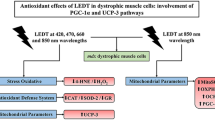
Abstract
This study is aimed at investigating the effects of LEDT, at multiple wavelengths, on intracellular calcium concentration; on transient receptor potential canonical channels; on calcium-binding protein; on myogenic factors; on myosin heavy chains; on Akt signaling pathway; on inflammatory markers; and on the angiogenic-inducing factor in dystrophic muscle cell culture experimental model. Dystrophic primary muscle cells were submitted to LEDT, at multiple wavelengths (420 nm, 470 nm, 660 nm, and 850 nm), and evaluated after 48 h for cytotoxic effects and intracellular calcium content. TRPC-1, TRPC-6, Calsequestrin, MyoD, Myogenin, MHC-slow, MHC-fast, p-AKT, p-mTOR, p-FoxO1, Myostatin, NF-κB, TNF-α, and VEGF levels were evaluated in dystrophic primary muscle cells by western blotting. The LEDT, at multiple wavelengths, treated-mdx muscle cells showed no cytotoxic effect and significant lower levels in [Ca2 +]i. The mdx muscle cells treated with LEDT showed a significant reduction of TRPC-1, NF-κB, TNF-α and MyoD levels and a significant increase of Myogenin, MHC-slow, p-AKT, p-mTOR, p-FoxO1 levels, and VEGF levels. Our findings suggest that different LEDT wavelengths modulate the Akt-signaling pathways and attenuate pathological events in dystrophic muscle cells, and a combined multiwavelength irradiation protocol may even provide a potentially therapeutic strategy for muscular dystrophies.
Graphical abstract










Similar content being viewed by others
References
Vanin, A. A., Verhagen, E., Barboza, S. D., Costa, L. O. P., & Leal-Junior, E. C. P. (2018). Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: A systematic review and meta-analysis. Lasers in Medical Science, 33(1), 181–214. https://doi.org/10.1007/s10103-017-2368-6
Rupel, K., Zupin, L., Colliva, A., Kamada, A., Poropat, A., Ottaviani, G., Gobbo, M., Fanfoni, L., Gratton, R., Santoro, M., di Lenarda, R., Biasotto, M., & Zacchigna, S. (2018). Photobiomodulation at multiple wavelengths differentially modulates oxidative stress in vitro and in vivo. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2018/6510159
Leal Junior, E. C. P., Lopes-Martins, R. A. B., Rossi, R. P., Marchi, T., Baroni, B. M., Godoi, V., Marcos, R. L., Ramos, L., & Bjordal, J. M. (2009). Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers in Surgery and Medicine, 41(8), 572–577. https://doi.org/10.1002/lsm.20810
Baroni, B. M., Leal Junior, E. C. P., Geremia, J. M., Diefenthaeler, F., & Vaz, M. A. (2010). Effect of light-emitting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomedicine and Laser Surgery, 28(5), 653–658. https://doi.org/10.1089/pho.2009.2688
Leal Junior, E. C., Godoi, V., Mancalossi, J. L., Rossi, R. P., Marchi, T., Parente, M., Grosselli, D., Generosi, R. A., Basso, M., Frigo, L., Tomazoni, S. S., Bjordal, J. M., & Lopes-Martins, R. A. B. (2011). Comparison between cold water immersion therapy (CWIT) and light emitting diode therapy (LEDT) in short-term skeletal muscle recovery after high-intensity exercise in athletes–preliminary results. Lasers in Medical Science, 26(4), 493–501. https://doi.org/10.1007/s10103-010-0866-x
Silveira, P. C. L., Ferreira, G. K., Zaccaron, R. P., Glaser, V., Remor, A. P., Mendes, C., Pinho, R. A., & Latini, A. (2019). Effects of photobiomodulation on mitochondria of brain, muscle, and C6 astroglioma cells. Medical Engineering & Physics, 71, 108–113. https://doi.org/10.1016/j.medengphy.2019.05.008
Silva, A. A. O., Leal-Junior, E. C. P., D’Avila, K. A. L., Serra, A. J., Albertini, R., França, C. M., Nishida, J. A., & Carvalho, P. T. C. (2015). Pre-exercise low-level laser therapy improves performance and levels of oxidative stress markers in mdx mice subjected to muscle fatigue by high-intensity exercise. Lasers in Medical Science, 30(6), 1719–1727. https://doi.org/10.1007/s10103-015-1777-7
Macedo, A. B., Mizobuti, D. S., Hermes, T. A., Mâncio, R. D., Pertille, A., Kido, L. A., Cagnon, V. H. A., & Minatel, E. (2020). Photobiomodulation therapy for attenuating the dystrophic phenotype of Mdx mice. Photochemistry and Photobiology, 96(1), 200–207. https://doi.org/10.1111/php.13179
Albuquerque-Pontes, G. M., Casalechi, H. L., Tomazoni, S. S., Serra, A. J., Ferreira, C. S. B., Brito, R. B. O., Melo, B. L., Vanin, A. A., Monteiro, K. K. D. S., Dellê, H., Frigo, L., Marcos, R. L., Carvalho, P. T. C., & Leal-Junior, E. C. P. (2018). Photobiomodulation therapy protects skeletal muscle and improves muscular function of mdx mice in a dose-dependent manner through modulation of dystrophin. Lasers in Medical Science, 33(4), 755–764. https://doi.org/10.1007/s10103-017-2405-5
Salam, E. A., Abdel-Meguid, I., & Korraa, S. (2007). Markers of oxidative stress and aging in Duchene muscular dystrophy patients and the possible ameliorating effect of He: Ne laser. Acta Myologica, 26(1), 14–21.
Macedo, A. B., Moraes, L. H. R., Mizobuti, D. S., Fogaça, A. R., Moraes, F. R. S., Hermes, T. A., Pertille, A., & Minatel, E. (2015). Low-level laser therapy (LLLT) in dystrophin-deficient muscle cells: effects on regeneration capacity, inflammation response and oxidative stress. PLoS ONE, 10(6), e0128567. https://doi.org/10.1371/journal.pone.0128567
Rando, T. A., & Blau, H. M. (1994). Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. Journal of Cell Biology, 125(6), 1275–1287. https://doi.org/10.1083/jcb.125.6.1275
Mizobuti, D. S., Fogaça, A. R., Moraes, F. R. S., Moraes, L. H. R., Mâncio, R. D., Hermes, T. A., Macedo, A. B., Valduga, A. H., Lourenço, C. C., Pereira, E. C. L., & Minatel, E. (2019). Coenzyme Q10 supplementation acts as antioxidant on dystrophic muscle cells. Cell Stress and Chaperones, 24(6), 1175–1185. https://doi.org/10.1007/s12192-019-01039-2
Borenfreund, E., & Puerner, J. A. (1985). A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). Journal of Tissue Culture Methods, 9, 7–9. https://doi.org/10.1007/BF01666038
Hoffman, E. P. (2020). The discovery of dystrophin, the protein product of the Duchenne muscular dystrophy gene. The FEBS Journal, 287(18), 3879–3887. https://doi.org/10.1111/febs.15466
Leal Junior, E. C. P., Lopes-Martins, R. A. B., Baroni, B. M., Marchi, T., Rossi, R. P., Grosselli, D., Generosi, R. A., Godoi, V., Basso, M., Mancalossi, J. L., Bjordal, J. M. (2009). Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomedicine and Laser Surgery, 27(4), 617-623. https://doi.org/10.1089/pho.2008.2350
Wu, S., & Xing, D. (2014). Intracellular signaling cascades following light irradiation. Laser & Photonics Reviews, 8(1), 115–130. https://doi.org/10.1002/lpor.201300015
Vandebrouck, C., Martin, D., Schoor, M. C., Debaix, H., & Gailly, P. (2002). Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. The Journal of Cell Biology, 158(6), 1089–1096. https://doi.org/10.1083/jcb.200203091
Gervásio, O. L., Whitehead, N. P., Yeung, E. W., Phillips, W. D., & Allen, D. G. (2008). TRPC1 binds to caveolin-3 and is regulated by Src kinase—role in Duchenne muscular dystrophy. Journal of Cell Science, 121(13), 2246–2255. https://doi.org/10.1242/jcs.032003
Franco, A., Jr., & Lansman, J. B. (1990). Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature, 344(6267), 670–673. https://doi.org/10.1038/344670a0
Vandebrouck, C., Duport, G., Cognard, C., & Raymond, G. (2001). Cationic channels in normal and dystrophic human myotubes. Neuromuscular Disorders, 11(1), 72–79. https://doi.org/10.1016/s0960-8966(00)00153-x
Huang, Y., Nagata, K., Tedford, C. E., & Hamblin, M. R. (2014). Low-level laser therapy (810 nm) protects primary cortical neurons against excitotoxicity in vitro. Journal of Biophotonics, 7(8), 656–664. https://doi.org/10.1002/jbio.201300125
Ryu, J. J., Yoo, S., Kim, K. Y., Park, J. S., Bang, S., Lee, S. H., Yang, T. J., Cho, H., & Hwang, S. W. (2010). Laser modulation of heat and capsaicin receptor TRPV1 leads to thermal antinociception. Journal of Dental Research, 89(12), 1455–1460. https://doi.org/10.1177/0022034510381394
Chung, H. S., Kim, G. E., Holewinski, R. J., Venkatraman, V., Zhu, G., Bedja, D., Kass, D. A., & Eyk, J. E. V. (2017). Transient receptor potential channel 6 regulates abnormal cardiac S-nitrosylation in Duchenne muscular dystrophy. Proceedings of the National Academy of Sciences of the United States of America, 114(50), 10763–10771. https://doi.org/10.1073/pnas.1712623114
Doran, P., Dowling, P., Lohan, J., McDonnell, K., Poetsch, S., & Ohlendieck, K. (2004). Subproteomics analysis of Ca+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle. European Journal of Biochemistry, 271(19), 3943–3952. https://doi.org/10.1111/j.1432-1033.2004.04332.x
Pertille, A., Carvalho, C. L. T., Matsumura, C. Y., Neto, H. S., & Marques, M. J. (2010). Calcium-binding proteins in skeletal muscles of the mdx mice: Potential role in the pathogenesis of Duchenne muscular dystrophy. International Journal of Experimental Pathology, 91(1), 63–71. https://doi.org/10.1111/j.1365-2613.2009.00688.x
Vieira, W. F., Kenzo-Kagawa, B., Alvares, L. E., Cogo, J. C., Baranauskas, V., & Cruz-Höfling, M. A. (2021). Exploring the ability of low-level laser irradiation to reduce myonecrosis and increase Myogenin transcription after Bothrops jararacussu envenomation. Photochemical & Photobiological Sciences, 20(4), 571–583. https://doi.org/10.1007/s43630-021-00041-x
Vatansever, F., Rodrigues, N. C., Assis, L. L., Peviani, S. S., Durigan, J. L., Moreira, F. M. A., Hamblin, M. R., & Parizotto, N. A. (2012). Low intensity laser therapy accelerates muscle regeneration in aged rats. Photonics Lasers in Medicine, 1(4), 287–297. https://doi.org/10.1515/plm-2012-0035
Podkalicka, P., Mucha, O., Dulak, J., & Loboda, A. (2019). Targeting angiogenesis in Duchenne muscular dystrophy. Cellular and Molecular Life Sciences, 76(8), 1507–1528. https://doi.org/10.1007/s00018-019-03006-7
Ferrara, N. (2002). Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Seminars in Oncology, 29(6), 10–14. https://doi.org/10.1053/sonc.2002.37264
Arsic, N., Zacchigna, S., Zentilin, L., Ramirez-Correa, G., Pattarini, L., Salvi, A., Sinagra, G., & Giacca, M. (2004). Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Molecular Therapy, 10(5), 844–854. https://doi.org/10.1016/j.ymthe.2004.08.007
Chalkiadaki, A., Igarashi, M., Nasamu, A. S., Knezevic, J., & Guarente, L. (2014). Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of duchenne muscular dystrophy. PLoS Genetics, 10(7), e1004490. https://doi.org/10.1371/journal.pgen.1004490
Webster, C., Silberstein, L., Hays, A. P., & Blau, H. M. (1988). Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell, 52(4), 503–513. https://doi.org/10.1016/0092-8674(88)90463-1
Miura, P., & Jasmin, B. J. (2006). Utrophin up-regulation for treating Duchenne or Becker muscular dystrophy: How close are we? Trends in Molecular Medicine, 12(3), 122–129. https://doi.org/10.1016/j.molmed.2006.01.002
Rommel, C., Bodine, S. C., Clarke, B. A., Rossman, R., Nunez, L., Stitt, T. N., Yancopoulos, G. D., & Glass, D. J. (2001). Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nature Cell Biology, 3(11), 1009–1013. https://doi.org/10.1038/ncb1101-1009
Kim, M. H., Kay, D. I., Rudra, R. T., Chen, B. M., Hsu, N., Izumiya, Y., Martinez, L., Spencer, M. J., Walsh, K., Grinnell, A. D., & Crosbie, R. H. (2001). Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function in dystrophin-deficient mdx mice. Human Molecular Genetics, 20(7), 1324–1338. https://doi.org/10.1093/hmg/ddr015
Bodine, S. C., Stit, T. N., Gonzalez, M., Kline, W. O., Stover, G. L., Bauerlein, R., Zlotchenko, E., Scrimgeour, A., Lawrence, J. C., Glass, D. J., & Yancopoulos, G. D. (2001). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology, 3(11), 1014–1019. https://doi.org/10.1038/ncb1101-1014
Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149(2), 274–293. https://doi.org/10.1016/j.cell.2012.03.017
Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. J., Blenis, J., & Greenberg, M. E. (1999). Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell, 96(6), 857–868. https://doi.org/10.1016/s0092-8674(00)80595-4
Hribal, M. L., Nakae, J., Kitamura, T., Shutter, J. R., & Accili, D. (2003). Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. The Journal of Cell Biology, 162(4), 535–541. https://doi.org/10.1083/jcb.200212107
Kitamura, T., Kitamura, Y. I., Funahashi, Y., Shawber, C. J., Castrillon, D. H., Kollipara, R., DePinho, R. A., Kitajewski, J., & Accili, D. (2007). A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. The Journal of Clinical Investigation, 117(9), 2477–2485. https://doi.org/10.1172/JCI32054
Allen, D. L., & Unterman, T. G. (2007). Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. American Journal of Physiology, 292(1), 188–199. https://doi.org/10.1152/ajpcell.00542.2005
Takahashi, A., Kureishi, Y., Yang, J., Luo, Z., Guo, K., Mukhopadhyay, D., Ivashchenko, Y., Branellec, D., & Walsh, K. (2002). Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Molecular and Cellular Biology, 22(13), 4803–4814. https://doi.org/10.1128/MCB.22.13.4803-4814.2002
Leal-Junior, E. C. P., Almeida, P., Tomazoni, S. S., Carvalho, P. T. C., Lopes-Martins, R. A. B., Frigo, L., Joensen, J., Johnson, M. I., & Bjordal, J. M. (2014). Superpulsed low-level laser therapy protects skeletal muscle of mdx mice against damage, inflammation and morphological changes delaying dystrophy progression. PLoS ONE, 9(3), e89453. https://doi.org/10.1371/journal.pone.0089453
Weichhart, T., Costantino, G., Poglitsch, M., Rosner, M., Zeyda, M., Stuhlmeier, K. M., Kolbe, T., Stulnig, T. M., Hörl, W. H., Hengstschläger, M., Müller, M., & Säemann, M. D. (2008). The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity, 29(4), 567–577. https://doi.org/10.1016/j.immuni.2008.08.012
Wang, D., Yin, Y., Yang, Y., Lv, P., Shi, Y., Lu, L., & Wei, L. (2014). Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. International Immunopharmacology, 19(2), 206–213. https://doi.org/10.1016/j.intimp.2014.02.002
Della Santa, G. M. L., Ferreira, M. C., Machado, T. P. G., Oliveira, M. X., & Santos, A. P. (2021). Effects of photobiomodulation therapy (LED 630 nm) on muscle and nerve histomorphometry after axonotmesis. Photochemistry and Photobiology, 97(5), 1116–1122. https://doi.org/10.1111/php.13415
Rohringer, S., Holnthoner, W., Chaudary, S., Slezak, P., Priglinger, E., Strassl, M., Pill, K., Mühleder, S., Redl, H., & Dungel, P. (2017). The impact of wavelengths of LED light-therapy on endothelial cells. Scientific Reports. https://doi.org/10.1038/s41598-017-11061-y
Wunsch, A., & Matuschka, K. (2014). A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Photomedicine and Laser Surgery, 32(2), 93–100. https://doi.org/10.1089/pho.2013.3616
Sorbellini, E., Rucco, M., & Rinaldi, F. (2018). Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: An update. Lasers in Medical Science, 33(7), 1431–1439. https://doi.org/10.1007/s10103-018-2584-8
Campos, G. R. S., de Moura, K. M. B., Barbosa, A. M., Zamuner, L. F., Nadur-Andrade, N., Dale, C. S., Gutiérrez, J. M., Chavantes, M. C., & Zamuner, S. R. (2018). Light-emitting diode (LED) therapy reduces local pathological changes induced by Bothrops asper snake venom. Toxicon, 152, 95–102. https://doi.org/10.1016/j.toxicon.2018.07.029
Ferraresi, C., dos Santos, R. V., Marques, G., Zangrande, M., Leonaldo, R., Hamblin, M. R., Bagnato, V. S., & Parizotto, N. A. (2015). Light-emitting diode therapy (LEDT) before matches prevents increase in creatine kinase with a light dose response in volleyball players. Lasers in Medical Science, 30(4), 1281–1287. https://doi.org/10.1007/s10103-015-1728-3
Tomazoni, S. S., Casalechi, H. L., Ferreira, C. S. B., Serra, A. J., Dellê, H., Brito, R. B. O., Melo, B. L., Vanin, A. A., Ribeiro, N. F., Pereira, A. L., Monteiro, K. K. D. S., Marcos, R. L., Carvalho, P. T. C., Frigo, L., & Leal-Junior, E. C. P. (2020). Can photobiomodulation therapy be an alternative to pharmacological therapies in decreasing the progression of skeletal muscle impairments of mdx mice? PLoS ONE, 15(8), e0236689. https://doi.org/10.1371/journal.pone.0236689
Acknowledgements
We thank Mrs. Deirdre Jane Donovan Giraldo for the English revision of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; #2020/09733-4 and #2009/05992-6), Coordenação de Pessoal de Nível Superior-Brasil (CAPES)—Finance Code 001, CNPq and FAEPEX. G.L.R, D.S.M, and C.C.L were the recipients of a CAPES fellowship. C.C. was the recipient of a CNPq fellowship. H.N.M.S is the recipient of a CNPq fellowship.
Author information
Authors and Affiliations
Contributions
The authors have contributed substantially to conception and design, acquisition of data, analysis, and interpretation of data. All authors participated in drafting the article, revised it critically for important intellectual content, and gave final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
da Rocha, G.L., Mizobuti, D.S., da Silva, H.N.M. et al. Multiple LEDT wavelengths modulate the Akt signaling pathways and attenuate pathological events in mdx dystrophic muscle cells. Photochem Photobiol Sci 21, 1257–1272 (2022). https://doi.org/10.1007/s43630-022-00216-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00216-0