Abstract
The ubiquitous presence of plastic brought on by the extensive use of plastic products calls for efficient and rapid plastic detection methods to detect and evaluate pollution. The commonly used Nile red dye takes many hours and is expensive while also not equally efficient across all the common plastic waste. To address this, we investigated the staining efficiency and optimized the ratio of a combined Coomassie brilliant blue and Methylene blue dye. In the optimisation process, Methanol-based Coomassie and Methylene blue dyes effectively stained the Polyethylene Terephthalate (PET), Polypropylene (PP), Polystyrene (PS), Linear Low-Density Polyethylene (LLDPE), Low-Density Polyethylene (LDPE), and Linear Low-Density Polyethylene (HDPE) plastics without compromising the plastic's integrity. Image analysis showed a generally better staining efficacy compared to Nile red. Through systematic experimentation, we identified specific optimal ratios of Coomassie (C) brilliant blue: Methylene (M) blue for various plastics: 5:5 (mass) for PVC, 7:3 (mass) for PET, and 8:2 (mass) for PP, LDPE, and HDPE. Additionally, the ratio of 10:0 (mass) was found suitable for PS and LLDPE. Given the cost-effectiveness, efficiency, and accessibility of the blue dyes in labs, the optimized ratio of the blue dyes makes it suitable for large-scale plastic staining across the six tested types of plastic, replacing Nile red.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plastic products have become ubiquitous due to their durability and low cost [1]. The “Plastic Europe” data showed that the world produced 390.7 million tons of plastic in 2021, when the COVID-19 pandemic peaked [2]. China is the largest plastic producer in the world, up to 32% of the global plastic production [2]. However, the exponential growth of plastic production has brought about major environmental concerns regarding plastic wastes [3, 4], especially when they are not generally biodegradable, taking many years to decompose. With plastics leeching into soil, water, and livestock, detecting small plastic particles and even nano-scale microplastics has become a priority for scientific researchers and environmentalists [5].
The detection methods of tiny plastic particles are classified into three categories: Microscopy, Spectroscopy, and Thermal analysis. Microscopy includes microscopic visual inspection, Scanning Electron Microscopy (SEM), and Scanning Electron Microscope coupled with energy-dispersive X-ray Spectroscopy (SEM–EDS). Spectroscopy includes Fourier Transform Infrared Spectrometer (FTIR) and Raman Spectroscopy. Thermal analysis includes Thermal Extraction Desorption-Gas Chromatography-Mass Spectrometry (TED-GC-MS) and Pyrolysis–Gas Chromatography–Mass Spectrometry (Py-GC-MS). A summary of the common methods is shown in Table 1.
Methods such as Py-GC-MS, FTIR, Raman spectroscopy, are often limited by high costs, complex sample pretreatment, and time-consuming procedures. Thus, visual inspection is still the primary method for detecting small plastic particles and microplastics. A recent review of the methods used to detect microplastics in water and sediment (N = 40) showed that a significant proportion (32.5%) were based on visualisation methods [14]. Visual examination permitted the identification of plastics quickly, relying on directly observable physical traits, either through the naked eye or aided by a microscope. It is a frequently used and an easily administered method for identifying and quantifying plastic particles prior to more detailed chemical characterization.
Staining is a convenient approach to facilitate visual identification with fluorescent microscopy dyes readily available for the detection of small plastic particles in optical/stereo microscopy methods. For example, Nile red (9-diethylamino-5H-benzo[α]phenoxazine-5-one), a hydrophobic fluorophore with a specific affinity for neutral lipids is a popular dye. Its unique properties enables in-situ staining, manifesting strong fluorescence solely in the presence of a hydrophobic environment. For plastic staining, it is typically used within the concentration range of 1 μg/mL to 1000 μg/mL (1 mg/mL) with staining times ranging between 5 min to 66 h [15]. Shim et al. developed a standardized method of using Nile red staining in identifying and quantifying microplastics [16], which was further improved and enhanced [17,18,19]. Besides Nile red, other fluorescent dyes were also discovered by many scientists for their plastic-staining function, for example, Tong et al. tested the staining effect of Rhodamine B on five types of plastics [20], while Karakolis et al. examined three textile dyes [21].
Fluorescence microscopy or spectroscopy (such as FTIR or Raman) is typically adopted to examine the plastic types and quantity following staining. Despite its useful application, the high cost of Nile red limits its widespread use. In the case of optical/stereo microscopy, it is considered a luxury to use fluorescent dyes. Therefore, an economical and sustainable dye to effectively stain plastics for naked-eye observation or optical/stereo microscopy is urgently needed. To solve this, the lower costing Coomassie brilliant blue and Methylene blue could be used as alternatives, especially as a combination. While Nile red powder costs around a couple of hundred US dollars per gram, Coomassie brilliant blue R250 powder is around US$1 and Methylene blue powder is around ~ US$2. Both Coomassie brilliant blue and Methylene blue are commonly used dyes in chemistry and cell biology, making them available in most laboratories, including in school labs. Coomassie brilliant blue is a triphenylmethane dye that is widely used for protein staining in polyacrylamide gels [22,23,24], while Methylene blue is a thiazine dye that has been used for staining a variety of biological specimens, such as bacteria, fungi, and blood cells [25]. See Fig. 1 for the chemical structures of the dyes.
Thus, even though the fluorescence staining method addresses the issues of slow spectral methods and the challenge of detecting small plastic particles (< 20 μm), the problem of high detection costs in terms of required consumables remain. Furthermore, the requirement of a fluorescence microscope for visualization limits its use. Therefore, this study aims to optimise the combination of both Coomassie brilliant blue and Methylene blue for various types of plastic staining in various solvents to provide a simple, efficient, and inexpensive solution for plastic detection. Common polymer compounds include Polyethylene Terephthalate (PET), Polyvinyl Chloride (PVC), Linear Low-Density Polyethylene (LLDPE), Low-Density Polyethylene (LDPE), High-Density Polyethylene (HDPE), Polypropylene (PP), and Polystyrene (PS) [2, 8] are targets for the dye ratio optimisation. The blue dyes are also compared to Nile red. For easy comparison of the effect on the plastics, the properties and chemical structure of tested plastics are shown in Table 2.
2 Materials and methods
2.1 Materials and reagents
Nile red powder (C20H18N2O2) of purity >98.0% was purchased from Macklin (Shanghai, China). Methanol sourced from Macklin (Shanghai, China), was of analytical grade with a purity of at least 99.5%. Acetone was obtained from Zhongxing Chemical Reagent Co., Ltd. (Zhejiang, China) with a purity of at least 99.5%. Methylene blue powder (purity ≥ 80.0%) was obtained from Wenzhou Overseas Chinese Chemical Reagent Co., Ltd. (Zhejiang, China). Coomassie brilliant blue R250 powder (purity ≥ 80.0%) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.1.1 Evaluating the staining of irregular-shaped plastic fragments
Polyethylene Terephthalate (PET) was collected from plastic mineral water bottles produced by Hangzhou Dingjin Food Co., Ltd. (Zhejiang, China). Polypropylene (PP) and Polystyrene (PS) were collected from the food packaging and fast-food boxes, respectively. Both were produced by Ruikang Houseware Co., Ltd. (Zhejiang, China). Polyethylene (PE) was collected from the food packaging bags made by Green Password Houseware Co., Ltd. (Zhejiang, China). High-Density Polyethylene (HDPE) was collected from the bottled milk bottle produced by Yiming Food Co., Ltd. (Zhejiang, China). All the plastics were cut into small pieces. The length and width of PET fragments were approximately 5.5 × 4.0 mm; PP fragments were 5.0 × 3.5 mm; PS fragments were 4.5 × 3.5 mm; PE fragments were 6.0 × 5.0 mm; HDPE fragments were 4.5 × 3.5 mm.
2.1.2 Staining of regular-shaped plastic particles
PET, PP, PS, LLDPE, LDPE, and HDPE plastic particles were all purchased from Usolf (Shandong, China). PVC plastic particles were purchased from Xinxicheng (Guangdong, China). The length, width, and height of PET particles were 3.3 × 2.7 × 2.0 mm, respectively; PP particles were 4.7 × 3.7 × 2.5 mm; PS particles were 4.0 × 3.0 × 3.3 mm; LLDPE particles were 5.0 × 4.3 × 2.5 mm; LDPE particles were 4.7 × 3.3 × 3.5 mm; and HDPE particles were 4.3 × 4.0 × 3.5 mm; The PVC particles had a diameter of 3.0 mm and a height of 3.0 mm.
2.1.3 Staining of microplastics
Microplastic PET, PVC, PP, PS, LLDPE, LDPE, and HDPE were purchased from Guangyuansuhua (Guangdong, China). All seven types of microplastics had an identical diameter of 150 µm. Filter papers were purchased from Changde BKMAM Biotechnology Co., Ltd. (Hunan, China). The mesh size of the filter papers was less than 120 µm.
2.2 Procedures
2.2.1 Optimization of solvent
To ensure optimal staining effectiveness, various organic solvents were tested according to the approach previously described by Maes et al. [15]. Acetone and Methanol were chosen to assess the impact of different solvents on the staining efficacy of Nile red dye, Coomassie brilliant blue, and Methylene blue (referred to as the CM blue dye hereafter). Nile red and the CM blue dye (comprising of Coomassie brilliant blue to Methylene blue mass ratio of 1:1) were dissolved in Acetone and Methanol, respectively, to reach a concentration of 1 mg/mL. Plastic fragments were incubated in a 200 μL solution of Nile red and CM blue dyes in both Acetone and Methanol solvents separately for 30 min at room temperature before the plastics were removed and rinsed with distilled water.
2.2.2 Validation of CM blue dye staining method in plastics fragments
In the preliminary test, Coomassie brilliant blue and Methylene blue were mixed 1:1 by mass in Methanol to examine whether the blue dye could stain plastic fragments. For the CM blue dye groups, five types of plastic fragments (PET, PP, PS, PE, and HDPE) were put into different centrifuge tubes, and 200 μL blue dye of 1 mg/mL concentration was added into each centrifuge tube to immerse the plastic fragments completely. Similarly, 200 μL of Nile red in Methanol with a 1 mg/mL concentration was used for the five types of plastic fragments. 200 μL of Methanol with dye was used as a control group to compare the staining effect of dyes on plastic fragments. All plastic fragments were washed with distilled water after a 30-min incubation at room temperature.
2.2.3 Optimization of blue dye proportion
For better homogenous staining, regular-shaped plastic was used in the cut plastics. The CM Blue dye was sub-divided into 11 groups according to the proportion of Coomassie brilliant blue to Methylene blue from C: M = 0:10 to C: M = 10:0 (C = Coomassie brilliant blue, M = Methylene blue). Nile red and CM blue dye storage solutions were prepared at 10 mg/mL in Methanol and diluted ten times to the working concentration of 1 mg/mL. For the CM blue dye, the total mass of Coomassie brilliant blue and Methylene blue was 1 mg/mL of powder in Methanol. All plastic particles were added to the 200 μL solution of Methanol, Nile red in Methanol, and CM blue dye in Methanol separately for 30 min at room temperature before rinsing with distilled water. All experiments were performed in three independent technical replicates.
2.2.4 The staining effect of CM blue dye on microplastics
To test the staining effect of blue dye on microplastics, seven types of microplastics were used in this experiment. Individual microplastics were stained with the optimized blue dye ratio: PET (C: M = 7:3), PVC (C: M = 5:5), PP (C: M = 8:2), PS (C: M = 10:0), LLDPE (C: M = 10:0), LDPE (C: M = 8:2), and HDPE (C: M = 8:2). 200 μL of 1 mg/mL CM blue dye submerged ~ 50 mg of the mentioned types of microplastics at room temperature for 30 min. Subsequently, the stained microplastics were filtered and washed with distilled water. The microplastics were collected and oven-dried at 80 ℃ for one hour.
Nile red solution in Methanol staining was compared with CM blue dye in Methanol as a control group to stain the seven types of microplastics (PET, PVC, PP, PS, LLDPE, LDPE, and HDPE). 50 mg of microplastics was incubated with 200 μL Methanol and Nile red solution in Methanol at 1 mg/mL concentration for 30 min, respectively. Following the incubation, the stained microplastics were placed on filter paper and washed with distilled water. After filtration, all microplastics were collected and oven-dried at 80 ℃ for one hour. All treatments were performed as three independent replicates.
2.2.5 Data analysis and statistics
ImageJ software was used to quantify the staining effect on the plastic. The grey value of the picture was measured by removing the background from the staining results of the plastic particles. To examine the variations in grey values between different groups following the plastic staining, Independent-Samples T-Test on independent samples was performed using SPSS version 26 (IBM Statistical Package).
3 Results
3.1 Effect of dye solvents on plastics
Figure 1 shows the effect of the dye solvents on plastic samples. When Acetone was used as a solvent, there was a clear alteration of the PS sample regardless of whether Nile red or CM blue dye was used. In contrast, plastic samples dyed with Methanol did not show shape alterations. Thus, Methanol was chosen as the solvent for the subsequent experiments.
3.2 CM blue dye staining efficacy on plastics
Figure 2 shows the staining results obtained by incubating the plastics with the CM blue dye and Nile red at a concentration of 1 mg/mL for the same duration. Visual examination showed a comparable staining effect between the CM blue dye and that of Nile red.
3.3 Optimization of the CM blue dye C: M ratio
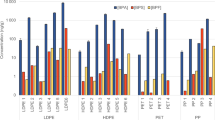
Figure 3 shows the staining effects of blue dye with different ratios of Coomassie brilliant blue to Methylene blue on the various plastic particles. Low proportions of Coomassie brilliant blue, e.g., C: M = 0:10, did not show observable staining on the plastic particles. As the amount portion of Methylene blue increased, the staining effect was more obvious across the various plastics. The Nile red dye demonstrated superior staining on PVC than all the CM blue dye ratio combinations.
The average grey values of each plastic particle after staining with different proportions of Coomassie brilliant blue: Methylene blue in blue dye (C: M ratio) are summarised in Table 2. Notably, the minimum grey value of PVC plastic occurred at the C: M ratio of 5:5; the minimum grey value of PET plastics occurred at the C: M ratio of 7:3; the minimum grey values of PP, LDPE, and HDPE plastics took place at the C: M ratio of 8:2; and the minimum grey values of PS and LLDPE plastics were observed at C: M ratio of 10:0.
The staining effect of Nile red solution (Methanol) on PVC particles was the most prominent, displaying significant differences from the Methanol group (p < 0.05). However, the grey values of the other Nile red groups (PET, PP, PS, LLDPE, LDPE, and HDPE) did not exhibit significant differences compared to the Methanol groups (p > 0.05). This indicated that the staining effect of Nile red on PET, PP, PS, LLDPE, LDPE, and HDPE plastic particles was negligible. In addition, for plastic other than PS, low portions of Coomassie brilliant blue in the blue dye yielded higher grey values than the Methanol control group. The results based on grey values were consistent with the visual observations.
3.4 CM blue dye on microplastics
Visual observation of the staining of the Nile red and CM blue dye on the microplastics showed more consistent dark staining by the CM blue dye than Nile red in Figs. 4 and 5.
Staining effect of the CM blue dye for the seven types of microplastics. Each type of microplastic is stained with the CM blue dye ratio at the lowest mean grey value optimized ratio from the results on the plastic particles: PET (C: M = 7:3), PVC (C: M = 5:5), PP (C: M = 8:2), PS (C: M = 10:0), LLDPE (C: M = 10:0), LDPE (C: M = 8:2), HDPE (C: M = 8:2)
Grey value measurements were utilised for more quantitative analysis of the staining effects and presented in Table 3. Except for PP, all microplastics in the Nile red groups (PET, PVC, PS, LLDPE, LDPE, and HDPE) showed significant differences in grey values compared to the unstained Methanol groups (p < 0.05). Similarly, the grey values of microplastics in all CM blue dye groups demonstrated significant differences from those in the unstained groups (p < 0.05). However, by comparing the grey values, it was observed that blue dye at the optimal ratios (PET, C: M = 7:3; PVC, C: M = 5:5; PP, LDPE, and HDPE, C: M = 8:2; PS and LLDPE, C: M = 10:0) exhibited a better staining effect on microplastics compared to Nile red. This finding is consistent with the results obtained from the experiments with plastic particles. It is worth noting that the CM blue dye showed a better staining effect on PVC microplastics compared to Nile red at a C: M ratio of 5:5, which differed from the staining results obtained from the experiments with plastic particles, probably due to post-production treatment and regularity of the sizes of the plastics (Table 4).
4 Discussions and future perspectives
This study sought to evaluate a more cost-effective and convenient way of staining plastics by optimising and evaluating the ratio of Coomassie brilliant blue and Methylene blue dyes compared to the Nile red dye. By testing the common types of plastics commonly found in products, we found different plastics to exhibit different optimal (Coomassie brilliant blue: Methylene blue) ratios in the combined blue dye. PVC exhibited the best staining results at C: M = 5:5 (mass) in the combined dye; PET at C: M = 7:3 (group); PP, LDPE, and HDPE at a C: M ratio of 8:2 (mass); and PS and LLDPE at C: M = 10:0 (mass). These optimised C: M ratios provide guidelines for enhancing the staining efficacy for different plastic types, emphasising the importance of tailoring the dye composition based on the specific plastic. While initial tests suggested the lower percentage Coomassie dyes to be better for staining, this was the case only for PS and LLDPE and for the other cases, some portion of Coomassie brilliant blue dye complimented the staining by Methylene blue, e.g., PVC.
The use of the optimised CM blue dye for plastic staining offers several advantages over existing methods. Firstly, it is more cost-effective than Nile red staining. At the optimal C: M ratios, the cost of using blue dye to detect PVC, PET, PP, LDPE, HDPE, PS, and LLDPE is about 1% that of Nile red. The availability and affordability of Coomassie brilliant blue and Methylene blue make the CM blue dye a more practical option for large-scale plastic detection projects, with the dyes responding likely to the different polymer natures. Secondly, the CM blue staining method required only 30 min incubation time, significantly reducing the time requirements of other detection methods, such as spectroscopy or thermal analysis. This efficiency is particularly valuable when dealing with many samples. Furthermore, the CM blue dye staining did not require fluorescence microscopy or other high-end equipment for visualisation. In fact, for microplastics characterised by their small size (< 5 mm) [5], a simple light microscope may already be sufficient.
Given the usage of both Coomassie and Methylene blue dyes in biology, it is necessary to note the limitation of looking for microplastics in tissues using the CM blue staining method. Organic compounds present can also lead to staining and can be found in many plastic wastes. While this can be mitigated by some cleaning and sample pre-treatment, such processes could be tedious and add to process costs. Nonetheless, the problem is also present with the more expensive Nile red alternative, thus the continued need for acid, alkali, or other digestions to degrade biotic substances without significantly altering the chemical or structural integrity of plastic particles persists [26].
It should be noted that the plastics used in everyday products may have additional additives or treatments, such as colouring in the product that may affect the staining results. A glimpse of such differences could be found in the slight differences in results here between the microplastics and the plastic particles. Thus, there may be room for further optimisations based on the additives and colouring to yield more visually observable staining.
Nonetheless, the demonstration of our analysis here using grey values and image processing from photos also demonstrates the possibility of automated detection of microplastics to be used for environmental monitoring of a range of applications, including water samples, sediments, soils, and biota. Such a piece of automated equipment could be built based on devices built on Microcontroller kits and 3D printing for the detection of blue particles or light absorption for an on-the-go quantification and detection [27, 28] or even smartphone applications leveraging on image processing of smartphone photos [29, 30] for higher throughput and quantitative processing.
5 Conclusions
With the cost of 1% of Nile red, we optimized the ratio of Coomassie brilliant blue and Methylene blue dyes in Methanol for a more cost-effective, faster, and efficient staining to detect plastic particles and microplastics that could be used for a wide range of environmental monitoring applications. The plastics that the dye had been optimised for include Polyethylene Terephthalate (PET), Polyvinyl Chloride (PVC), Linear Low-Density Polyethylene (LLDPE), Low-Density Polyethylene (LDPE), High-Density Polyethylene (HDPE), Polypropylene (PP), and Polystyrene (PS) that could be used in place of the commonly used Nile red solution.
Data availability
The photos of all the data are shown in the manuscript. Image J calculations are available upon reasonable request to the corresponding author.
References
Rodrigues M, Abrantes N, Gonçalves F, Nogueira H, Marques J, Gonçalves A. Impacts of plastic products used in daily life on the environment and human health: what is known? Environ Toxicol Pharmacol. 2019;72: 103239.
Plastic Europe, Plastics—The Facts 2022. 2022. https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/. Accessed 19 Mar 2023.
Halfar J, Brožová K, Čabanová K, Heviánková S, Kašpárková A, Olšovská E. Disparities in methods used to determine microplastics in the aquatic environment: a review of legislation, sampling process and instrumental analysis. Int J Environ Res Public Health. 2021;18(14):7608.
Krüger L, Casado-Coy N, Valle C, Ramos M, Sánchez-Jerez P, Gago J, Carretero O, Beltran-Sanahuja A, Sanz-Lazaro C. Plastic debris accumulation in the seabed derived from coastal fish farming. Environ Pollut. 2020;257: 113336.
Hurley RR, Lusher AL, Olsen M, Nizzetto L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ Sci Technol. 2018;52(13):7409–17.
Morgado V, Gomes L, da Silva RJB, Palma C. Validated spreadsheet for the identification of PE, PET, PP and PS microplastics by micro-ATR-FTIR spectra with known uncertainty. Talanta. 2021;234:122624.
Woo H, Seo K, Choi Y, Kim J, Tanaka M, Lee K, Choi J. Methods of analyzing microsized plastics in the environment. Appl Sci. 2021;11(22):10640.
Van Cauwenberghe L, Claessens M, Vandegehuchte MB, Janssen CR. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ Pollut. 2015;199:10–7.
Baruah A, Sharma A, Sharma S, Nagraik R. An insight into different microplastic detection methods. Int J Environ Sci Technol. 2022;19(6):5721–30.
Wang Z-M, Wagner J, Ghosal S, Bedi G, Wall S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci Total Environ. 2017;603:616–26.
Jung MR, Horgen FD, Orski SV, Rodriguez V, Beers KL, Balazs GH, Jones TT, Work TM, Brignac KC, Royer S-J. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar Pollut Bull. 2018;127:704–16.
Collard F, Gilbert B, Eppe G, Parmentier E, Das K. Detection of anthropogenic particles in fish stomachs: an isolation method adapted to identification by Raman spectroscopy. Arch Environ Contam Toxicol. 2015;69:331–9.
Funck M, Yildirim A, Nickel C, Schram J, Schmidt TC, Tuerk J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS. MethodsX. 2020;7: 100778.
Prata JC, da Costa JP, Duarte AC, Rocha-Santos T. Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC, Trends Anal Chem. 2019;110:150–9.
Maes T, Jessop R, Wellner N, Haupt K, Mayes AG. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile red. Sci Rep. 2017;7(1):44501.
Shim WJ, Song YK, Hong SH, Jang M. Identification and quantification of microplastics using Nile Red staining. Mar Pollut Bull. 2016;113(1–2):469–76.
Meyers N, Catarino AI, Declercq AM, Brenan A, Devriese L, Vandegehuchte M, De Witte B, Janssen C, Everaert G. Microplastic detection and identification by Nile red staining: towards a semi-automated, cost-and time-effective technique. Sci Total Environ. 2022;823: 153441.
Sturm MT, Horn H, Schuhen K. The potential of fluorescent dyes—comparative study of Nile red and three derivatives for the detection of microplastics. Anal Bioanal Chem. 2021;413:1059–71.
Lv L, Qu J, Yu Z, Chen D, Zhou C, Hong P, Sun S, Li C. A simple method for detecting and quantifying microplastics utilizing fluorescent dyes-Safranine T, fluorescein isophosphate, Nile red based on thermal expansion and contraction property. Environ Pollut. 2019;255: 113283.
Tong H, Jiang Q, Zhong X, Hu X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ Sci Pollut Res. 2021;28:4209–15.
Karakolis EG, Nguyen B, You JB, Rochman CM, Sinton D. Fluorescent dyes for visualizing microplastic particles and fibers in laboratory-based studies. Environ Sci Technol Lett. 2019;6(6):334–40.
Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9(6):255–62.
Sim JZ, Nguyen PV, Lee HK, Gan SKE. Republication GelApp: Mobile gel electrophoresis analyzer. Sci Phone Apps Mob Dev. 2019;5:4. https://doi.org/10.30943/2019/23122019.
Ling WL, Lua WH, Gan SKE. Fast reversible single-step method for enhanced band contrast of polyacrylamide gels for automated detection. Electrophoresis. 2015;17:1–4. https://doi.org/10.1002/elps.201500094.
Wainwright M. Methylene blue derivatives—suitable photoantimicrobials for blood product disinfection? Int J Antimicrob Agents. 2000;16(4):381–94.
Tirkey A, Upadhyay LSB. Microplastics: an overview on separation, identification and characterization of microplastics. Mar Pollut Bull. 2021;170: 112604.
Poh JJ, Wu WL, Goh NWJ, Tan SMX, Gan SKE. Spectrophotometer on-the-go: the development of a 2-IN-1 UV-Vis portable arduino-based spectrophotometer. Sensors and Actuat A Phys. 2021;325: 112698. https://doi.org/10.1016/j.sna.2021.112698.
Gan SKE. Editorial: “Set My Scientists Free”—scientific phone apps and DIY equipment during lockdowns. Sci Phone Apps Mob Dev. 2020;6(4):1–3. https://doi.org/10.30943/2020/14042020.
Nah CHK, Wu WL, Gan SKE, Wong SWG. ‘Antigen rapid test’ image-processing based machine learning algorithm for ART buddy. Sci Phone Apps Mob Dev. 2022;8:1. https://doi.org/10.30943/Trove/2022/28032022.
Chew ZY, Wu WL, Gan SKE. Application notes—APD LAMP diagnostic app: automated colorimetric analysis and documentation. Sci Phone Apps Mob Dev. 2021;7:1. https://doi.org/10.30943/2021/17032021.
Funding
This work was supported by the Wenzhou Science and Technology Bureau, Key Lab Program, Wenzhou Municipal Key Laboratory for Applied Biomedical and Biopharmaceutical Informatics, Wenke Jiji (2021) No. 4, to Wenzhou-Kean University.
Author information
Authors and Affiliations
Contributions
LL performed the experiments and wrote the initial draft. LYXD assisted in the image analysis and the editing, XCT assisted in the planning of the experiments, YKK assisted in the editing and writing, YY provided the resources and supervised XCT and LYXD. SKEG conceived the project, provided the resources for the experiments, supervised the entire project, and revised and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The work described here is part of a patent 2023052200897250 filed by Wenzhou Kean University on 22 May 2023 to the China National Intellectual Property Administration to which most of the authors in this article are also inventors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, L., Dong, L., Tian, X. et al. Optimizing the ratio of coomassie and methylene blue dyes for a cost-effective and rapid staining of PET, PVC, PP, PS, LLDPE, LDPE, and HDPE. Discov Sustain 5, 74 (2024). https://doi.org/10.1007/s43621-024-00261-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43621-024-00261-y