Other studies on groundwater in the Kumasi metropolis and the Ashanti region reported pH values that were slightly acidic and comparable to those obtained in this study [36, 37]. In contrast, Opafola et al. [38] reported that sachet water sold within a tertiary institution in southwestern Nigeria had pH values within the WHO guidelines. The difference in pH could result from the water source for sachet water production.
The electrical conductivity values ranged from 2.24 to 43.6 µS/cm, consistent with previous studies on sachet drinking water in Ghana [23, 39]. The total dissolved solids’ concentration ranged from 2 to 42 mg/L, with a median value of 11.45 mg/L. This falls within the acceptable range set by the WHO, indicating that the samples are safe for consumption.
The concentrations of calcium, sodium, potassium, and magnesium ions were also within the acceptable range for drinking water according to WHO guidelines.
All the anion concentrations were within the WHO-acceptable concentrations. Fluoride was present in concentrations of up to 0.15 mg/L in some samples. This falls within the acceptable range set by the WHO. Exposure to high concentrations of fluoride in water can lead to dental and skeletal fluorosis [20,21,22, 40].
Table 3 shows the results of Pearson’s correlation analysis of the physicochemical properties of sachet water. There is a strong positive correlation between total dissolved solids (TDS) and electrical conductivity (EC), as indicated by Pearson’s correlation analysis with a correlation coefficient (r) of 1.00. This means that as TDS increases, the electrical conductivity of the water also increases. The relationship can be attributed to the fact that TDS measures the total amount of inorganic and organic substances dissolved in water, including dissolved salts and minerals, contributing to its electrical conductivity.
The results indicate a positive correlation between calcium (Ca2+) ions and electrical conductivity (EC) with a correlation coefficient (r) of 0.57. An increased Ca2+ ion concentration in water will likely increase the EC value. Similarly, the positive correlation between Ca2+ ions and total dissolved solids (TDS) with a correlation coefficient (r) of 0.57 suggests that an increase in the concentration of Ca2+ ions in the sachet water will likely increase the TDS value of the water.
There was a positive correlation between calcium and total hardness with a coefficient (r) of 0.52, indicating that as the concentration of calcium increases, the total hardness of the water also tends to increase. This relationship is expected, as calcium is one of the significant contributors to water hardness, along with magnesium.
The strong positive correlations in this study suggest a significant relationship between these physicochemical properties of sachet water. Specifically, the positive correlations between EC and Cl− ions, TDS and Cl− ions, and Ca2+ and total hardness suggest that the concentration of these ions may be related to the water’s overall mineral content. The positive correlations between alkalinity and F− ions and NO3− ions and EC/TDS suggest that the water source may influence these properties.
The weighted arithmetic water quality index method estimated the water quality index (Table 4). Table 4 shows the water quality index. The water quality index was calculated to be 6.21, indicating the water is of excellent quality and safe for human consumption regarding its physicochemical properties. The WQI is based on selected criteria, but overall water quality considers additional factors like biology and microbiology.
The microbiological properties of the sachet water samples are presented in Table 5. The results indicate that 67% of the samples were contaminated with pathogens. Samples S8, S12, S18, and S29 were contaminated with E. coli, fecal and total coliforms. Only ten brands were free from microbes. Obiri-Danso et al. [41], reported that sachet water sold on the streets of Kumasi contained pathogens such as E. coli, enterococci, Pseudomonas aeruginosa, and Aeromonas hydrophila. Awuah et al. [26] reported similar results, indicating that 82% of the sachet water samples contained E. coli, Salmonella, and other coliforms, corroborating results presented in this work.
The microbiological properties of water are an essential aspect of its quality because the presence of some microorganisms can pose a severe risk to human health.
Since some samples contained E.coli, fecal and total coliforms, the packaging materials, production methods, or water sources were likely contaminated. Poor hygiene and inappropriate treatment procedures used during production are blamed for contaminating sachet water with hazardous germs. The finding of E.coli, fecal and total coliforms in the water samples shows that drinking tainted sachet water carries a risk of contracting waterborne infections. Therefore, it is crucial to guarantee that sachet water’s manufacturing and packaging procedures are clean and pollutants-free. The brands of sachet water that were found to be free from microbes in this study could be a model for other producers to follow to ensure safe and high-quality sachet water production.
4 Conclusion
The results of this study show that the physicochemical properties of the drinking water samples from sachets are within the WHO-acceptable range. The median pH value was slightly below the advised range, which is of concern because low pH levels can lead to the corroding of pipes and plumbing fixtures, which releases dangerous metals like lead into the water. The study also discovered that the water samples had low microbiological quality, with hazardous pathogens such as E. coli and other coliforms in 67% of the samples.
Positive correlations between some of the physicochemical parameters show a significant connection between these variables. The physicochemical properties of the water samples may be acceptable, but other factors like biological and microbiological qualities must also be considered to assess the overall water quality.
The discovery of E.coli, fecal and total coliforms in water samples emphasizes the need to maintain proper hygiene and adequate treatment processes during sachet water production to produce safe and high-quality sachet water.
Data availability
Supplementary data will be available from the corresponding author upon request.
References
Hall RP, Van Koppen B, Van Houweling E. The human right to water: the importance of domestic and productive water rights. Sci Eng Ethics. 2014;20:849–68. https://doi.org/10.1007/S11948-013-9499-3.
Langford M. The United Nations concept of water as a human right: a new paradigm for old problems? Int J Water Resour Dev. 2005;21:273–82. https://doi.org/10.1080/07900620500035887.
Bogale GG. Hotspots of unimproved sources of drinking water in Ethiopia: mapping and spatial analysis of Ethiopia demographic and health survey data 2016. BMC Public Health. 2020;20:1–8. https://doi.org/10.1186/S12889-020-08957-2.
Raimi M, Ayibatonbira A, Anu B, et al. ‘Digging Deeper’ evidence on water crisis and its solution in Nigeria for Bayelsa State: a study of current scenario. Int J Hydro. 2023. https://doi.org/10.2139/ssrn.3422483.
Bhushan B. Design of water harvesting towers and projections for water collection from fog and condensation. Philos Trans R Soc A Math Phys Eng Sci. 2020;378:1–37.
Amuakwa-Mensah F, Klege RA, Adom PK, Köhlin G. COVID-19 and handwashing: implications for water use in sub-Saharan Africa. Water Resour Econ. 2021;36:100189. https://doi.org/10.1016/j.wre.2021.100189.
Nyika J, Dinka MO. Water challenges in rural sub-Saharan Africa. Cham: Springer; 2023. p. 39–55.
UNICEF. Water | UNICEF Ghana. 2019. https://www.unicef.org/ghana/water
Lutz W, Kc S. Dimensions of global population projections: what do we know about future population trends and structures? Philos Trans R Soc B Biol Sci. 2010;365:2779–91. https://doi.org/10.1098/RSTB.2010.0133.
Martínez-Santos P. Does 91% of the world’s population really have sustainable access to safe drinking water? Int J Water Resour Dev. 2017;33:514–33. https://doi.org/10.1080/07900627.2017.1298517.
Emenike CP, Tenebe IT, Omole DO, et al. Accessing safe drinking water in sub-Saharan Africa: issues and challenges in South–West Nigeria. Sustain Cities Soc. 2017;30:263–72. https://doi.org/10.1016/j.scs.2017.01.005.
Rodríguez-Izquierdo E, Alvarado-Velázquez J, García-Meneses PM, et al. Inequality, water accessibility, and health impacts in Chiapas, Mexico. Reg Environ Change. 2023;23:23. https://doi.org/10.1007/s10113-022-01993-1.
Perveen S, Amar-Ul-Haque. Drinking water quality monitoring, assessment and management in Pakistan: a review. Heliyon. 2023. https://doi.org/10.1016/j.heliyon.2023.e13872.
Amosah J, Lukman T, Atanga RA. Portable water sources in rural communities the experience of Togmaa community in the Wa West District. Am J Arts Hum Sci. 2023;2:8–14. https://doi.org/10.54536/ajahs.v2i1.1158.
Bazaanah P, Mothapo RA. Sustainability of drinking water and sanitation delivery systems in rural communities of the Lepelle Nkumpi Local Municipality, South Africa. Environ Dev Sustain. 2023. https://doi.org/10.1007/S10668-023-03190-4.
Funari E, Manganelli M, Sinisi L. Impact of climate change on waterborne diseases. Ann Ist Super Sanita. 2012;48:473–87. https://doi.org/10.4415/ANN_12_04_13.
Joy C, Sundar GN, Narmadha D. Artificial intelligence based test systems to resist waterborne diseases by early and rapid identification of pathogens: a review. SN Comput Sci. 2023;4:4. https://doi.org/10.1007/S42979-022-01620-0.
Woolf AD, Stierman BD, Barnett ED, et al. Drinking water from private wells and risks to children. Pediatrics. 2023;151:e2022060645. https://doi.org/10.1542/peds.2022-060645
Takuissu GR, Kenmoe S, Ebogo-Belobo JT, et al. Occurrence of hepatitis a virus in water matrices: a systematic review and meta-analysis. Environ Res Public Health. 2023;20:1054.
Srivastava S, Flora SJS. Fluoride in drinking water and skeletal fluorosis: a review of the global impact. Curr Environ Health Rep. 2020;7:140–6. https://doi.org/10.1007/s40572-020-00270-9.
Guissouma W, Hakami O, Al-Rajab AJ, Tarhouni J. Risk assessment of fluoride exposure in drinking water of Tunisia. Chemosphere. 2017;177:102–8. https://doi.org/10.1016/j.chemosphere.2017.03.011.
Qian J, Susheela DAK, Mudgal A, Keast G. Fluoride in water: an overview. Water Front. 1999;1:11–3.
Cheabu BSN, Ephraim JH. Sachet water quality in Obuasi, Ashanti Region, Ghana. J Biol Agric Healthc. 2014;4:37–42.
Addo HO, Amegah KE, Xonam TA, et al. Consumer preference and quality of sachet water sold and consumed in the Sunyani Municipality of Ghana. Biomed Res Int. 2020;2020:1. https://doi.org/10.1155/2020/3865895.
Mosi L, Adadey SM, Sowah SA, Yeboah C. Microbiological assessment of sachet water pure water from five regions in Ghana. AAS Open Res. 2019;1:12. https://doi.org/10.12688/aasopenres.12837.2.
Awuah E, Gyasi SF, Anipa HK, Adjei A. Microbial quality of sachet and bagged drinking water: a case study in Kumasi, Ghana. Res J Microbiol. 2014;9:199–207. https://doi.org/10.3923/jm.2014.199.207.
Kusi LY, Agbeblewu S, Nyarku KM. Challenges and prospects confronting commercial water production and distribution industry: a case study of the Cape Coast Metropolis. Int J Manag Sci. 2015;5:544–55.
Bosompem C, Stemn E, Fei-Baffoe B. Multi-criteria GIS-based siting of transfer station for municipal solid waste: the case of Kumasi Metropolitan Area, Ghana. Waste Manag Res. 2016;34:1054–63. https://doi.org/10.1177/0734242X16658363.
Bolor VK, Boadi NO, Borquaye LS, Afful S. Human risk assessment of organochlorine pesticide residues in vegetables from Kumasi, Ghana. J Chem. 2018;2018:1–11. https://doi.org/10.1155/2018/3269065Research.
Darko G, Dodd M, Nkansah MA, et al. Distribution and ecological risks of toxic metals in the topsoils in the Kumasi metropolis, Ghana. Cogent Environ Sci. 2017;3:1354965. https://doi.org/10.1080/23311843.2017.1354965.
Okeahialam BN. Article commentary: the cold dusty harmattan: a season of Anguish for cardiologists and patients. Environ Health Insights. 2016. https://doi.org/10.4137/EHI.S38350.
Beutler M, Wiltshire KH, Meyer B, et al. APHA (2005), Standard methods for the examination of water and wastewater, Washington DC: American Public Health Association. Ahmad, SR, and DM Reynolds (1999), Monitoring of water quality using fluorescence technique: prospect of on-line process control. Dissolved Oxyg Dyn Model Case Study A Subtrop Shallow Lake. 1999;217:95.
Brenner KP, Rankin CC, Roybal YR, et al. New medium for the simultaneous detection of total coliforms and Escherichia coli in water. Appl Environ Microbiol. 1993;59:3534–44. https://doi.org/10.1128/aem.59.11.3534-3544.1993.
APHA. Standard Methods for the examination of water and wastewater. Washington, DC: APHA; 2012.
Călmuc VA, Călmuc M, Țopa CM, et al. Various methods for calculating the water quality index. Ann “Dunarea Jos” Univ Galati Fascicle II Math Physics Theor Mech. 2018;41:171–8.
Boadi NO, Saah SA, Baa-poku F, et al. Safety of borehole water as an alternative drinking water source. Sci Afr. 2020;10: e00657. https://doi.org/10.1016/j.sciaf.2020.e00657.
Nkansah MA, Boadi NO, Badu M. Assessment of the quality of water from hand-dug wells in Ghana. Environ Health Insights. 2010;4:7–12.
Opafola OT, Oladepo KT, Ajibade FO, David AO. Potability assessment of packaged sachet water sold within a tertiary institution in southwestern Nigeria. J King Saud Univ Sci. 2020;32:1999–2004. https://doi.org/10.1016/j.jksus.2020.02.004.
Malik A, Saah SA, Boadi NO. Physicochemical and microbial properties of sachet water in the Kumasi Metropolis of Ghana. Ghana J Chem. 2014;1:1–11. https://doi.org/10.1044/leader.PPL.19102014.18.
Sunkari ED, Zango MS, Korboe HM. Comparative analysis of fluoride concentrations in groundwaters in Northern and Southern Ghana: implications for the contaminant sources. Earth Syst Environ. 2018;2:103–17. https://doi.org/10.1007/s41748-018-0044-z.
Obiri-Danso K, Okore-Hanson AK, Jones K. The microbiological quality of drinking water sold on the streets in Kumasi. Ghana. 2003;37:334–9. https://doi.org/10.1046/j.1472-765X.2003.01403.x.
Acknowledgements
The authors would like to thank the Department of Chemistry, Kwame Nkrumah University of Science and Technology, Kumasi, for providing resources for this research.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NOB and SAA wrote the main manuscript. MB, FB-P, FO and POS edited the manuscript. All reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boadi, N.O., Saah, S.A., Badu, M. et al. Assessment of sachet water quality in Kumasi, Ghana. Discov Water 3, 24 (2023). https://doi.org/10.1007/s43832-023-00048-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00048-8