Abstract
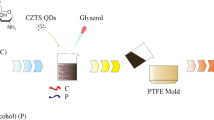
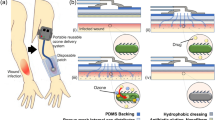
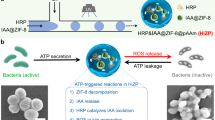
The search for new materials that can be applied in the treatment of injured human tissues has led to the development of new dressings. Membranes have potential as dressing materials because they can be fitted to and interact with the tissue surface. In this study, we analyze the morphological properties and wettability of latex membranes, along with the incorporation of the photosensitizer methylene blue, in the context of the utility of the membranes in curative applications involving photodynamic therapy (PDT). It was observed that deposition of the photosensitizer into latex membranes increased both the surface roughness and wettability. Antifungal testing indicated that antimicrobial PDT assisted by the latex membranes incorporating methylene blue effectively inactivated Candida albicans.




Similar content being viewed by others
References
Burn prevention: success stories and lessons learned - World Health Organization - March 2011 (ISBN 978 92 4 150118 7). https://www.who.int/publications/i/item/9789241501187
Khoo, T. L., Halim, A. S., Saad, A. Z. M., & Dorai, A. A. (2010). The application of glycerol-preserved skin allograft in the treatment of burn injuries: An analysis based on indications. Burns, 36, 897–904. https://doi.org/10.1016/j.burns.2009.03.007
Church, D., Elsayed, S., Reid, O., Winston, B., & Lindsay, R. (2006). Burn wound infections. Clinical Microbiology Reviews, 19, 403–434. https://doi.org/10.1128/CMR.19.2.403-434.2006
Wasiak, J., Cleland, H., & Campbell, F. (2008). Dressings for superficial and partial thickness burns. In J. Wasiak (Ed.), Cochrane database systematic reviews. Chichester: Wiley. https://doi.org/10.1002/14651858.CD002106.pub3
Wattanakaroon, W., Akanitkul, P., Kaowkanya, W., & Phoudee, W. (2017). Albumin-natural rubber latex composite as a dermal wound dressing. Materials Today: Proceedings, 4, 6633–6640. https://doi.org/10.1016/j.matpr.2017.06.178
Silva, A. J., Silva, J. R., de Souza, N. C., & Souto, P. C. S. (2014). Membranes from latex with propolis for biomedical applications. Materials Letters, 116, 235–238. https://doi.org/10.1016/j.matlet.2013.11.045
Krupp, T., dos Santos, B. D., Gama, L. A., Silva, J. R., Arrais-Silva, W. W., de Souza, N. C., Américo, M. F., & de Souza Souto, P. C. (2019). Natural rubber—propolis membrane improves wound healing in second-degree burning model. International Journal of Biological Macromolecules, 131, 980–988. https://doi.org/10.1016/j.ijbiomac.2019.03.147
Jori, G., Fabris, C., Soncin, M., Ferro, S., Coppellotti, O., Dei, D., Fantetti, L., Chiti, G., & Roncucci, G. (2006). Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers in Surgery and Medicine, 38, 468–481. https://doi.org/10.1002/lsm.20361
Mroz, P., Huang, Y., Szokalska, A., Zhiyentayev, T., Janjua, S., Nifli, A.-P., Sherwood, M. E., Ruzié, C., Borbas, K. E., Fan, D., Krayer, M., Balasubramanian, T., Yang, E., Kee, H., Kirmaier, C., Diers, J., Bocian, D., Holten, D., Lindsey, J. S., & Hamblin, M. R. (2010). Stable synthetic bacteriochlorins overcome the resistance of melanoma to photodynamic therapy. The FASEB Journal, 24, 3160–3170. https://doi.org/10.1096/fj.09-152587
Choi, K.-H., Wang, K.-K., Oh, S.-L., Im, J.-E., Kim, B.-J., Park, J.-C., Choi, D., Kim, H.-K., & Kim, Y.-R. (2010). Singlet oxygen generating nanolayer coatings on NiTi alloy for photodynamic application. Surface & Coatings Technology, 205, S62–S67. https://doi.org/10.1016/j.surfcoat.2010.04.033
Moore, E. C., Padiglione, A. A., Wasiak, J., Paul, E., & Cleland, H. (2010). Candida in burns: Risk factors and outcomes. Journal of Burn Care & Research, 31, 257–263. https://doi.org/10.1097/BCR.0b013e3181d0f536
Acar, A., Uygur, F., Diktaş, H., Evinç, R., Lkür, E. Ü., Öncül, O., & Görenek, L. (2011). Comparison of silver-coated dressing (ActicoatW), chlorhexidine acetate 0.5% (BactigrassW) and nystatin for topical antifungal effect in Candida albicans-contaminated, full-skin-thickness rat burn wounds. Burns, 37, 882–885. https://doi.org/10.1016/j.burns.2011.01.024
Gomes, D. J. C., de Souza, N. C., & Silva, J. R. (2013). Using a monocular optical microscope to assemble a wetting contact angle analyser. Measurement, 46, 3623–3627. https://doi.org/10.1016/j.measurement.2013.07.010
Costa Pedro, M. F., Kalck, A. S., dos Santos, K. F., Sousa, M. S., Romio, K. B., Souto, P. C. S., Silva, J. R., & de Souza, N. C. (2018). Immobilization of triclosan and erythrosine in layer-by-layer films applied to inactivation of microorganisms. Photodiagnosis Photodynamic Therapy, 22, 158–165. https://doi.org/10.1016/j.pdpdt.2018.04.012
Peloi, L. S., Soares, R. R. S., Biondo, C. E. G., Souza, V. R., & Hioka, N. (2008). Elza Kimura, Photodynamic effect of light-emitting diode light on cell growth inhibition induced by methylene blue. Journal of Biosciences, 33, 231–237. https://doi.org/10.1007/s12038-008-0040-9
Tardivo, J. P., Giglio, A. D., de Oliveira, C. S., Gabrielli, D. S., Junqueira, H. C., Tada, D. B., Severino, D., de Fátima Turchiello, R., & Baptista, M. S. (2005). Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis and Photodynamic Therapy, 2, 175–191. https://doi.org/10.1016/S1572-1000(05)00097-9
Pasquetti, M., Chiavassa, E., Tizzani, P., Danesi, P., & APeano, . (2015). Agar diffusion procedures for susceptibility testing of malassezia pachydermatis: evaluation of Mueller-Hinton agar plus 2 % glucose and 0.5 µg/ml methylene blue as the test medium. Mycopathologia, 180, 153–158. https://doi.org/10.1007/s11046-015-9913-2
Tolnai, S. (1975). A method for viable cell count. Tissue Culture Association Manual, 1, 37–38. https://doi.org/10.1007/BF00914435
Chermsirivathana, S. (1952). A rapid method of staining for fungus and monilial infection. The Journal of Investigative Dermatology, 19, 7. https://doi.org/10.1038/jid.1952.60
Webb, R. J., Berger, L., Skerratt, L. F., & Roberts, A. A. (2019). A rapid and inexpensive viability assay for zoospores and zoosporangia of Batrachochytrium dendrobatidis. Journal of Microbiological Methods, 165, 105688. https://doi.org/10.1016/j.mimet.2019.105688
Ge, X., Gao, M., Situ, B., Feng, W., He, B., He, X., Li, S., Ou, Z., Zhong, Y., Lin, Y., Ye, X., Hu, X., Tang, B. Z., & Zheng, L. (2020). One-step, rapid fluorescence sensing of fungal viability based on a bioprobe with aggregation-induced emission characteristics. Materials Chemistry Frontiers. https://doi.org/10.1039/C9QM00732F
Kwolek-Mirek, M., & Zadrag-Tecza, R. (2014). Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Research, 14, 1068. https://doi.org/10.1111/1567-1364.12202
dos Santos, K. F., Sousa, M. S., Valverde, J. V. P., Olivati, C. A., Souto, P. C. S., Silva, J. R., & de Souza, N. C. (2019). Fractal analysis and mathematical models for the investigation of photothermal inactivation of Candida albicans using carbon nanotubes. Colloids and Surfaces B: Biointerfaces, 180, 393–400. https://doi.org/10.1016/j.colsurfb.2019.05.002
Romio, K. B., dos Santos, K. F., da Silva, R. J., Pedro, M. F. C., Kalck, A. S., da Silva Sousa, M., Possamai, L. M., Souto, P. C. S., Silva, J. R., & de Souza, N. C. (2017). Incorporation of triclosan and acridine orange into liposomes for evaluating the susceptibility of Candida albicans. Journal Photochemistry Photobiology B: Biology, 173, 514–521. https://doi.org/10.1016/j.jphotobiol.2017.06.034
Strugger, S. (1948). Fluorescence microscope examination of bacteria in soil. Canadian Journal Research, 26c(2), 188–193. https://doi.org/10.1139/cjr48c-019
Guo, R., McGoverin, C., Swift, S., & Vanholsbeeck, F. (2017). A rapid and low-cost estimation of bacteria counts in solutionusing fluorescence spectroscopy. Analytical and Bioanalytical Chemistry, 409, 3959–3967. https://doi.org/10.1007/s00216-017-0347-1
Martino, R. F., Davicino, R. C., Mattar, M. A., Casali, Y. A., Correa, S. G., & Micalizzi, B. (2011). In vivo effect of three fractions of Larrea divaricata Cav (jarilla) on the innate immune system: Macrophage response against Candida albicans. Mycoses, 54, e718–e725. https://doi.org/10.1111/j.1439-0507.2010.02006.x
Paramanantham, P., Antony, A. P., Sruthil Lal, S. B., Sharan, A., Syed, A., Ahmed, M., Alarfaj, A. A., Busi, S., Maaza, M., & Kaviyarasu, K. (2018). Antimicrobial photodynamic inactivation of fungal biofilm using amino functionalized mesoporus silica-rose bengal nanoconjugate against Candida albicans. Scientific African. https://doi.org/10.1016/j.sciaf.2018.e00007
Chick, E. W. (1961). Acridine orange fluorescent stain for fungi. Archives of Dermatology, 83, 305–309. https://doi.org/10.1001/archderm.1961.01580080135015
Denny, M. W. (2008). The Intrigue of the Interface. Science, 320, 931–934. https://doi.org/10.1126/science.1156023
Petty, M. C. (1996). Langmuir-Blodgett films : An introduction (p. 234). Cambridge: Cambridge University Press.
de Souza, N. C., Flores, J. C. J., & Silva, J. R. (2009). Layer-by-layer films from tartrazine dye with bovine serum albumin. Chemical Physics Letters, 484(1–3), 33–36. https://doi.org/10.1016/j.cplett.2009.10.065
de Souza, N. C., Cavalheri, A. S., Brito, J. B., Job, A. E., Oliveira, O. N., Jr., Giacometti, J. A., & Silva, J. R. (2012). Photoinduced orientation in natural rubber. Chemical Physics Letters, 531, 110–113. https://doi.org/10.1016/j.cplett.2012.01.070
Usacheva, M. N., Teichert, M. C., & Biel, M. A. (2003). The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. Journal of Photochemistry and Photobiology B: Biology, 71(1–3), 87–98. https://doi.org/10.1016/j.jphotobiol.2003.06.002
Bartlett, J. A., & Indig, G. L. (1999). Effect of self-association and protein binding on the photochemical reactivity of triarylmethanes. Implications of noncovalent interactions on the competition between photosensitization mechanisms type I and type II. Photochemistry and Photobiology, 70(4), 490–498. https://doi.org/10.1111/j.1751-1097.1999.tb08243.x
Shin, S., Seo, J., Han, H., Kang, S., Kim, H., & Lee, T. (2016). Bio-Inspired extreme wetting surfaces for biomedical applications. Materials, 9, 116–141. https://doi.org/10.3390/ma9020116
Falde, E. J., Yohe, S. T., Colson, Y. L., & Grinstaff, M. W. (2016). Superhydrophobic materials for biomedical applications. Biomaterials, 104, 87–103. https://doi.org/10.1016/j.biomaterials.2016.06.050
Kamoun, E. A., Kenawy, E. R. S., & Chen, X. (2017). A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. Journal of Advanced Research, 8(3), 217–233. https://doi.org/10.1016/j.jare.2017.01.005
Barabási, A.-L., & Stanley, H. E. (1995). Fractal concepts in surface growth. New York: Press Syndicate of the University of Cambridge.
Gorza, F. D. S., da Silva, R. J., Trescher, T. F., Pedro, G. C., de Sousa, M. A. O., Souto, P. C. S., Silva, J. R., & de Souza, N. C. (2016). Immobilization of chlorophyll by using layer-by-layer technique for controlled release systems and photodynamic inactivation. Photodiagnosis and Photodynamic Therapy, 15, 147–155. https://doi.org/10.1016/j.pdpdt.2016.06.010
Acknowledgements
This work was supported by CNPq (Brazil). Oliveira de Sousa, dos Santos, Sousa, de Faria, Ribeiro and Rocha would like to thank CAPES for the scholarship. Valverde would like to thank CNPq for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O. Sousa, M.A., de Faria, M.A.C., Ribeiro, R.P. et al. Latex membranes with methylene blue dye for antimicrobial photodynamic therapy. Photochem Photobiol Sci 20, 1027–1032 (2021). https://doi.org/10.1007/s43630-021-00077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-021-00077-z