Abstract
The kidneys play a pivotal role in elimination of most drugs; therefore, a comprehensive understanding of renal physiology and pathology is important for those involved in drug development. High filtration capacity and metabolic activity make the kidneys vulnerable to drug-induced nephrotoxicity (DIN). Acute DIN may manifest on a background of renal impairment that has resulted from underlying disease, previously administered nephrotoxic medications, congenital renal abnormalities, or the natural aging process. The ability of the kidneys to compensate for DIN depends on the degree of pre-insult renal function. Therefore, it can be difficult to identify. The discovery and development of novel biomarkers that can diagnose kidney damage earlier and more accurately than current clinical measures and may be effective in detecting DIN. The goal of this manuscript is to provide a pragmatic and evidence-based supportive guidance for the early identification and management of DIN during the drug development process for clinical trial participants of all ages. The overall objective is to minimize the impact of DIN on kidney function and to collect renal safety data enabling risk analysis and mitigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
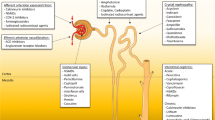
The kidneys are complex organs with many functions including blood cleansing through glomerular filtration, tubular reabsorption, and tubular secretion.
Glomerular ultrafiltration of plasma begins in the 9th week of gestation. During gestation, glomerular filtration rate (GFR) increases in parallel with gestational age until a large increase occurs at the time of completion of nephrogenesis, which is achieved at approximately 35–36 weeks of gestation [1]. Of note, certain maternal medications can influence renal development in utero. One example of this is the use of non-steroidal anti-inflammatory drugs after 20 weeks of gestation can result in prostaglandin receptor blockade as the mechanism for reduced renal perfusion with resultant oligohydramnios, leading to neonatal renal impairment [2].
Most drugs and their metabolites are eliminated in the urine by glomerular filtration and/or tubular secretion. The ultrafiltrate formed in the glomeruli is modified through tubular transport, mostly in the proximal tubule. In the context of nephrotoxicity, tubular cell uptake of potentially nephrotoxic drugs occurs via the apical pathways via endocytosis/pinocytosis and other passive or active transport. Alternatively, uptake can occur through the basolateral membranes of the proximal tubules via the peritubular capillaries [3].
The kidneys are especially predisposed and vulnerable to the toxic effects of drugs and their metabolites as they can reach high levels of local exposure in renal tubules and interstitium, which could lead to DIN [4]. For example, some drugs (e.g., aminoglycosides, cyclosporine, cisplatin, amphotericin B) have direct proximal tubular cell toxicity, and their use is associated with an increased risk of kidney damage. Therefore, drug-induced renal effects are an important consideration in drug development that require prediction, vigilance, early detection, and adequate risk mitigation.
While AKI often resolves if the underlying etiology is corrected, it may also lead to chronic, irreversible histologic changes within the kidneys. Signs and symptoms of nephrotoxicity can vary and span a broad spectrum, reflecting damage to different nephron segments, potentially resulting in proteinuria, hematuria, oliguria, dysregulated acid–base balance, and/or electrolyte abnormalities. Ultimately, DIN can present as either acute kidney injury (AKI) or chronic kidney disease (CKD).
The kidneys have significant functional reserve; and while their regenerative capacity is limited, they can adapt, as exemplified by the ability to donate one kidney, and can compensate for mild degrees of renal impairment by increasing function in other segments of a nephron (i.e., hypertrophy), or by increasing function of other healthy nephrons (i.e., recruitment). The kidneys increasingly depend on functional reserve capacity with increasing age. Each DIN event further decreases the reserve capacity and thus, potentially leads to an earlier onset of functional renal impairment, even in response to mild injury that may be difficult to detect with clinical testing.
In this manuscript, the authors provide a comprehensive overview of DIN, including a classification schema to allow for appropriate identification and mitigation of DIN based on patient population, intrinsic pharmaceutical agent composition, pharmacokinetic properties, and results of preclinical studies. Further, monitoring and mitigation strategies are presented, including caveats pertaining to special populations such as children and cancer patients.
Diagnosis and Classification of AKI and CKD
AKI can span a spectrum from mild forms to more advanced injury necessitating renal replacement therapy (RRT). Clinically, AKI is characterized by a reduction in renal function resulting in a failure to maintain fluid, electrolyte, and/or acid–base homeostasis. The acute loss in renal function may manifest as accumulation of end products of nitrogen metabolism, oliguria, metabolic acidosis, and/or electrolyte abnormalities [5].
In 2012, the KDIGO working group combined the RIFLE [6] and AKIN [7] classifications to establish one classification of AKI for practice, research, and public health (Table 1) [8]. AKI can be due to pre-renal, renal, or post-renal etiologies. AKI often has an abrupt onset and is in principle reversible. Therefore, early identification and prompt management are critical. Once the diagnosis of AKI has been confirmed or suspected, the patient should receive immediate adequate specialized treatment to minimize the potential to become chronic and irreversible.
Urine output is one of the most easily measured parameters and, as such, is an important indicator of renal function used in the modern classification of AKI [6]. It may be a more sensitive index of changes in renal hemodynamics than biochemical markers of solute clearance. However, it has insufficient sensitivity and specificity as a marker of renal function in patients with AKI due to impaired free water and solute excretion. In patients with severe AKI, urine output could be normal or even elevated due to tubulo-interstitial injury [9].
Laboratory assessment of renal function even in the presence of considerable renal damage, serum creatinine (SCr), blood urea nitrogen (BUN), and estimated glomerular filtration rate (eGFR) may remain stable, as seen in the slow, progressive, and asymptomatic renal function decline that commonly occurs in the elderly as the total number of functioning nephrons gradually decreases with age. A disadvantage is that changes in SCr occur no earlier than 24 to 48 h after kidney damage, which delays the diagnosis of DIN, increasing the risk of irreversible kidney damage. Further, muscle mass also decreases with age and thus only small increases in creatinine may underestimate the magnitude of renal injury. A biomarker representing the number of functional nephrons is not yet available.
Chronic kidney disease is defined as either kidney damage or decreased eGFR for at least 3 months leading to long-term, irreversible renal damage and is staged based on severity (Table 2). Kidneys with chronic damage are often more susceptible to acute injury. Early or mild degrees of renal impairment are seldom associated with significant long-term serum creatinine increases or clinical manifestations; hence measurement of creatinine may not be sufficient for identifying Stage 1 and Stage 2 CKD. In such cases, the presence of one or more of the markers listed in Table 2 may help in identifying early CKD.
A measured GFR is considered the best marker of kidney function; however, pragmatically, eGFR is useful to determine the prognosis of kidney disease, anticipate complications, and adapt drug dosage. Glomerular filtration is the main mechanism responsible for drug elimination; therefore, drug renal clearance will depend mainly on the rate of glomerular filtration. For infants, this rate is a function of gestational and post-natal ages. Thus, even outside the context of AKI, eGFR is important for correct drug dosing. Pharmacological adaptation (such as reduced dosage or longer administration intervals) is then essential to limit drug toxicity in patient with renal dysfunction. While glomerular filtration is often responsible for drug elimination, tubular excretion may also contribute and markers including serum electrolytes should be monitored. With a decline in GFR, markers such as urine output, SCr, BUN, and cystatin C change significantly, and each marker has unique advantages and some limitations for detecting changes in glomerular filtration (Tables 3, 4).
Causes of Drug-Induced Nephrotoxicity
Drug-induced nephrotoxicity may be the result of a combination of contributing factors including the Investigational Medicinal Product (IMP) mechanism of action (both on-target and off-target), the specific study population (e.g., in the elderly, patients with polycystic kidney disease), the presence of existing renal impairment at treatment initiation, individual genetic predisposition, and/or concomitant medications. It may manifest immediately following drug exposure (e.g., contrast agents), after days to weeks (e.g., non-steroidal anti-inflammatory drugs (NSAIDs), or aminoglycoside antibiotics), or only after extended exposure to the offending agent (e.g., phenacetin or cyclosporine), with high variability among agents and their nephrotoxic manifestations. As transient and modest degrees of DIN may remain asymptomatic and therefore undetected, renal function assessments should be planned to identify the early- and delayed-onset of DIN. Nephrotoxicity, manifesting as impaired renal function, may have different etiologies (Table 1).
CKD that has resulted from disease, drugs used in prior treatments, congenital renal abnormalities, or the natural aging process may hinder the kidneys’ ability to compensate for DIN, depending on baseline functional impairment/reserve. In general, drug classes have specific patterns of nephrotoxicity, which can be categorized by their effects on renal function (e.g., altered GFR due to angiotensin-converting enzyme (ACE) inhibition, diabetes insipidus, renal Fanconi syndrome, pseudo-hypo-aldosteronism), or structural abnormalities causing injury to specific areas in the kidney (e.g., the glomerulus, proximal or distal convoluted tubule). Tubulo-interstitial damage is the most common form of direct DIN. In contrast, idiosyncratic nephrotoxicity may present with a range of clinical syndromes including immune-mediated renal injury (e.g., gold therapy-induced membranous glomerulonephritis, drug-induced lupus-like syndrome, drug-induced glomerulosclerosis, protein deposits, calculi, infections secondary to immunosuppression).
DIN generally occurs more frequently in the presence of risk factors that increase patient vulnerability to nephrotoxicity. In particular, patients aged > 65 years or with pre-existing renal impairment regardless of age are at higher risk for DIN. Other concomitant diseases and conditions increase patient vulnerability to DIN, including medical conditions that cause renal hypoxia or decrease renal perfusion [10]. These factors need to be taken into consideration before allowing a high risk patient to be treated with a potentially nephrotoxic drug.
Several drugs and different contrast agents have the potential to cause or exacerbate renal injury and should be avoided during clinical trials (Table 5). If this is not possible, renal monitoring should be adapted to ensure patient safety. At the very minimum, all medications, including herbal supplements, should be documented in the concomitant medication list. Certain drugs may compete for renal tubular excretion, e.g., penicillin (acids), procainamide (bases), and all glucuronides, leading to drug interaction and potential toxicity. The risk of renal injury escalates in the presence of multiple risk factors. Patients with one or more risk factors (e.g., diabetes, cardiac failure, and myeloma) should be specifically monitored to identify the changes in renal function during long-term studies, in particular during dose escalation, when changes are made to co-medication, or during changes in patient volume status. This may include more frequent safety assessment in some or all of the patients in a trial.
Assessing Drug Potential for Nephrotoxicity
Preclinical Evaluation and Biomarkers
During preclinical drug safety evaluation, nephrotoxicity is generally assessed in vivo, while in vitro evaluation is used to explore renal drug transport and metabolism and to study the mechanism of DIN. Renal toxicity of an IMP can manifest as a functional change, as overt renal lesions, or as injury associated with histopathological changes, frequently depending on the dose and duration of exposure. The effect may be regional or may be diffused and affected several regions, resulting in specific clinical manifestations, urinalysis changes, and/or biomarker patterns. Histopathological signs of DIN include the following:
-
Glomerular podocyte foot process effacement
-
Glomerular sclerosis
-
Glomerular epithelial crescent formation
-
Acute tubular necrosis, vacuolization, obstruction
-
Interstitial inflammation
-
Tubular atrophy
-
Tubulitis
-
Nephrocalcinosis
-
Renal papillary necrosis
-
Renal artery or renal vein hypertrophy, anatomical change, dilation, or rupture
-
Renal vasculitis or thrombosis, or vascular occlusion or vascular obliteration
-
Renal intravascular/perivascular inflammatory changes/infiltrates
-
Urinary calculi
-
Crystal deposition
Other preclinical findings that may indicate nephrotoxicity include dose-dependent increases in serum creatinine, microscopic or gross hematuria, proteinuria, metabolic acidosis, or urinary crystal formation/excretion. Monitoring of biomarkers that allow early identification of the onset and severity of DIN and that track the reversal of DIN after drug withdrawal may additionally provide mechanistic insight into IMP-related renal toxicity. As a result, novel preclinical biomarkers of renal toxicity have been developed (Table 6).
Due to variability and multi-dimensional urine composition in human clinical setting, interpretation of urinary kidney biomarkers evaluated in preclinical models will profit from additional clinical assessments in humans. Urinary creatinine should be measured to normalize (creatinine-index) biomarker concentrations and assessment of sensitivity and specificity of each biomarker is important during transitioning to clinical use.
Clinical Evaluation
Assessing an IMP’s potential to cause nephrotoxicity must consider the following factors:
-
The IMP-specific preclinical or early clinical signals of nephrotoxicity
-
The clinical predictive value of animal models, at relevant exposure levels
-
Pre-existing conditions in the patient population that may predispose to DIN
-
Interaction with concomitant medications
-
Renal effects of other IMPs in the same drug class
When transitioning from preclinical to clinical development, the first step is to determine the IMP’s potential to cause nephrotoxicity. Based on our drug development experience, we have developed classification nomenclature for IMPs, namely DIN-L1 (Level 1) if there is no known potential to cause DIN, or DIN-L2 (Level 2) if there is evidence of or potential for DIN (Table 7). As DIN may be caused by indirect mechanisms or manifest only after longer clinical exposure, it is recommended that DIN-L1 IMPs with a new mechanism of action (MoA) that is not well characterized or are planned to be used in patients with pre-existing nephropathy, are conservatively classified as DIN-L2 during early phase clinical studies.
Even though a compound may initially be classified as either DIN-L1 or DIN-L2, reassessment of renal effects should occur after Phase 2 and Phase 3 studies. Appropriate renal monitoring parameters should be included in respective clinical trial protocols. An IMP’s potential to cause DIN should be reassessed regularly during the development lifecycle, at clinical trial phase transition, and when relevant safety data become known, considering all available preclinical and clinical evidence (i.e., laboratory values, adverse event profile, biomarkers), and internal or external safety data from similar drugs. Should DIN manifest in a DIN-L1 IMP in a specific patient population (e.g., the elderly, pediatrics, first in human or in patients with diabetes, cardiac failure, or chronic kidney disease), a reassessment should be conducted based on available data to determine if the IMP should be reclassified to DIN-L2 and the monitoring plan updated. Early studies with DIN-L2 IMPs may exclude subjects > 65 years, subjects with certain concomitant diseases predisposing to renal impairment (e.g., hepatic or cardiac failure), and the use of potentially nephrotoxic co-medication until the renal safety of the IMP has been established.
In the absence of any clinical findings of DIN during early phase clinical studies, a DIN-L2 IMP can be reclassified to DIN-L1 after careful consideration (note that DIN may only manifest after 6 to 12 months’ treatment). Drug development teams are advised to include renal safety monitoring in long-term studies in the target population if applicable (may not be applicable in limited drug exposure, short-term treatment, or in oncology, where other criteria may apply), and in consultation with renal experts and relevant safety board(s). The IMP’s potential to cause nephrotoxicity will impact the choice of appropriate level of safety monitoring, the clinical trial eligibility criteria and discontinuation criteria, and the selection of specific renal safety markers to be measured/monitored.
Renal Monitoring During the Clinical Development Program
Proactive monitoring for potential nephrotoxicity allows for timely detection and enhances patient safety throughout the clinical program. A monitoring program consists of selected renal safety assessments performed with the appropriate assessment frequency. Depending on whether the IMP is DIN-L1 or DIN-L2, the development phase, and the target patient population risk profile, monitoring requirements will vary from Base to Observation to Intense Monitoring (Table 8). Monitoring may need to be increased from Base to Observation or Intense for any of the following situations:
-
Inclusion of a new at-risk patient population
-
Addition of concomitant nephrotoxic medication, or
-
Identification of a potential renal safety signal
The collection of pharmacokinetic (PK) samples is recommended, especially for DIN-L2 IMPs, or when renal events occur, to determine whether nephrotoxicity correlates with drug exposure levels. PK sampling should be performed at steady state at regular intervals depending on the DIN monitoring level. PK sampling is also recommended following dose adjustment, or in the presence of other risk factors that may increase the risk for nephrotoxicity. Some PK parameters to be considered should include CMax, AUC, elimination half-life, protein binding or association with albumin or bilirubin (BR), PkA, the use of parametric analysis, or population pharmacokinetics (popPK) models stratified for CrCL (creatinine clearance). Health authority guidelines should be referenced to determine the most applicable parameters.
The collection and storage of additional plasma and urinary samples in early phase clinical studies that will allow the team to evaluate genetics and biomarkers at a later time for DIN-L2 IMPs in consultation with the relevant experts is recommended. These samples may be evaluated during the study to monitor patient safety, at study completion, or at a later stage as required. For DIN-L2 IMPs in particular, prospectively collected urine samples may become important if companion diagnostic tests for IMP nephrotoxicity have been or will be developed and validated.
Additional laboratory and clinical assessment (e.g.; evaluation of the patient hemodynamic status) may provide information regarding specific aspects of kidney function, for example, renal causes for electrolyte or water imbalances. Study teams should be mindful of the storage requirements for certain tests (e.g., alkaline urine needed to prevent B2-microglobulin degradation or required centrifugation for certain assays).
Considerations for Clinical Trial Design and Protocols
As noted above, study eligibility criteria and monitoring will be determined by the IMP level (DIN-L1 or DIN-L2), development phase, and the presence of any population-related risk factors of nephrotoxicity, including concomitant medications, the underlying disease, the current CKD stage, and comorbid conditions. Eligibility criteria and clinical assessments should follow a company’s guidance for clinical trial protocols.
During protocol and assessment schedule creation, the IMP’s monitoring level should be applied to select the appropriate tests and measurement frequency. In addition, if any renal adverse event is reported, additional testing, including urinalysis and blood testing, should be performed.
Definition of Renal Event
A renal event is defined as abnormal clinical signs and/or symptoms and/or laboratory abnormalities that reflect impaired renal function, or a confirmed change in urine composition such as the presence of protein, glucose, or blood.
While the most sensitive diagnosis of a renal event is a confirmed increase in serum creatinine of ≥ 25% compared to baseline (corresponding to a decrease of eGFR by approx. 20%), eGFR is preferred in evaluating for renal events as transient increases in serum creatinine may occur as a result of non-renal factors, such as changes in hydration status, diet or exercise. Therefore, confirmation of an event with a second assessment is required to ensure a renal etiology of the event. Since creatinine is often measured frequently, it should be diligently evaluated as well. An increase of 25–50% in serum creatinine even in normal ranges may not necessarily qualify as an adverse event, but should be evaluated to better clarify the occurrence.
In the presence of low baseline serum creatinine values, such as those with low muscle mass (e.g., pediatric population or cancer patients), detecting a 25% change from baseline may be limited by assay sensitivity. Similarly, changes in muscle mass will affect creatinine clearance; decreasing muscle mass will impede the ability to identify a renal event, while increased muscle mass or muscle breakdown may increase creatinine levels and trigger false events. If muscle mass changes during the course of the study, it is advised to define renal events by changes in eGFR rather than serum creatinine changes.
Any positive dipstick finding should be followed up by microscopy if the sample is adequately preserved and timing is same day; otherwise, another fresh sample needs to be obtained as soon as possible. Urine protein present on a dipstick should be quantified using protein:creatinine ratio (PCR) measurement.
Management and Follow-Up
Upon diagnosis and confirmation of a renal event, some general procedures, and some event specific activities, as illustrated in Table 9, are indicated depending on the severity of the event and the clinical status of the patient. Whenever a renal event is identified, a detailed patient history and examination, including the parameters below are indicated to identify and treat the patient:
-
Blood pressure
-
Signs and symptoms such as fever, headache, shortness of breath, cardiac murmur, back or abdominal pain, hepatomegaly, dysuria or hematuria, edema
-
Changes in body weight, fluid intake, voiding pattern, or urine output
-
Concomitant events such as trauma, surgical procedures, cardiac or hepatic failure, nephrotoxin administration, or other diseases or causes, e.g., dehydration, tumor lysis.
Special Populations
Pediatrics
Clinical trials in children are usually performed after the adult population has been studied; however, they may be conducted first or simulataneously with adults if the disease state primarily or frequently occurs in children. In either scenario, pediatric studies require additional consideration. In general, renal function (eGFR) in the child is considered to be comparable to the adult after two years of age. However, muscle mass, and therefore serum creatinine and measured GFR should be corrected for body surface area which increases with increasing age reaching adult values when growth during the second decade of life.
Disorders increasing the risk of CKD, and DIN in children and adolescents include the following:
-
Family history of genetic kidney disease
-
Low birth weight
-
Teratogens: ACE inibitors, Angiotensin receptor blockers (ARBs), congenital infections, NSAIDs
-
Underlying renal disease including renal dysplasia/hypoplasia
-
Urologic disorders—especially obstructive uropathies
-
History of perinatal hypoxemia, maternal oligohydramnios, or other acute renal insult
Renal events in the pediatric population should be defined as 25% decrease in eGFR [25] using age-specific normal values for eGFR and serum/urinary normal values. AKI can best be assessed using KDIGO criteria [26]. The Schwartz age-specific eGFR formula [23] should be used to calculate eGFR in children. Since the most common cause of secondary hypertension in children is renal disease, blood pressure should be monitored in accordance with the Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents [27]. Proteinuria may be seen in children in a benign condition called benign orthostatic (postural) proteinuria, which is not associated with renal dysfunction and is considered a normal physiologic variant. Thus, whenever assessing a positive dipstick for proteinuria in children, a first morning sample should be used to eliminate this potentially complicating variable.
Cancer Patients
Chronic kidney disease is prevalent in cancer patients regardless of tumor type [28, 29]. Disease progression and compromised renal function in cancer patients resulting in decreased clearance rates may lead to higher drug exposure. In addition, cancer patients are frequently exposed to potentially nephrotoxic chemotherapeutic agents (i.e., cisplatin).
Prediction of DIN in Cancer Patients and Oncology IMP Classification
Therapeutic drug exposure is an important determinant of DIN in oncology patients and may vary significantly between treatment regimens. Due to higher levels of drug exposure during preclinical evaluation, preclinical renal toxicity signals may not translate into clinical nephrotoxicity. In other words, even though an IMP could be classified as DIN-L2 based on preclinical toxicity findings at high exposure levels, DIN may not occur in patients at lower exposure levels. In addition, modern molecular targeted oncology therapeutic IMPs (e.g., tyrosine kinase inhibitors) generally lack tissue specificity and selectivity and may directly or indirectly affect multiple organs, including the renal system [30]. Therefore, unless a specific mechanism for potential renal toxicity can be identified or suspected, DIN risks may be difficult to predict.
In IMPs whose drug exposures inducing preclinical DIN are unlikely to be achieved in humans; and provided a sufficient safety margin has been established when assessing the therapeutic drug concentrations, DIN-L1 monitoring may be appropriate. Such a decision will be based on the early safety data in the context of predicted therapeutic use, consideration of factors that may increase drug exposure (e.g., hepatic or renal impairment), feasible risk mitigation strategy, and on DIN potential in the target population. For IMPs assigned to DIN-L2 prior to human testing, a re-classification to L1 may be considered on the basis of a conservative risk assessment of the preclinical evidence, if there is adequate evidence to determine that no clinical or sub-clinical renal toxicity signal is identified at the completion of the Phase I oncology trial(s).
Mitigation Strategies for DIN in Cancer Patients
Pre-existing renal impairment is a risk factor for DIN, where the risk of drug-induced renal toxicity is proportional to the deterioration of renal function in cancer patients. Therefore, caution should be exercised in treating cancer patients with existing renal impairment, especially for DIN-L2 IMPs. In addition, adequate hydration remains fundamental for all patients receiving IMPs with nephrotoxic potential. Changes in renal function during and after drug administration should be correlated with drug exposure levels, and dose interruption or reduction is recommended to mitigate the risk of potential renal toxicity. The renal function monitoring frequency may also need to be adapted based on the accumulated renal safety information for the IMP and indication. In specific situations where the accurate assessment is imperative to know an actual GFR to determine accurate dosing or in situations where even small changes in GFR are significant, GFR should be measured using radionucleotide techniques (e.g., iohexol) as these are more precise than creatinine or eGFR (Table 3).
Investigational Medicinal Product discontinuation
The decision to discontinue the IMP, temporarily or permanently, in any individual patient is made by the investigator based on patient safety and the risk–benefit profile of the treatment. From a renal vantage point,
-
Consider discontinuing or interrupting study treatment for a patient if individual eGFR decreases ≥ 50% compared to baseline (and is considered clinically significant), or in the event of treatment-emergent quantified proteinuria (ACR > 1000 mg/g or > 100 mg/mmol; PCR ≥ 2 g/g or > 200 mg/mmol), unless the event is deemed not drug related, related to natural disease progression, or if the benefit/risk assessment supports continuing treatment.
-
A renal event leading to patient discontinuation should be followed up until event resolution (Serum Cr within 10% of baseline, PCR < 1 g/g Cr, ACR < 300 mg/g Cr) or stabilizes.
DIN-related modifications to the trial protocol should be based on IMP level (DIN-L1 or DIN-L2), the known safety profile of the IMP, and the overall risk–benefit profile. Guidance from relevant experts (e.g., nephrologists) is recommended for DIN-L2 or DIN-L1 IMPs in a patient population with an increased risk for DIN.
Conclusions
Given that DIN may influence the choice as to whether or not a specific therapy should be prescribed, the benefit-risk of the compound should be considered to determine whether the disease for which a drug is being developed is severe enough that some degree of kidney damage may be acceptable, particularly for drugs where changes in kidney function are mechanism-dependent and no alternative therapy is available. If reversibility of kidney damage is indeed established, continued dosing of the IMP could be considered, but with more frequent safety assessments to determine if kidney function is stabilizing or further deteriorating. If deterioration of renal function is observed, the drug should be discontinued. DIN in drug development should be anticipated through preclinical studies considering validated biomarkers and by identifying risk factors. The clinical trial team should focus on identification of subjects at risk of DIN, and the development of clear protocols to proactively define the necessary steps to reduce or eliminate this risk for patients enrolled in clinical trials.
Current definitions of renal injury are based on changes in SCr that relate to changes in GFR and not to renal injury itself. These classifications need to be refined for DIN, or a new classification may be needed explicitly for DIN. Another disadvantage is that changes in SCr occur not earlier than 24 to 48 h after kidney damage, which delays the diagnosis of DIN, increasing the risk of irreversible kidney damage. A major obstacle to earlier diagnosis of kidney damage, irrespective of etiology, is a lack of validated biomarkers to predict damage to the kidneys holistically as well as different nephron segments. Intensive research and collaboration between academia, the pharmaceutical industry, and health authorities are currently focused on the development and qualification of renal safety biomarkers. Those identified by the Nephrotoxicity Working Group of the PSTC have received approval from the Food and Drug Administration and European Medicines Agency for use in preclinical and clinical Phase I studies. It is expected that their validation and integration into different phases of clinical drug development will facilitate the early detection of DIN, leading to the development of appropriate risk mitigation and minimization strategies. Developing more sensitive methods to predict DIN in preclinical studies and early clinical detection of kidney damage would help ensure patient safety and facilitate informed decisions during drug development.
References
Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Semin Nephrol. 2009;29(4):321–37.
Antonucci R, Fanos V. NSAIDs, prostaglandins and the neonatal kidney. J Matern Fetal Neonatal Med. 2009;22(Suppl 3):23–6.
Perazella MA. Pharmacology behind Common Drug Nephrotoxicities. Clin J Am Soc Nephrol. 2018;13(12):1897–908.
Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol. 2006;2(2):80–91.
Longo D, Fauci A, Kasper D, et al. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill Professional; 2011.
Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12.
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Kidney Disease: Improving Global Outcomes (KDIGO). Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1.
Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med. 2008;36(4 Suppl):S152–8.
Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, Mehta R. Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care. 2005;11(6):533–6.
Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28(5):830–8.
Ribichini F, Gambaro G, Graziani MS, et al. Comparison of serum creatinine and cystatin C for early diagnosis of contrast-induced nephropathy after coronary angiography and interventions. Clin Chem. 2012;58(2):458–64.
Abrahamson M, Olafsson I, Palsdottir A, et al. Structure and expression of the human cystatin C gene. Biochem J. 1990;268(2):287–94.
Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10(6):476–82.
Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–22.
Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–21.
Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28(5):455–62.
Sayer J, McCarthy MP, Schmidt JD. Identification and significance of dysmorphic versus isomorphic hematuria. J Urol. 1990;143(3):545–8.
Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–49.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011 Sep 20;155(6):408]. Ann Intern Med. 2009;150(9):604–612.
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–63.
Counahan R, Chantler C, Ghazali S, Kirkwood B, Rose F, Barratt TM. Estimation of glomerular filtration rate from plasma creatinine concentration in children. Arch Dis Child. 1976;51(11):875–8.
Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37.
Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–93.
Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–35.
Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–61.
Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents [published correction appears in Pediatrics. 2017;140(6):e20173035] [published correction appears in Pediatrics. 2018;142(3):e20181739]. Pediatrics. 2017;140(3):e20171904.
Launay-Vacher V, Chatelut E, Lichtman SM, et al. Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Ann Oncol. 2007;18(8):1314–21.
Aapro M, Launay-Vacher V. Importance of monitoring renal function in patients with cancer. Cancer Treat Rev. 2012;38(3):235–40.
Xiong Y, Wang Q, Liu Y, et al. Renal adverse reactions of tyrosine kinase inhibitors in the treatment of tumours: a Bayesian network meta-analysis. Front Pharmacol. 2022;13:1023660.
Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–54.
Carroll MF, Temte JL. Proteinuria in adults: a diagnostic approach. Am Fam Phys. 2000;62(6):1333–40.
Baum N, Dichoso CC, Carlton CE. Blood urea nitrogen and serum creatinine. Physiology and interpretations. Urology. 1975;5(5):583–8.
Funding
No financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript’s conception, design, and approval.
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of Novartis. Ronald Portman, Victor Dong, Nicholas J Webb, and Deepa H. Chand hold Novartis stocks or stock options.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antognini, N., Portman, R., Dong, V. et al. Detection, Monitoring, and Mitigation of Drug-Induced Nephrotoxicity: A Pragmatic Approach. Ther Innov Regul Sci 58, 286–302 (2024). https://doi.org/10.1007/s43441-023-00599-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00599-x