Abstract
In this study, dual-phase (DP, ferrite + martensite) microstructures were obtained by performing intercritical heat treatments (IHT) at 750 and 800 °C followed by quenching. Decreasing the IHT temperature from 800 to 750 °C leads to: (i) a decrease in the volume fraction of austenite (martensite after quenching) from 0.68 to 0.36; (ii) ~ 100 °C decrease in martensite start temperature (Ms), mainly due to the higher carbon content of austenite and its smaller grains at 750 °C; (iii) a reduction in the block size of martensite from 1.9 to 1.2 μm as measured by EBSD. Having a higher carbon content and a finer block size, the localized microhardness of martensite islands increases from 380 HV (800 °C) to 504 HV (750 °C). Moreover, despite the different volume fractions of martensite obtained in DP microstructures, the hardness of the steels remained unchanged by changing the IHT temperature (~ 234 to 238 HV). Applying lower IHT temperature (lower fraction of martensite), the impact energy even decreased from 12 to 9 J due to the brittleness of the martensite phase. The results of the tensile tests indicate that by increasing the IHT temperature, the yield and ultimate tensile strengths of the DP steel increase from 493 to 770 MPa, and from 908 to 1080 MPa, respectively, while the total elongation decreases from 9.8 to 4.5%. In contrast to the normalized sample, formation of martensite in the DP steels could eliminate the yield point phenomenon in the tensile curves, as it generates free dislocations in adjacent ferrite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Intercritical heat treatments (IHT) is one of the successful practical processes employed to improve the mechanical properties of low carbon steels by promoting the formation of a dual-phase (DP) microstructure. In this heat treatment, the steel is usually heated up from room temperature (direct IHT) or cooled from the single-phase austenite region (continuous IHT) to a temperature within the intercritical zone (between A1 and A3) and held for some time. At this temperature, a ferrite + austenite microstructure is established. Depending on the quenching temperature and cooling rate, the austenite transforms into martensite and/or bainite [1, 2]. Hereafter, the DP steels will be referred to the steels with a ferrite + martensite microstructure obtained through the direct IHT.
Among the IHT variables, its temperature can be considered as the most important parameter affecting the microstructure and thus mechanical properties of DP steels, since it controls the amount and carbon content of austenite in the two-phase region [3, 4]. The C content of austenite, thus the Ms (martensite start) temperature, determines the morphology of transformed martensite (termed as lath and plate) [5]. Lath martensite, which forms in low- and medium-carbon steels, consists of parallel arrays or lath-shaped crystals. Current views hold that, when austenite is transformed to martensite, a prior austenite grain divides into martensite packets. The packets divide into several blocks, which contain a group of laths with similar orientations [6, 7] (Fig. 1).
Mechanical properties of DP steels arise from the dispersion of a hard phase (martensite) in a ductile matrix (ferrite) and all the related phenomena that accompany this coexistence [8, 9]. For instance, it is known that the UTS is closely related to the work hardening behavior of the DP steel (especially of the ferrite phase), which comprises different stages: (i) motion of the stored dislocations and rapid work hardening of ferrite, (ii) constraint of plastic flow of ferrite by martensite; (iii) yielding of martensite and occurrence of plastic deformation in both ferrite and martensite phases [10, 11]. However, martensite never reaches its UTS when the necking of DP steels occurs [12]. Besides, according to EBSD [13] and TEM [14] observations, it has been reported that the dislocation density in ferrite can be inhomogeneous, with a highest density close to the martensite patches. In fact, transformation of austenite into martensite would generate dislocations in the adjacent ferrite. These areas in ferrite may act differently during the tensile test. Therefore, ferrite condition (e.g., its dislocation density and grain size) should be taken into account when evaluating the tensile strength of a DP steel [15, 16].
Apart from the ferrite condition, performing the IHT at higher temperature increases the amount of austenite (martensite after quenching), while, due to lower carbon content of the austenite, the hardness/strength of the transformed martensite would be lower. Therefore, opposing effects of IHT temperature on the strength/hardness of the DP steel would be expected.
Due to the complications mentioned above, over the past decades, researchers have developed approaches toward explaining the properties of the DP steels and tried to find the decisive parameters affecting the mechanical properties of DP steels. For instance, as an early report, Davies [17] stated that the strength of a DP structure is mainly dependent on the ferrite grain size and the volume fraction of martensite (Vm), where the influence of the strength of martensite has not been highlighted. Later, Chen and Cheng [12] reported that the tensile strength of DP steels could be predicted using the law of mixtures, in which both Vm and strength of the phases affect this property. While, in 1999, Bag et al. [18] claimed that the models developed on the basis of a rule/law of mixtures are inadequate in the determination of the tensile properties of DP steels, especially those with Vm > 0.55; in such a case, they observed a decrease in steel strength and explained it based on the drop of the martensite strength. It has been stated elsewhere that the strength raises as Vm increases, unless when martensite becomes a continuous phase [19].
Despite the common use of intercritical heat treatment, to the authors’ knowledge, the mechanical properties of the DP steels have not been discussed based on the all effective microstructural parameters (e.g., ferrite condition, and volume fraction and properties of each phase) and deeper investigations on the microstructures and the regarded phenomena seem to be necessary. For instance, for better understanding the mechanical properties of cast DP steels, it is essential to know how different carbon contents or amounts of austenite would affect the block size of martensite. Because, now it is believed that the block/packet size of martensite (as regions separated by high angle boundaries) mostly controls the dislocation movement and strength of the martensite phase; in contrast, martensite laths (previously considered as the main effective feature), separated by low angle boundaries, are considered to be transparent to dislocations [20]. Moreover, the dislocation state in ferrite can be affected by the transformation of austenite into martensite. This would help to understand the tensile behavior (i.e., yielding and work hardening) of the cast DP steel. Hereupon, this work was carried out to study and clarify the effect of the IHT temperature on the microstructure and mechanical properties of cast DP microalloyed steels, which may provide scientific insights in practical applications. With this aim, a low-carbon cast microalloyed steel has been subjected to IHT at two different temperatures; the transformations were carefully studied by a high-resolution dilatometer and explained according to previously developed dilatometry models as well as predictions provided by ThermoCalc software. The DP structures produced were further characterized by providing EBSD maps to explain the mechanical properties of the studied steel.
2 Experimental procedures
2.1 Production and history of materials
A low-carbon cast microalloyed steel was produced as test material for this investigation. The steelmaking process has been carried out using an induction furnace in an open atmosphere. The dimensions of the Y-block ingot and the casting process were described in our previous study in detail [21]. The chemical composition of the studied steel, measured by Optical Emission Spectrometry (OES: ARL 3460), is given in Table 1. The amount of O and N in the steel was 93 and 112 ppm, respectively, which has been measured using a gas analyzing equipment (model: LECO TC-436 AR).
This steel also contains around 200 ppm of Rare-Earth elements (RE: La + Ce). The amount of RE was measured by Inductively Coupled Plasma (ICP: Optima 7300 DV) technique. By adjusting the chemical composition (addition of RE), the possible detrimental effects of the cast defects/microstructure on the mechanical properties have been diminished. In this sense, our previous studies showed that the addition of 200 ppm of RE to such a low-carbon steel, in the normalized condition (as-received condition for the current study), could: (1) refine the microstructure [22]; (2) modify the inclusions [21]; and (3) thus improve the toughness [23] and tensile properties, while the steel has still confronted the yield point phenomenon [24]. Therefore, this alloy, as a clean steel with a fine initial microstructure, has been selected for this investigation. The yield point phenomenon, which has been promoted by RE addition in the normalized condition, is considered as a drawback of low-carbon steels. Therefore, the selection of this composition was also intended to see how obtaining a DP structure would affect the yielding behavior of such a steel.
The ingot of the microalloyed steel was homogenized (furnace cooling after 5 h at 1100 °C). Then, the ingot was cut into smaller parts with approximate dimensions of 15 × 15 × 150 mm3. Before applying intercritical heat treatment, the cut samples were normalized at 950 °C for 30 min followed by air cooling.
2.2 Dilatometry experiments and intercritical heat treatments
To design the IHT, critical transformation temperatures during continuous heating conditions (Ac1 and Ac3) have been estimated using high-resolution dilatometry (dilatometer model used: DIL 805 A/D Quenching & Deformation dilatometer from TA instruments). For the dilatometry experiments, cylindrical samples of 4 mm in diameter and 10 mm in length were prepared from the normalized steel. The determination of Ac1 and Ac3 temperatures, which define the two-phase region, is of great importance for choosing the IHT temperatures of this study. To estimate these temperatures under conditions as close as possible to equilibrium (quasi-equilibrium conditions [25]), very slow heating at 0.05 °C/s was employed in the dilatometer to reach a full austenitization temperature (1050 °C). Thermodynamic calculations were carried out using ThermoCalc® (TC; using TCFE8 database) to predict equilibrium Ae1, Ae3 temperatures and the carbon content of the austenite (γ) at each equilibrium temperature within the θ + α + γ phase field (θ = cementite). To undertake these predictions, the system was allowed to contain the following phases: austenite, ferrite, cementite, NbC, and the presence of a miscibility gap whose composition was close to carbide VC was unavoidable.
The above-mentioned dilatometer was also used to undertake the intercritical heat treatments on the samples whose microstructure will be subsequently characterized and also to estimate the Ms temperature after quenching the steel at 100 °C/s to room temperature, using a helium gas flow, from the IHT and fully austenitic (1050 °C) temperatures.
The samples intended for the tensile and impact Charpy tests had larger dimensions than the dilatometry samples and were heat-treated using a muffle furnace (model: AZAR M11L). In this case, the samples were placed in the furnace once the target IHT temperature was reached and held for different holding times (e.g., 20, 30, and 45 min). After undertaking a detailed investigation on the influence of the holding time on the microstructure of samples, it was found that microstructures obtained after holding for 45 min were almost identical to those achieved after the heat treatments performed in the dilatometer, in terms of fraction of the phases (i.e., ferrite and martensite). Having similar phase fractions at IHT temperature would also imply the same carbon content of austenite at that temperature.
2.3 Metallography and microstructural examinations
For the metallographic characterization of the as-received and heat-treated microstructures, the samples were ground and then carefully polished with diamond pastes, down to 1 µm, using a lubricant (a mix of ethanol and DP-Lubricant Blue). The polished surface of the samples was etched with Nital-2% for a few (5–10) seconds to reveal the microstructure of the IHT and normalized samples. The volume fraction of the phases present in the microstructure has been estimated readily by the help of an image analyzer software (ImageJ 1.47v), which was conducted on at least five images taken by an Optical Microscope (OM, NIKON ECLIPSE LV150N) at a given magnification. The volume fraction of the phases was used to estimate the carbon content of phases (especially austenite) according to the following expression (disregarding the formation of other phases like carbides):
where \({C}_{\gamma }\), \({C}_{\alpha }\), and \({C}_{0}\) stand for the carbon content of austenite, ferrite, and the nominal carbon content of the steel, respectively, while, \({f}_{\gamma }\) and \({f}_{\alpha }\) are the austenite and ferrite volume fractions, respectively.
The microstructure of the intercritically heat-treated samples was further examined under a Scanning Electron Microscope (SEM, QUANTA 450). Besides, fractography studies were carried out using this SEM on the fracture surfaces of impact samples to understand the mode and causes of failure.
Figure 1 shows that lath martensite has a hierarchical microstructure composed of packets, blocks, and laths. Among these features, Morito et al. [26] have emphasized the importance of the martensite block size in analyzing the property–structure relationship in steels. They believed that the block size, not the lath/packet size or PAGS (as previously thought), is the parameter that influences most the mechanical behavior of lath martensite. Besides, Shibata et al. [27] also concluded that block boundaries are the most effective barriers to strengthen the lath martensite. Therefore, in this study, the block size of martensite is measured, since it is the most important feature of martensite affecting its properties. The block size was estimated using Electron Backscatter Diffraction (EBSD) measurements. For this reason, the polished samples have experienced one more preparation step by applying final polishing using a suspension of silicon chloride in ethanol. An SEM (model, JEOL-JSM-7600F) was utilized to obtain the EBSD maps. Oxford-instruments system (HKL Channel 5) was employed to analyze the EBSD results and to create the maps. The local misorientations in the microstructure, Band Contrast (BC) images, and Inverse Pole Figure (IPF) maps of the IHT samples have been generated using a magnification of 1000 × and a step size of 0.15 µm. The block size/width of the martensite was measured from the boundary maps, provided by the EBSD results, according to the line intersect method (ASTM: E112 standard) and an average was reported. For this measurement, in the boundary maps, only high angle boundaries (> 10º) would be represented. Different colors were used to show the boundaries with different misorientations. Therefore, the boundaries with misorientations between 10 and 49° (grain boundaries) were distinguishable from the ones belonged to misorientations higher than 49° (block boundaries); the boundaries between 10 and 49º delimit equiaxed grains (ferrite) and the ones higher than 49º define microstructures with an elongated or lath shape (martensite) [28, 29].
It should also be mentioned that the misorientation range for the packet boundaries might feasibly overlap with that of block boundaries. This does not cause any problem in measuring the block size of martensite, since a packet boundary could be also considered as a block boundary (see Fig. 1). Moreover, to prevent the interference of grain boundaries from the block size measurement, the intersecting lines were drawn within each martensite island.
2.4 Mechanical testing
Mechanical tests have been performed on the heat-treated samples to elucidate the influences of different DP structures on the mechanical behavior of the cast steels. Aimed at measuring the hardness of the individual phases (ferrite and martensite) present in the DP steels, microhardness tests were conducted on the surface of the IHT samples using a very small load (10 g) and a loading time of 10 s. A testing machine (Wilson Tukon 1202) with a Vickers indenter was employed to perform this test. The hardness value reported for each phase was the average of at least five independent measurements. It is worth mentioning that for obtaining accurate hardness values, the indentations were made leaving a distance three times the length of the diagonal of the microindentation to the boundary between martensite and ferrite. Besides, to measure the hardness of the IHT samples, at least five independent measurements were carried out by means of a KARL FRANK GMBH machine with a Vickers indenter using a load of 30 kgf and a loading time of 12 s.
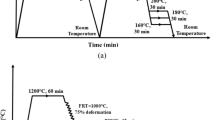
Three impact tests were performed (for each condition) at room temperature using a WOLPERT testing machine (model AMSLER D-6700). The Charpy V-notch specimens with dimensions of 10 × 10 × 55 mm3 were prepared from intercritically heat-treated parts, according to ASTM: E23 standard.
A GOTECH AL-7000 LA 30 machine was employed to perform tensile tests at room temperature, at a constant strain rate of 5 × 10–4 s−1. Tensile samples were prepared according to ASTM E8-04 standard and carefully grinded before performing the tests. The reduced section of the tensile samples was 6 mm in diameter and 36 mm in length. A pair of grips shown in our previous study was used to carry out the tensile tests [24]; using those grips on the tensile samples (also schematically shown in that work) ensures that the samples would not slide over the grips. The load–displacement (measured from the crosshead motion) was recorded continuously and converted to engineering stress–strain curves. The tensile properties (YS: yield strength, UTS: ultimate tensile strength and elongation) estimated from these curves were provided as the average of three values measured for each condition.
3 Results and discussion
3.1 Initial microstructure
The microstructure of the normalized samples, as the initial microstructure before undertaking the IHT heat treatments, is shown in Fig. 2, which is composed of ferrite and pearlite. The amount of pearlite estimated by image analysis is around 21% (the dark area); the remaining white matrix is ferrite. More details about this microstructure are provided in our recent work [22].
3.2 Austenite formation: determination of the transformation temperatures by dilatometry and ThermoCalc Software
Figure 3 demonstrates the temperature evolution of the relative change in length during slow heating rate (HR, 0.05 °C/s) up to 1050 °C. The inset in this figure provides a more detailed description of the contraction observed due to the formation of the austenite (γ). According to this figure, the initial microstructure [ferrite (α) + pearlite (P)] expands quasi-linearly up to a temperature (Ac1) where a first contraction due to the fast transformation from pearlite to high carbon austenite (γ′), P + α → γ′ + α occurs, followed by a second consecutive contraction as a result of the α + γ’ → γ transformation [30, 31]. From the dilatometry plot, the transition temperature between both transformations (inflection point) has been estimated to be around 775 ± 5 °C. Following the dilatometry model published by one of the authors [30], which assumes that no overlapping takes place between both transformations, the temperature evolution of the volume fraction of austenite and its carbon content has been estimated from the dilatometry experimental data in Fig. 3 and represented in Fig. 4 (black dash line). In addition, the same predictions obtained using TC are presented for comparison in Fig. 4 (black solid line), which considers cementite (θ) instead of pearlite. Table 2 summarizes the location of the transformation temperatures as estimated using dilatometry (Ac1, Ac3) and the Thermo-Calc software (Ae1, Ae3). These results show that non-equilibrium heating conditions, even if very low rates are considered, provide much slower transformation kinetics and higher transformation temperatures that those predicted by the TC. Besides, the carbon content of the austenite (Cγ) has also been predicted by the TC (red solid line) and dilatometry data (red dash line). During the equilibrium cementite dissolution, Cγ increases slightly up to a maximum value (\({C}_{\gamma }^{\mathrm{max}}\)=0.65 wt% at T = 709 °C) and then decreases monotonously during the α + γ’ → γ transformation down to temperature Ae3, at which the average carbon content of the austenite equals the nominal carbon composition of the steel (0.16 wt%). The blue dot lines in Fig. 4 establish the temperature that separates the P/θ + α → γ’ + α and α + γ’ → γ transformations.
3.3 Design of the intercritical heat treatments
Based on the results shown in Fig. 4, two different IHT temperatures were selected, one closer to Ae1 (750 °C) and one closer to Ae3 (800 °C). The expected amount of austenite transformed intercritically at these two temperatures, as estimated from dilatometry and predicted by the TC, is provided in Table 3. The value estimated from metallographic measurements is the volume fraction of martensite, assumed equal to that of austenite at high temperature. The intercritical heat treatments designed according to these results include heating at 5 °C/s to the targeted IHT temperatures (750 and 800 °C), a holding time of 10 min at those temperatures, followed by a fast cooling (at 100 °C/s) to room temperature. The terminology to be used throughout the manuscript for these samples intercritically annealed at 750 and 800 °C will be IHT750 and IHT800, respectively.
3.4 The intercritical heat treatments (IHT): dilatometry experiments
Figure 5a shows relative change in length recorded as a function of the temperature during continuous heating at 5 °C/s up to 750, 800, and 1050 °C (full austenitization) for comparison. Figure 5b describes the relative change in length as a function of time during heating, isothermal holding (intercritical treatment), and cooling; in this plot, the focus has been placed on the isothermal holding stage, represented by the solid lines. In addition, Fig. 5c provides a clearer picture of the transition between the continuous heating and isothermal stages.
Relative change in length recorded: a as a function of the temperature during continuous heating at 5 °C/s up to 750, 800, and 1050 °C and b as a function of time during heating, isothermal holding, and cooling. Plots shown in c provide a clearer picture of the transition between the continuous heating and isothermal stages
The results in Fig. 5 show that during heating up to 750 °C, the transformation P + α → γ has just started, but most of it takes place during the isothermal holding (Fig. 5b, c). On the contrary, at 800 °C, upon heating, all the pearlite seems to be dissolved (clear contraction) and also part of the α + γ′ → γ transformation has already started, continuing this transformation during the isothermal holding. This implies that austenite formation, as a diffusional transformation, requires time to take place. In both, the IHT750 and IHT800 samples, the contraction is sharper at the start, during which pearlite dissolution takes place, and then, it is continuously decreasing in a more progressive way during the α + γ′ → γ until an asymptotic value should be obtained once the equilibrium conditions have been reached.
Figure 6a shows the temperature evolution of the relative change in length during quenching (cooling rate, CR: 100 °C/s) from the intercritical temperatures investigated (750, 800 °C); the austenitization temperature of 1050 °C has been also represented for comparison. The derivative of the dilatometric signal with respect to the temperature is displayed in Fig. 6b, which helps to stablish more precisely the location of the Ms temperature for each condition; these have been pinpointed by arrows in Fig. 6b. In these plots, the dilatometry curves obtained for IHT750 and IHT800 have been offsetting in the positive direction to provide a better comparison and visualization of the results.
During quenching from the IHT temperature and in the absence of any phase transformation (pearlite, ferrite, bainite, or precipitation), a linear contraction should be expected before reaching the Ms temperature. This is clearly the case for sample IHT750 which undergoes a gradual thermal contraction until it reaches the Ms temperature, estimated to be around 292 °C. For condition IHT800, the Ms was estimated to be 388 °C. On the other hand, fast cooling from 1050 °C seems to transform the sample slightly to bainite before reaching the Ms temperature (a small expansion is observed, which is more obvious in the derivative plot in Fig. 6b). The approximate location of the Bs (bainite start temperature) has been also located and pointed out with an arrow (Bs = 570 °C), while the Ms temperature has been observed around 408 °C.
Regarding the values found for the Ms temperature, it is known that raising the carbon content of austenite stabilizes this phase and drops this critical transformation temperature. The value found after heating to 1050 °C (= 408 °C) is somehow close to that found after heating at 800 °C (388 °C) which was initially unexpected as the carbon content of the austenite after these two heat treatments is supposed to be significantly different (0.16 wt% and 0.25 wt% at 1050 and 800 °C, respectively, according to the TC). However, in fully austenitized sample, the preformation of bainite would enrich the remaining untransformed austenite in carbon, reducing the Ms temperature. Finally, the austenite formed intercritically at 750 °C would have a much higher carbon content (0.43 wt%, according to the TC) than the one at 800 °C; therefore, a much lower Ms was expected as also experimentally determined by dilatometry (Fig. 6b). Another possible factor affecting the martensitic transformation and the location of the Ms temperature is the prior austenite grain size (PAGS); as reducing the PAGS would lower the Ms temperature [32].
3.5 Microstructure of IHT samples
3.5.1 Martensite volume fraction and carbon content
Figure 7 shows OM (low magnification) and SEM (higher magnification) images of the dual phase microstructures obtained after performing the IHT at 750 °C (Fig. 7a, c) and 800 °C (Fig. 7b, d). In the OM images, ferrite (α) is the white phase and martensite (α’) is the darker phase. The volume fraction of martensite was measured on randomly selected OM images and has been included in Table 3 for comparison with the results of the dilatometry model and TC predictions. According to the metallographic measurements, the volume fraction of martensite in the microstructure of the IHT750 and IHT800 samples is 0.36 and 0.68, respectively. Comparing the amount of martensite transformed and that of pearlite (0.21) in the initial/normalized microstructure (Fig. 2), it can be concluded that all the pearlite and a fraction of ferrite (0.15) have transformed to austenite during the IHT at 750 °C. For IHT800 sample, it seems that the whole pearlite plus a significant volume fraction of ferrite (0.47) transformed to austenite (martensite, after cooling).
Bearing in mind that the solubility of C in ferrite is negligible compared to that of austenite (according to the TC, \({C}_{\alpha }\approx\) 5 × 10–3 wt% at 750–800 °C), using Eq. 1, the austenite in IHT750 sample is estimated to have about ~ 0.44 wt%, which is more than twice the C content of austenite when the steel is fully austenitized. Making a similar calculation for IHT800 sample gives the amount of ~ 0.24 wt% C.
3.5.2 Martensitic microstructure: influence of the PAGS and its carbon content
In previous subsection, the results showed that increasing the IHT temperature leads to a higher volume fraction of martensite, while its carbon content is reduced. Employing higher IHT temperatures, the austenite islands would grow further into ferrite grains, feasibly making larger grains. These consequences would alter the Ms temperature (Fig. 6). It is also known that the carbon content of the austenite determines the type of martensite transformed [5]. In this work, the carbon variation between the IHT samples will not change the anticipated lath morphology. However, the difference in the C content of austenite may influence the lath and block size of martensite. Hence, EBSD technique has been employed to characterize the martensitic microstructure in the IHT samples.
Figures 8 and 9 illustrate the EBSD results: (a) band contrast (BC) image, (b) inverse pole figure (IPF) map, and (c) grain boundary maps, corresponding to the microstructures of the IHT750 and IHT800 samples, respectively. In the BC images, the martensite is easily identified as it exhibits a blurred pattern in this imaging mode. The IPF map shows the crystal (grain) orientations (Figs. 8b, 9b). Regarding the boundary maps shown in this figure, as it was described in Sect. 2.3, the blue lines represent the boundaries with misorientations between 10 and 49° (grain boundaries), while the brown/reddish lines refer to misorientations higher than 49° (block boundaries); there are some grain boundaries disconnected from the boundary network. This can be due to either lack of proper indexing of the boundaries or the existence of grain boundary carbides on these locations. Besides, according to the heat treatment applied on the samples (IHT), the grain boundaries appeared here (the blue lines) are considered to be ferrite grain boundaries.
Table 4 gives the results of the average block size for the both IHT samples according to the methodology described in Sect. 2.3. It can be apprehended from this table that the block size increases about 60% when IHT temperature is raised from 750 to 800 °C. There are two main factors refining the microstructure of lath martensite; a reduction in the PAGS [26, 33] and an increase in the carbon content of austenite [20, 34]. It is reported that the blocks even have a stronger dependence on the PAGS than the martensite laths [35]. The effect of IHT temperature on the PAGS would depend on the nucleation and growth rates of austenite in the two-phase region. In this regard, Li et al. [36] studied the in situ formation of austenite during intercritical annealing treatments in a low-carbon low-alloyed steel using dilatometry and confocal laser scanning microscopy. Savran et al. [37] used three-dimensional X-ray diffraction (3D-XRD) at a synchrotron source to also provide in situ observations of the austenite nucleation and growth. In agreement with another work [31], they believe that once pearlite had transformed, the austenitizing process is accomplished mainly via the migration of the existing ferrite/austenite interfaces rather than by the nucleation of new grains in the ferrite phase. Therefore, from the experimental evidence available, it can be suggested that most of austenite nuclei would formed during the pearlite-to-austenite transformation, and thus, when the IHT temperature increases, the austenite grains grow further toward the adjacent ferrite, making larger grains, which probably took place in IHT800 sample (with raising the IHT temperature).
Apart from the influence of the PAGS on the block size of martensite, it has been observed that increasing the carbon content of austenite would reduce its Ms temperature and strengthen this phase, refining the martensitic microstructure (packet, block, and lath size) due to the higher resistance against this transformation [6]. In fact, when the C content increases, the strain associated with the formation of martensite would not be easily relieved by plastic accommodation in the austenite matrix, reducing the size of laths and blocks. Therefore, the higher average carbon content of austenite obtained at lower IHT temperatures would result in the refinement of the block size of martensite (Table 4).
3.6 Mechanical properties of the IHT samples
3.6.1 Microhardness measurement of ferrite and martensite
Table 5 summarizes the microhardness of ferrite and martensite in IHT750 and IHT800 samples. This table shows that when the IHT temperature decreases from 800 to 750 °C, the hardness of the martensite phase increases significantly from 380 to 504 HV. Huang et al. [34] indicated that the supersaturation degree of C and the dislocation density of martensite are the main effective parameters strengthening the martensitic structure rather than the packet/block size of this phase; although all these parameters are coupled as it has been discussed above. On the contrary, Gao et al. [20] discussed that there is strong evidence to support that carbon is generally segregated to dislocations and lath boundaries. Thus, its influence should be taken into account indirectly through the increase in the dislocation density and the refinement of the microstructure. Since all these microstructural and compositional factors (related to martensite) are coupled together, it is difficult to separate the individual hardening contributions and formulate regression equations, as it can be done for polygonal ferrite [12, 19]. In summary, the hardness increase observed in the IHT750 sample would be a result of having a higher density of dislocations and finer block size (Table 4) mainly due to the higher carbon content.
The microhardness of the ferrite phase in the DP structures is also given in Table 5. The results show that the ferrite hardness is similar for both IHT conditions. Because, the carbon solubility in ferrite is very low and does not change significantly with temperature (in the intercritical range) as it changes in the austenite. Besides, it is not clear to what extent the stress accommodation in ferrite (due to formation of martensite) would affect the ferrite hardness. This effect is expected to be significant only close to the ferrite/martensite boundary, which will be further discussed in the following subsection.
3.6.2 Tensile behavior
Figure 10 shows representative tensile curves of the samples intercritically heat-treated (IHT750, IHT800) and a normalized sample, for comparison. As it was mentioned in introduction, in a recent work of the authors, it has been shown that the studied steel (in normalized condition) underwent the yield point phenomenon [24], which is associated with the occurrence of Lüders bands. This phenomenon and its promotion by the RE addition in the cast condition have been a weak point of the normalized steel. On the contrary, one can see in Fig. 10 that the DP microstructures do not show the yield point phenomenon. The inset in this figure shows the Lüders deformation (1.3 ± 0.1%) with more details.
The average characteristic mechanical properties estimated from the tensile tests performed in normalized and IHT conditions are given in Table 6. It can be apprehended that increasing the IHT temperature led to a significant increase (from 493 to 770 MPa) in the yield strength of the produced cast DP steel. The UTS of the DP steel also increases from 908 to 1080 MPa with the IHT temperature. In the terms of ductility, contrary to the normalized condition, the DP microstructures shows very little necking, specially condition IHT800, and break-up just after reaching the UTS. The strength enhancement in the IHT800 is accompanied by a reduction in the total elongation, decreasing from 9.8 to 4.5%. The very small post-uniform elongation and brittle response beyond the UTS can be due to the large difference in strength between the ferrite and martensite phases [16].
Regarding the difference between the yielding behavior of the normalized and DP steels, the continuous yielding of the DP steels can be well explained based on the generation of dislocations during volume expansion associated with the transformation of austenite to martensite. In this way, dislocation sources in ferrite are activated and the density of free dislocations increases. The presence of free mobile dislocations would lead to a smooth transition from the elastic to the plastic regions [11]. Because, it is believed that, during the martensite formation, the adjacent ferrite will enter into the plastic state even when the volume fraction of transformed martensite is as low as 1% [38]. Therefore, around the martensite islands, there are many preferentially yielding zones in ferrite.
The YS of the DP steel is closely correlated with ferrite yielding [10], which itself is influenced by some parameters such as grain size, precipitation state, dislocation density, and atoms in solid solution. It is well known that a finer ferrite grain, df, results in a higher yield stress [15]. This is based on the fact that the development and distribution of stresses in DP steels mainly depend on this parameter (df). In the case of the DP microstructures under investigation, when the IHT temperature increases, the austenite volume fraction increases which consumes the ferrite matrix, making the ferrite regions effectively smaller. Therefore, compared to the IHT750 sample, the ferrite grains present in IHT800 samples are expected to be finer. This, in turn, could enhance the yield strength of the DP steel. In addition, kernel average misorientation (KAM) maps, obtained after performing EBSD measurements of the DP microstructures (Fig. 11), show that ferrite grains in IHT800 sample have higher KAM value, near the boundaries with martensite. The higher KAM value suggests that higher fraction of transformed martensite (in IHT800 sample) induces a higher dislocation density into the ferrite nearby which contributes to the strengthening of this phase and to the increase in the YS.
Apart from the parameters described above, it has been reported that the dissolution of nano-precipitates in DP steels would lower the YS, though it will put more solute atoms into the matrix [16]. Increasing the intercritical temperatures may lead to partial dissolution of fine precipitates and their reprecipitation might be suppressed by quick cooling from the IHT temperature [19]. Therefore, the ferrite phase heat-treated at lower IHT temperature (in IHT750 sample) could be more strengthened by nano-precipitates (likely V-precipitation [24]), which is in contrast to the results shown in Table 6.
Regarding the UTS of the DP microstructures and contrary to an earlier report [18] where UTS decreases when the martensite volume fraction exceeds 0.55, Table 6 shows that the UTS of IHT800 sample with a martensite volume fraction of 0.68 is higher than that of IHT750 sample (Vm: 0.36). As it was mentioned in the introduction, there are different approaches explaining the contributions of DP structures to the tensile strength of DP steels: (i) martensite volume fraction and its strength are the main parameters affecting the UTS; regarding this approach, it can be stated that the strength/hardness of martensite is lower in IHT800 sample (Table 5), while the martensite volume fraction is higher in that sample (Table 3). In fact, these two factors act in an opposing way to determine the tensile strength of the DP steel. (ii) Martensite volume fraction plus ferrite condition (even after yielding) have strong contributions to determination of the UTS. In DP steels, ferrite mostly undertakes the deformation. Thus, the work hardening behavior of this phase can greatly affect the UTS. Bergström et al. [39] also assumes that that the plastic deformation is localized to the ferrite and smaller volume fraction and grain size of ferrite (IHT800 in this study) result in a higher work hardening rate. It should be also mentioned that the dislocation density within the ferrite grains increases as the volume fraction of martensite increases, with highest density near the boundaries (Fig. 11). The areas with higher dislocation density have a higher work hardening rate. A detailed dislocation-based theory for work hardening behavior of DP steels can be found in the literature [39].
In addition to these parameters, it has been demonstrated that in early stage of deformation where martensite is still un-deformed, compared to the martensite islands with high aspect ratio, the equiaxed martensite islands are less sufficient in receiving stresses from the ferrite phase [40]. Otherwise, in general (after the first stage of deformation), obtaining scattered fine martensite islands with a higher volume fraction would improve the strength by increasing the contact area between the two phases (ferrite and martensite) [18], facilitating the stress transfer [11]. On contrary, Mazinani et al. [41] believed that the yield and tensile strengths are affected by the volume fraction of martensite but not by its morphology. To the authors’ knowledge, it is not still clear to what extent the morphology of martensite islands would contribute to the strength property.
Table 6 also shows the uniform (UE) and total elongation (TE) for the cast steels under investigation. There is not a big difference between these two parameters for each steel which is due to the very small plastic deformation after necking. It can be deduced from this table that, compared to IHT800, IHT750 sample has higher elongation. This has been expected, since the ferrite matrix, volume fraction of which is higher in IHT750 sample, provides a good plasticity/ductility in the DP steel.
3.6.3 Hardness and impact properties
Table 7 demonstrates the results of the hardness measurement for the cast DP steels obtained through the IHT. It can be realized from these results that the hardness of the IHT750 sample is similar to the hardness level obtained for IHT800 sample. This seems to contradict the previous reports [8, 42] which show that the hardness increases with martensite volume fraction; their view had taken no account of the austenite carbon content which is reduced when the IHT temperature is elevated, reducing the martensite hardness. In this investigation, the hardness value obtained could be explained based on the results of microhardness measurements (Table 5) together with the volume fractions obtained for each phase (Table 3). Because, where the fraction of martensite is higher (intercritically heat-treated at higher temperature), the hardness of that martensite is lower.
Further investigations in a wider range of IHT temperatures might be necessary to understand the effects of IHT temperature (different Vm) on the hardness of cast DP steels.
Table 7 also gives the results of impact test for IHT samples. It can be seen that increasing the IHT temperature from 750 to 800 °C caused the impact toughness to increase from 9 to 12 J. Fractography studies were conducted on the fracture surfaces of the IHT samples to understand the reasons behind the failure of these samples. Figure 12a, b shows the fracture surfaces of the impact samples. Comparing the micrographs shown in this figure, one can see that the size of facets in IHT800 is finer than that observed in the IHT750 sample. In martensitic steels, the facet size is closely related to the block size of martensite [43], which is finer in IHT750 sample (Table 4). While, in DP steels, the amount and distribution of the ferrite phase can also affect the facet size on the fracture surface. Therefore, in this case, it is not clear whether the facet size is governed by the block size of martensite or by the ferrite phase. Figure 12a (more clearly in the magnified image) shows some microcracks probably formed at the interface between ferrite and martensite in IHT750 sample. Similar to the IHT750 sample (Fig. 12a), Fig. 12b shows the same microcracks formed in IHT800 sample. Although it is not easy to compare the number of crack initiation sites in the IHT samples, according to previous reports, the number of these sites increases with an increase in martensite volume fraction [18]. From these points of view, the impact toughness of IHT750 sample should be higher than that of IHT800 sample (in contrast to Table 7). Therefore, there should be other effects counteracting and lowering the toughness of IHT750 sample. To elucidate it, the martensite structure appeared on the fracture surface of IHT750 sample was further inspected at higher magnifications.
Figure 13 demonstrates that some martensite islands have been fractured in the IHT750 sample during the impact loading. It is known that steels with high carbon content are more prone to microcracking [44]. Therefore, even though having a lower martensite volume fraction should improve the toughness, the higher carbon content of the martensite present in this condition makes the martensite harder and more brittle. Therefore, in addition to the influence of the volume fraction of martensite, its carbon content would strongly affect the impact properties of DP steels. Accordingly, it seems that the brittleness of martensite present in IHT750 sample led to a premature failure, lowering the toughness of this sample. Applying tempering treatment on the DP structures produced can be an alternative way to decrease the brittleness of martensite and accordingly enhance the toughness of the cast DP steels under investigation.
4 Conclusions
The results presented in this study answer the questions proposed at the beginning of this paper and clarify some issues which have not been clearly discussed in the published reports. The outcomes of performing the IHT at different temperatures can be summarized as follows:
-
1.
Decreasing the IHT temperature from 800 to 750 °C led to a lower volume fraction of martensite (Vm, decreased from 0.68 to 0.36), while the C content of austenite increased from 0.24 to 0.44 wt%. The higher C content and smaller PAGS (at lower IHT temperature) can be the reasons for the significant change in the Ms temperature, as Ms values of 388 and 292 °C were obtained for IHT800 and IHT750 samples, respectively. For the fully austenitized sample, with a nominal C content of 0.16 wt%, Ms was found to be around 408 °C.
-
2.
The block size of martensite (measured by EBSD) decreased significantly from 1.9 (in IHT800) to 1.2 μm (in IHT750) due to the change in the C content and size of austenite grains in the intercritical region. Accordingly, the hardness of the martensite phase greatly increased from 380 to 504 HV.
-
3.
The results of tensile tests demonstrated that YS and UTS of the DP steel increase as the IHT temperature is elevated (higher Vm), which is mainly due to the volume fraction of martensite and the ferrite condition (i.e., its dislocation density and grain size); the results of EBSD showed that dislocation density in ferrite increases with an increase in the Vm.
-
4.
An important benefit gained from the DP structure was the annihilation of the yield point phenomenon, which has been previously observed in the normalized steel and promoted by RE addition. This can be explained according to the presence of free dislocations in ferrite phase generated by martensitic transformation.
-
5.
The results of hardness measurement demonstrated that this property does not necessarily increase by applying higher IHT temperature, since the hardness of the martensite (with higher Vm) drops significantly.
-
6.
It was also clarified that the impact energy of the DP samples can be higher when the steel is heat-treated at higher IHT temperature, despite the higher volume fraction of martensite. Fractography inspections suggested that the martensite in the DP sample heat-treated at lower IHT temperature (IHT750) could be harder/more brittle due to its higher C content.
References
Voigt RC. Analysis of intercritical heat treatment of cast steels. J Heat Treat. 1989;7(2):95–105. https://doi.org/10.1007/bf02833194.
Radwanski K, Kuziak R, Rozmus R. Structure and mechanical properties of dual-phase steel following heat treatment simulations reproducing a continuous annealing line. Arch Civ Mech Eng. 2019;19(2):453–68. https://doi.org/10.1016/j.acme.2018.12.006.
Yang Y-G, Mi Z-L, Xu M, Xiu Q, Li J, Jiang H-T. Impact of intercritical annealing temperature and strain state on mechanical stability of retained austenite in medium Mn steel. Mater Sci Eng A. 2018;725:389–97. https://doi.org/10.1016/j.msea.2018.04.041.
Gorain N, Walunj MG, Soni MK, Kumar BR. Effect of continuous annealing process on various structure parameters of martensite of dual-phase steels. Arch Civ Mech Eng. 2020;20(1):29. https://doi.org/10.1007/s43452-020-00022-4.
Krauss G. Martensite in steel: strength and structure. Mater Sci Eng A. 1999;273–275(Supplement C):40–57. https://doi.org/10.1016/S0921-5093(99)00288-9.
Morito S, Tanaka H, Konishi R, Furuhara T, Maki T. The morphology and crystallography of lath martensite in Fe-C alloys. Acta Mater. 2003;51(6):1789–99. https://doi.org/10.1016/S1359-6454(02)00577-3.
Krauss G. Steels: processing, structure, and performance. ASM International; 2005.
de la Concepción VL, Lorusso HN, Svoboda HG. Effect of carbon content on microstructure and mechanical properties of dual phase steels. Proc Mater Sci. 2015;8:1047–56. https://doi.org/10.1016/j.mspro.2015.04.167.
Kalhor A, Soleimani M, Mirzadeh H, Uthaisangsuk V. A review of recent progress in mechanical and corrosion properties of dual phase steels. Arch Civ Mech Eng. 2020;20(3):85. https://doi.org/10.1007/s43452-020-00088-0.
Jahanara AH, Mazaheri Y, Sheikhi M. Correlation of ferrite and martensite micromechanical behavior with mechanical properties of ultrafine grained dual phase steels. Mater Sci Eng A. 2019;764:138206. https://doi.org/10.1016/j.msea.2019.138206.
Ahmad E, Manzoor T, Ziai MMA, Hussain N. Effect of martensite morphology on tensile deformation of dual-phase steel. J Mater Eng Perform. 2012;21(3):382–7. https://doi.org/10.1007/s11665-011-9934-z.
Chen H-C, Cheng G-H. Effect of martensite strength on the tensile strength of dual phase steels. J Mater Sci. 1989;24(6):1991–4. https://doi.org/10.1007/bf02385411.
Calcagnotto M, Ponge D, Demir E, Raabe D. Orientation gradients and geometrically necessary dislocations in ultrafine grained dual-phase steels studied by 2D and 3D EBSD. Mater Sci Eng A. 2010;527(10):2738–46. https://doi.org/10.1016/j.msea.2010.01.004.
Korzekwa DA, Matlock DK, Krauss G. Dislocation substructure as a function of strain in a dual-phase steel. Metall Mater Trans A. 1984;15(6):1221. https://doi.org/10.1007/bf02644716.
Peng-Heng C, Preban AG. The effect of ferrite grain size and martensite volume fraction on the tensile properties of dual phase steel. Acta Metall. 1985;33(5):897–903. https://doi.org/10.1016/0001-6160(85)90114-2.
Kamikawa N, Hirohashi M, Sato Y, Chandiran E, Miyamoto G, Furuhara T. Tensile behavior of ferrite-martensite dual phase steels with nano-precipitation of vanadium carbides. SIJ Int. 2015;55(8):1781–90. https://doi.org/10.2355/isijinternational.ISIJINT-2015-106.
Davies RG. Influence of martensite composition and content on the properties of dual phase steels. Metall Trans A. 1978;9(5):671–9. https://doi.org/10.1007/bf02659924.
Bag A, Ray KK, Dwarakadasa ES. Influence of martensite content and morphology on tensile and impact properties of high-martensite dual-phase steels. Metall Mater Trans A. 1999;30(5):1193–202. https://doi.org/10.1007/s11661-999-0269-4.
Džupon M, Parilák Ľ, Kollárová M, Sinaiová I. Dual phase ferrite-martensitic steel micro-alloyed with V-Nb. Metalurgija. 2007;46(1):15–20.
Gao G, Gao B, Gui X, Hu J, He J, Tan Z, et al. Correlation between microstructure and yield strength of as-quenched and Q&P steels with different carbon content (0.06–0.42 wt% C). Mater Sci Eng A. 2019;753:1–10. https://doi.org/10.1016/j.msea.2019.03.018.
Torkamani H, Raygan S, Garcia Mateo C, Rassizadehghani J, Vivas J, Palizdar Y, et al. The influence of La and Ce addition on inclusion modification in cast niobium microalloyed steels. Metals. 2017;7(9):377–95. https://doi.org/10.3390/met7090377.
Torkamani H, Raygan S, Garcia-Mateo C, Rassizadehghani J, Palizdar Y, San-Martin D. Evolution of pearlite microstructure in low-carbon cast microalloyed steel due to the addition of La and Ce. Metall Mater Trans A. 2018;49(10):4495–508. https://doi.org/10.1007/s11661-018-4796-8.
Torkamani H, Raygan S, Garcia Mateo C, Rassizadehghani J, Palizdar Y, San-Martin D. Contributions of rare earth element (La, Ce) addition to the impact toughness of low carbon cast niobium microalloyed steels. Met Mater Int. 2018. https://doi.org/10.1007/s12540-018-0084-9.
Torkamani H, Raygan S, Garcia Mateo C, Rassizadehghani J, Palizdar Y, San-Martin D. Tensile behavior of normalized low-carbon Nb-microalloyed steel in the presence of rare earth elements. Mater Sci Eng A. 2019;749:56–64. https://doi.org/10.1016/j.msea.2019.02.005.
Garcia C, Alvarez LF, Carsi M. Effects of heat-treatment parameters on non-equilibrium transformations and properties of X45Cr13 and X60Cr14MoV martensitic stainless steels. Weld Int. 1992;6(8):612–21. https://doi.org/10.1080/09507119209548251.
Morito S, Yoshida H, Maki T, Huang X. Effect of block size on the strength of lath martensite in low-carbon steels. Mater Sci Eng A. 2006;438–440:237–40. https://doi.org/10.1016/j.msea.2005.12.048.
Shibata A, Nagoshi T, Sone M, Morito S, Higo Y. Evaluation of the block boundary and sub-block boundary strengths of ferrous lath martensite using a micro-bending test. Mater Sci Eng A. 2010;527(29):7538–44. https://doi.org/10.1016/j.msea.2010.08.026.
Vivas J, Capdevila C, Altstadt E, Houska M, Sabirov I, San-Martín D. Microstructural degradation and creep fracture behavior of conventionally and thermomechanically treated 9% chromium heat resistant steel. Met Mater Int. 2019;25(2):343–52. https://doi.org/10.1007/s12540-018-0192-6.
Chen H, Yang R, Al-Abedy HK, Li H, Sun W, Jones IA. Characterisation of deformation process and fracture mechanisms of P91 steel at 600 °C in small punch tensile testing. Mater Charact. 2020;168:110514. https://doi.org/10.1016/j.matchar.2020.110514.
San Martín D, Rivera-Díaz-del-Castillo PEJ, García-de-Andrés C. In situ study of austenite formation by dilatometry in a low-carbon microalloyed steel. Scr Mater. 2008;58(10):926–9. https://doi.org/10.1016/j.scriptamat.2008.01.019.
San Martín D, de Cock T, García-Junceda A, Caballero FG, Capdevila C, de Andrés CG. Effect of heating rate on reaustenitisation of low-carbon niobium microalloyed steel. Mater Sci Technol. 2008;24(3):266–72. https://doi.org/10.1179/174328408X265640.
García-Junceda A, Capdevila C, Caballero FG, de Andrés CG. Dependence of martensite start temperature on fine austenite grain size. Scr Mater. 2008;58(2):134–7. https://doi.org/10.1016/j.scriptamat.2007.09.017.
Galindo-Nava EI, Rivera-Díaz-del-Castillo PEJ. A model for the microstructure behaviour and strength evolution in lath martensite. Acta Mater. 2015;98:81–93. https://doi.org/10.1016/j.actamat.2015.07.018.
Huang F, Yang L, Rong Y, Guo Z. Effect of carbon content and microstructural refinement on the mechanical property of quenching-partitioning–tempering-treated steels. Mater Res Innov. 2015;19(4):54–8. https://doi.org/10.1179/1432891715Z.0000000001516.
Simm T, Sun L, McAdam S, Hill P, Rawson M, Perkins K. The influence of lath, block and prior austenite grain (PAG) size on the tensile, creep and fatigue properties of novel maraging steel. Materials. 2017;10(7):730–45. https://doi.org/10.3390/ma10070730.
Li S-C, Kang Y-L, Zhu G-M, Kuang S. Austenite formation during intercritical annealing in C-Mn cold-rolled dual phase steel. J Central S Univ. 2015;22(4):1203–11. https://doi.org/10.1007/s11771-015-2634-3.
Savran VI, Offerman SE, Sietsma J. Austenite nucleation and growth observed on the level of individual grains by three-dimensional X-ray diffraction microscopy. Metall Mater Trans A. 2010;41(3):583–91. https://doi.org/10.1007/s11661-009-0142-5.
Bhattacharyya A, Sakaki T, Weng GJ. The influence of martensite shape, concentration, and phase transformation strain on the deformation behavior of stable dual-phase steels. Metall Trans A. 1993;24(2):301–14. https://doi.org/10.1007/bf02657317.
Bergström Y, Granbom Y, Sterkenburg D. A dislocation-based theory for the deformation hardening behavior of DP steels: impact of martensite content and ferrite grain size. J Metall. 2010. https://doi.org/10.1155/2010/647198.
Sun S, Pugh M. Properties of thermomechanically processed dual-phase steels containing fibrous martensite. Mater Sci Eng A. 2002;335(1):298–308. https://doi.org/10.1016/S0921-5093(01)01942-6.
Mazinani M, Poole WJ. Effect of martensite plasticity on the deformation behavior of a low-carbon dual-phase steel. Metall Mater Trans A. 2007;38(2):328–39. https://doi.org/10.1007/s11661-006-9023-3.
Topçu O, Übeyli M, Demir T. On the hardenability of an intercritically heat treated microalloyed steel. Instrum Sci Technol. 2010;38(2):178–86. https://doi.org/10.1080/10739141003594552.
Zhang C, Wang Q, Ren J, Li R, Wang M, Zhang F, et al. Effect of martensitic morphology on mechanical properties of an as-quenched and tempered 25CrMo48V steel. Mater Sci Eng A. 2012;534:339–46. https://doi.org/10.1016/j.msea.2011.11.078.
Totten GE, Narazaki M, Blackwood RR, Jarvis LM. Failures related to heat treating operations: failure analysis and prevention. In: Becker WT, Shipley RJ, editors. ASM International; 2002, vol 11, pp. 192–223. https://doi.org/10.31399/asm.hb.v11.a0003510.
Acknowledgements
The authors are grateful to the Phase Transformations and Microscopy labs from CENIM-CSIC. Mr. Javier Vara Miñambres from the Phase Transformations lab (CENIM-CSIC) is gratefully acknowledged for his continuous experimental support. J. Vivas acknowledges financial support in the form of a FPI Grant BES-2014-069863 from the Ministerio de Economia y Competitividad (MINECO).
Funding
Open access funding provided by Lulea University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torkamani, H., Raygan, S., Garcia Mateo, C. et al. Low-carbon cast microalloyed steel intercritically heat-treated at different temperatures: microstructure and mechanical properties. Archiv.Civ.Mech.Eng 21, 70 (2021). https://doi.org/10.1007/s43452-021-00222-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-021-00222-6