Abstract
Inflammatory bowel disease (IBD) is an autoimmune disease mediated by immune disorder and termed as one of the most refractory diseases by the Word Health Organization. Its morbidity has increased steadily over the past half century worldwide. Environmental, genetic, infectious, and immune factors are integral to the pathogenesis of IBD. Commonly known as the king of herbs, ginseng has been consumed in many countries for the past 2000 years. Its active ingredient ginsenosides, as the most prominent saponins of ginseng, have a wide range of pharmacological effects. Recent studies have confirmed that the active components of Panax ginseng have anti-inflammatory and immunomodulatory effects on IBD, including regulating the balance of immune cells, inhibiting the expression of cytokines, as well as activating Toll-like receptor 4, Nuclear factor-kappa B (NF-κB), nucleotide-binding oligomerization domain-like receptor (NLRP), mitogen-activated protein kinase signaling, and so on. Accumulated evidence indicates that ginsenosides may serve as a potential novel therapeutic drug or health product additive in IBD prevention and treatment in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory intestinal disease with unknown etiologies and pathogenesis, including ulcerative colitis (UC) and Crohn's disease (CD). The visible clinical symptoms of patients with IBD are abdominal pain, diarrhea, and rectal bleeding [1]. According to the World Health Organization, IBD is one of the most refractory diseases around the world. The incidence and death rates for IBD have increased over the last half century, especially in newly industrialized countries [2,3,4]. Many investigations showed that psychological disorders and malnutrition are observed in patients with IBD, which not only increase the economic burden on patients but also impact negatively on their quality of life [5]. Currently, 25% of drugs used to treat IBD are made from herbs, while 10% are made from microbial sources. As the king of all herbs, ginsenosides, the main active components of Panax ginseng belonging to the family Araliaceae, have various biological and pharmacological effects, such as anti-inflammatory, immunomodulatory, antioxidant, and others. Since ancient times, ginsenosides have been used as food additives in soups and tea drinks in Southeast Asian countries, such as China and have developed into functional foods to prevent inflammation. Previous in vitro and in vivo studies demonstrated that the pharmacological effects of ginsenoside on IBD involved the regulation of immune cell differentiation, cytokine secretion, and inflammatory signal activation. Recent studies focused on the role of natural compound ginsenoside in treating IBD. The findings indicated that the potential therapeutic effects of ginsenosides on IBD were partially mediated by regulating the balance of immune status, cytokine expression, and activation of inflammation-related signaling pathways.
Ginsenosides
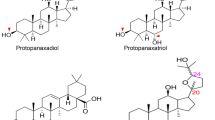
Ginseng, the dried root or rhizome of Panax ginseng belonging to the family Araliaceae, was first referred to in the book Sheng Nong's Herbal Classic [6]. In China, it is known as the "king of all herbs." In the West, it is called Panax ginseng C.A. Meyer, "Panax," which is a Greek word meaning "cure all diseases." Noteworthily, ginsenosides are the main active components of ginseng herbal medicine. They are extracted from roots, stems, leaves, flowers, and fruits of ginseng. They have pharmacological properties, including anti-inflammatory, antitumor, antifibrotic, and glucose lowering [7]. Ginsenosides are polysaccharide derivatives mainly comprising semi-acetal hydroxyl groups of sugars and nonsugar compounds. More than 100 kinds of ginsenosides have been isolated and identified to date [7]. Ginsenosides have a similar chemical structure and are composed of triterpenoid saponins with 30 carbon atoms. They have three main categories based on the structural differences: A, B, and C (Fig. 1). Type A (20 (s)-protopanaxadiol-type saponins] and type B [20 (s)-protopanaxatriol-type saponins) are dammarane-type tetracyclic triterpenoid saponins; most ginsenosides belong to this kind of saponins. C-type saponins are oleanane-type pentacyclic triterpenoid saponins, such as ginsenoside R, which are relatively common in the nature (Table 1).
Pharmacological effects of ginsenosides
For more than 2000 years, ginseng has been regarded in traditional Chinese medicine as a panacea for prolonging life, which can effectively relieve mental stress and physical fatigue [8]. It is not only widely used in the clinic, but also consumed as food materials or additives, health products, and other functional foods in daily life, such as ginseng wine, preserved ginseng fruit, ginseng biscuits, ginseng essence oral liquid, and other products. Sun et al. confirmed that the long-term intake of ginsenosides from the ginseng extract could promote the production of intestinal probiotics and the secretion of anti-inflammatory factors (IL-4 and IL-10), and immunoglobulin (Ig) A (IgA), by nontargeted gas chromatography with time-of-flight mass spectrometry (GC-TOFMS) metabonomics analysis of serum, cecum, and ileum contents [9]. These results showed that long-term administration of ginsenosides had positive effects on host intestinal metabolism, immunity, and intestinal flora balance. With the scientific and technological progress and commodity globalization, ginseng is widely used all over the world. The edible and medicinal values of ginseng have been one of the hotspots of the current new drug research and development because ginsenosides have a variety of pharmacological activities, such as antitumor, anti-inflammatory, analgesic, and antiaging, besides regulating immune homeostasis.
A large body of evidence shows that some monomer components of ginsenosides have protective effects in multiple organs, tissues, and systems (Table 2). Ginsenosides protect against arrhythmia, myocardial hypertrophy, cardiomyocyte apoptosis, myocardial ischemia–reperfusion injury, and heart failure in the cardiovascular system. It can be used to treat neurodegenerative diseases, improve memory function, protect brain tissue, regulate metabolism, treat diabetes and obesity, and regulate insulin levels. Also, it can effectively prevent and control lung cancer, esophageal cancer, gastric cancer, liver cancer, and breast cancer by inducing cancer cell apoptosis and inhibiting cancer cell proliferation. In addition, ginsenosides also have many other effects, such as whitening [10], relieving itching [11], anti-inflammation [12], antivirus [13], and regulating immunity. Among these, the role of ginsenosides in maintaining immune homeostasis is directly or indirectly realized by extensive and clear regulation of immune cells (Table 3). The immunomodulatory effect of ginsenosides is critical in the process of IBD treatment.
Pathogenesis of IBD
An intestinal barrier is very important to maintain host health, and is one of the most metabolically dynamic systems. It is the first line of defense against the invasion of potential pathogens and maintaining immunity homeostasis. The intestinal barrier is composed of physical barrier, immune barrier, and biochemical barrier formed by mucopolysaccharides secreted by intestinal epithelial cells and innate and acquired immune cells and mediated by immune mediators; these barriers work in a coordinated manner. IBD is believed to be a chronic and nonspecific intestinal inflammation caused by intestinal mucosal barrier disorder under the combined action of immunity, genetic, infection, and environmental factors [14, 15]. The underdevelopment and damage of the intestinal physical barrier and immune barrier leads to the onset of IBD. Further, the pathogenesis of IBD is closely related to intestinal endothelial cells and intestinal immune cells [dendritic cells (DCs), macrophages, neutrophils, T lymphocytes, and B lymphocytes] and the levels of secreted cytokines.
Intestinal epithelial cells
The first line of defense of the gastrointestinal tract against antigen invasion is composed of intestinal epithelial cells (IECs); it is located between the lamina propria immune cells and microorganisms in the intestinal lumen. These mature IECs include mucus-secreting goblet cells, hormone-producing Paneth cells, mechano-sensing tuft cells, and nutrient-absorbing enterocytes. These cells participate in antigen presentation and immune response by secreting mucins, antimicrobial peptides (AMPs), and reactive oxygen species (ROS) [16]. Under antigen stimulation, IECs have the potential to secrete cytokines, which not only recruit immune cells to the sites of injured mucosa to participate in immune response but also directly induce the overexpression of inflammatory cytokines (tumor necrosis factor-α, interleukin-1 beta, and IL-6), thus leading to the occurrence and aggravation of IBD. As an indispensable part of the mucosal barrier, IECs play an important role in maintaining the integrity and dynamic balance of the epithelial barrier [17]. When the epithelial cells undergo excessive apoptosis or tight junctions of the gut are damaged, intestinal microorganisms enter the mucosal layer through intestinal leakage, resulting in the continuous stimulation of antigens, massive recruitment of immune cells, and excessive release of inflammatory mediators [18].
Intestinal immune cells
Intestinal innate immune cells involve macrophages, dendritic cells, lamina propria lymphoid cells, and neutrophils, which can respond quickly to various invasive pathogenic microorganisms. However, when innate immune cells are dysregulated, they secrete large amounts of pro-inflammatory cytokines, causing intestinal tissue damage. M1 macrophages secrete pro-inflammatory cytokines (NF-α, IL-1β, and IL-6), while M2 macrophages secrete anti-inflammatory cytokines (IL-4 and IL-10). When the M1/M2 ratio is severely unbalanced, inflammatory cytokine storms cause inflammatory damage in the gut. In IBD biopsy tissues, macrophages recognize pathogenic microorganisms secreting inflammatory cytokines (IL-1α, IL-1β, and TNF-α), aggravating intestinal inflammation [19]. Therefore, macrophages may be an effective therapeutic approach to alleviate IBD [20]. Dendritic cells, full-time antigen-presenting cells located in the lamina propria, can efficiently process intestinal antigens and present them for T cells to induce Th17 cell differentiation. During the pathogenesis of IBD, DC inhibitors promote the occurrence of enteritis. CD103 + DCs can promote the differentiation of Treg cells and inhibit the inflammatory response, while CX3CR1 + DCs exacerbate the inflammatory response [21]. Innate lymphoid cells directly or indirectly affect macrophage and dendritic cell differentiation by secreting cytokines and other mediators to exert early immune surveillance and immunomodulatory functions [22, 23]. Neutrophils are important in inflammation and tissue damage in IBD. Recent studies suggested that IBD symptoms improved significantly and neutrophils were promoted from N1 to N2 phenotype when extracellular regulated protein kinases (ERK) protein phosphorylation was inhibited [24].
In addition, adaptive immune cells such as T helper (Th) cells (Th1, Th2, Th17, and Th9 cells) and B cells are also critical in IBD. Studies have confirmed that Th cells are directly or indirectly involved in the intestinal immune response of IBD, resulting in the aggravation or relief of intestinal mucosal inflammation. Naive CD4 + T cells are stimulated by exogenous antigens and then differentiate into various subsets, such as Th1, Th2, Th7, Th9, Th10, Th17, and Treg cells [25]. IBD are classified into two types based on the different sources of cytokines in inflammatory tissues of intestinal mucosa: Th1-cell-mediated CD and Th2-cell-mediated UC. In patients with CD, Th1 cells secrete large amounts of TNF-α and IFN-γ; thus, affecting the secretion of TNF-α by macrophages in the gut and thus exacerbating the inflammatory response. Th2 cells in the mucosal tissues of patients with UC secrete a large amount of IL-5 and IL-13; thus, promoting the apoptosis of IECs and destroying the intestinal mucosal barrier. The balance of Th17 and Treg cells is important in the induction and regulation of intestinal inflammation, and efforts are underway to determine their role in IBD [26]. CD4 + T cells differentiate into Th17 cells after activation of the signal transduction and transcriptional activator 3 (STAT3) pathway, thus promoting Th17 cells to secrete excessive IL-17 and aggravating inflammatory response [27]. Zheng et al. found that the number of Foxp3 + Treg cells decreased significantly in patients with UC compared with healthy individuals [28]. Th9 cells are newly discovered effector T cells stimulated by IL-4 and TGF-β through transcription factors, such as PU.1. Interferon regulatory factor 4 secretes IL-9 and hence destroys the intestinal mucosal barrier by inhibiting the proliferation of IECs and downregulating the expression of cell tight junction proteins [29]. Meanwhile, B cells are important effector cells in the acquired immune system, which mediate humoral immunity by secreting antibodies. Likewise, they also supply opsonins for the maturity and functioning of antigen-presenting cells to develop T cells. B cells are involved in the pathogenesis of IBD. B cells promote chemotaxis and migration of neutrophils to the site of tissue inflammation and aggravate inflammatory injury in the peripheral blood of patients with CD, followed by high expression of Toll-like receptor 2 (TLR2) and IL-8 on the surface of B cells, indicating that the immune activity of B cells was enhanced during IBD morbidity [30]. Breg-like cells (IL-33 + Breg) isolated from mice with IBD inhibited the expansion and functioning of immune effector cells and effectively prevented the development of spontaneous colitis in IL-10 − / − mice after adoptive metastasis [31]. These results indicated that adaptive immune cells were the key to the treatment of IBD, and targeting the differentiation of T lymphocytes and B lymphocytes might be an effective strategy for treating IBD.
Cytokines
Cytokines are small-molecule proteins with a wide range of biological activities. They are produced by immune and nonimmune cells stimulated by antigens, mitogen, or other factors. They are divided into anti-inflammatory cytokines and pro-inflammatory cytokines. The imbalance between them is an important reason for induced intestinal mucosal injury, intestinal barrier dysfunction, and persistent intestinal inflammation. The breakdown of the intestinal barrier attributes the most to the overproduction of pro-inflammatory cytokines, such as TNF-α, IL-1α, and IFN-γ, which are triggered by the activation of the activating protein 1/mitogen-activated protein kinase (AP-1/MAPK) pathway [32]. In vivo studies found that the downregulated activation of IL-1R and TLR effectively reduced chronic colonic inflammatory injury in mice [33], which was closely related to pro-inflammatory cytokines. Similarly, clinical studies showed that retinoic acid maintained intestinal inflammation by upregulating pro-inflammatory cytokines (IL-17 and IFN-γ) [34]. In addition, anti-inflammatory cytokines (IL-10 and TGF-β1) also played an important role in repairing intestinal inflammatory injury. Huber and his workmates reported that IL-10 suppressed the excessive immune response by inhibiting the polarization of Th1 and Th17 cells to alleviate IBD [35]. In vivo and in vitro studies showed that TGF-β1 was a negative regulator of mucosal inflammation. The activation of TGF-β1 led to a decrease in the expression of pro-inflammatory cytokines in mice with colitis or patients with IBD, thus effectively relieving the clinical symptoms of CD [36]. Interestingly, some cytokines, such as IL-33, have dual immunomodulatory effects. Lopetuso and his workmates reported that IL-33 promoted the recovery of acute colitis by inducing miR-320 to stimulate epithelial regeneration and repair in experimental colitis [37]. In contrast, Zhu showed that IL-33 induced DSS-induced colitis in mice by promoting Th2 response and inhibiting Th1 response in the mesenteric lymph node (MLN) [38]. To sum up, the cytokine played a decisive role in the occurrence and pathogenesis of IBD. It also suggested that the balance of cytokines might be an effective target for IBD treatment.
Effects of ginsenoside on IBD
Clinically, the therapeutic agents for IBD include cyclosporin, corticosteroids, 5-aminosalicylic acid (Mesalamine), mercaptopurine, antitumor necrosis factor (TNF −) monoclonal antibody, azathioprine, and so on. These drugs are expensive and also have obvious side effects and poor tolerance [39]. Yet, natural medicines are cheap, readily available, and highly effective. Notably, relevant studies reported that ginsenosides, the main active components of Panax ginseng, had anti-inflammatory and immunomodulatory effects on IBD, including improving body weight, decreasing disease activity index (DAI), and the index of colonic weight and body weight or colonic length, restoring pathological damage in colonic mucosa, and reducing microscopic, and macroscopic injury scores [40]. In vivo experiments showed that ginsenosides not only promoted the proliferation of intestinal mucosal epithelium, but also regulated the differentiation of immune cells and the secretion of inflammatory mediators; thus, effectively relieving the symptoms of IBD [41]. In vitro cell experiments showed that ginsenosides could effectively inhibit HT-29 cells to secrete pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) under lipopolysaccharide (LPS) stimulation [6]. The mechanism of ginsenoside treating IBD was reviewed in this study, thus providing the reference for the clinical application of IBD.
Ginsenosides improved IBD by regulating the balance of immune cells
With the help of innate immunity, adaptive immunity is activated, and their combined action establishes and maintains the immune homeostasis. As an autoimmune disease, IBD is caused by multiple factors, including environment, genetic predisposition, and immune dysregulation, leading to abnormal differentiation of autoreactive T and B lymphocytes. Yang et al. found that ginsenoside Rd alleviated the symptoms of TNBS-induced animal UC by inhibiting neutrophil infiltration and improving the antioxidant capacity of damaged colon tissue [42]. As shown in Fig. 2a, 20 (S)-protopanaxatriol, Rg1 metabolite of ginsenoside inhibited the binding of TLR4 to LPS on macrophages, restored the balance of Th17/Treg cells, and thus relieved inflammatory diseases such as colitis [43]. Lee et al. used ginsenoside Re to treat TNBS-induced colitis in mice and found that ginsenoside Re could also inhibit the binding of LPS to macrophage membrane TLR4 and further effectively treat inflammation [44]. Moreover, Ginseng berry extract (GB) inhibited the activation of infiltrating T cells, neutrophils, dendritic cells, and macrophages in the colon of mice with DSS-induced colitis and effectively relieved IBD. At the same time, GB promoted the migration of CD103 + CD11c + DCs and the proliferation and differentiation of Foxp3 + Treg cells in the colon of mice with colitis [45]. Fermented red ginseng alleviated TNBS-induced colitis by inhibiting the activation of macrophages and regulating the differentiation of Th1 and Treg cells [46]. To sum up, ginseng and ginsenosides clearly had the potential as an effective target for treating IBD by interfering with the proliferation and differentiation of immune cells.
Ginsenosides improved IBD by regulating cytokine expression
Both CD and UC are characterized by increased expression of IL-1β, IL-6, IL-8, TNF-α, IL-16, and various T-cell chemokines [47, 48]. As shown in Fig. 2e, ginsenoside Rg1 has been reported to inhibit the release of pro-inflammatory cytokines (IL-1β and TNF-α) by upregulating the expression of NLRP2 and then weaken the inflammatory response of DSS-induced colitis in mice [49]. Ahn et al. showed that ginsenoside Rf significantly decreased the production of IL-1β, IL-6, TNF-α, NO, and ROS in IBD (Fig. 2b), indicating that ginsenoside Rf could treat IBD by inhibiting the expression of inflammatory factors [6]. Some animal experiments by Wang and his workmates showed that ginsenoside Rg3 could significantly ameliorate DSS-induced colitis by inhibiting the expression of pro-inflammatory cytokines (IL-1β and IL-6) [40]. After mice with TNBS-induced colitis were treated by intragastric administration of ginsenoside Rb1, the levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) declined, while the levels of anti-inflammatory cytokines (IL-10) increased [50]. Li et al. found that ginsenoside metabolite K relieved histopathological damage in mice with DSS-induced colitis, decreased myeloperoxidase (MPO) activity (Fig. 2b), reduced the production of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α), and increased the production of IL-10 in colon tissue and peripheral blood [51]. Ginsenoside Rh2 significantly decreased the expression of IL-6, TNF-α, and IFN-γ in mice with DSS-induced colitis. Ginsenoside also regulated the levels of inflammatory cytokines; thus, playing a positive role in treating IBD.
Ginsenosides improved IBD by regulating inflammatory signaling pathway
Many inflammatory signaling pathways are vital in IBD, such as NF-κB, NLRP3, MAPK, AMP-activated kinase (AMPK), and TGF-β/Smad signaling pathway [52, 53]. As shown in Fig. 2d, ginsenoside Rf [44], ginsenoside Rb1 [54], and ginsenoside compound K [55] had regulatory effects on these inflammatory signaling pathways in the process of IBD treatment. Ginsenoside Rd drove autophagy to degrade NLRP3 inflammatory bodies through the AMPK-ULK1-p62 signal axis and downregulated the secretion of IL-1β by inhibiting macrophages to finally treat acute colitis in mice [56]. Furthermore, some studies showed that the protective effect of ginsenoside Rd on TNBS-induced recurrent colitis might be realized by regulating the activation of JNK and p38, reducing leukocyte aggregation, and downregulating the expression of TNF-α, IL-1β, IL-6, and other pro-inflammatory cytokines [57]. The TGF-β signal is considered to be one of the essential anti-inflammatory signaling pathways. Ginsenoside Rh2 may increase the phosphorylation of downstream small mother against decapentaplegic (Smad) signaling by activating the TGF-β signaling pathway, inhibit the activation of pro-inflammatory signal pathways, such as NF-κB and MAPK, and significantly relieve the symptoms of IBD [58]. The interaction between pro-inflammatory and anti-inflammatory signaling pathways determines the occurrence and development of IBD. Ginsenosides and their metabolites regulate a variety of inflammatory signaling pathways with their multitarget characteristics so as to effectively alleviate IBD.
Conclusions
In summary, the potential protective effects of ginsenosides on IBD treatment are very definite and effective. They are the main active components of Panax ginseng. IBD is typically characterized by severe destruction of intestinal barrier function, including excessive apoptosis of IECs, excessive secretion of cytokines, and imbalance of immune status. Aminosalicylates, corticosteroids, immunosuppressive drugs, and monoclonal antibodies to TNF-α are well-established pharmacological therapies for IBD. However, these drugs have not always been effective against IBD and have some side effects. Recent studies focused on the effects of natural anti-inflammatory drug ginsenosides on IBD treatment, largely due to the safety, reliability, and affordability of ginsenosides in clinic. Ginsenosides are important in inhibiting TLR4/NF-κB/NLRP signal transduction, regulating inflammatory cytokine expression, and inducing immune cell maturation and differentiation to relieve the inflammatory injury in the colonic mucosa of patients with colitis. The effects of ginsenosides on immunoregulation and intestinal epithelial regeneration aim to enhance the intestinal mucosal barrier function. Hence, ginsenosides might serve as a promising new drug for treating IBD.
Ginsenosides should be developed and widely used in the future to alleviate inflammatory injury in the colonic mucosa of patients with IBD owing to its therapeutic effect, less side effects, and high acceptability.
Abbreviations
- AMPK:
-
AMP-activated kinase
- CD:
-
Crohn’s disease
- DSS:
-
Dextran sulfate sodium
- ERK:
-
Extracellular regulated protein kinases
- GC-TOFMS:
-
Gas chromatography with time-of-flight mass spectrometry
- HT-29:
-
Colorectal cancer cells
- IBD:
-
Inflammatory bowel disease
- IFN-γ:
-
Interferon-γ
- ILs:
-
Interleukins
- JNK:
-
C-Jun n-terminal kinase
- MAPK:
-
Mitogen-activated protein kinases
- NF-κB:
-
Nuclear factor-kappa B
- NLRP:
-
Nucleotide-binding oligomerization domain-like receptor protein
- NO:
-
Nitric oxide
- PU.1:
-
Purine-rich nucleic acid-binding protein
- TGF-β:
-
Transforming growth factor-beta
- TLR4:
-
Toll-like receptor 4
- TNBS:
-
2,4,6-Trinitrobenzene sulfonic acid
- TNF-α:
-
Tumor necrosis factor-α
- UC:
-
Ulcerative colitis
References
Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–32.
Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152(313–321):e312.
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–78.
Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741–63.
Cao Q, Huang YH, Jiang M, Dai C. The prevalence and risk factors of psychological disorders, malnutrition and quality of life in IBD patients. Scand J Gastroenterol. 2019;54:1458–66.
Sun XY, Sun FJ. Shennong classic of materia medica. Beijing: Commercial Press; 1955. p. 9.
Shin BK, Kwon SW, Park JH. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–98.
Patel S, Rauf A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed Pharmacother. 2017;85:120–7.
Sun Y, Chen S, Wei R, Xie X, Wang C, Fan S, et al. Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 2018;9:3547–56.
Liu XY, Xiao YK, Hwang E, Haeng JJ, Yi TH. Antiphotoaging and antimelanogenesis properties of ginsenoside C-Y, a ginsenoside Rb2 metabolite from American Ginseng PDD-ginsenoside. Photochem Photobiol. 2019;95:1412–23.
Lee WJ, Kim YS, Shim WS. Korean Red Ginseng extract and ginsenoside Rg3 have anti-pruritic effects on chloroquine-induced itch by inhibition of MrgprA3/TRPA1-mediated pathway. J Ginseng Res. 2018;42:470–5.
Jang M, Lee MJ, Choi JH, Kim EJ, Nah SY, Kim HJ, et al. Ginsenoside Rb1 attenuates acute inflammatory nociception by inhibition of neuronal ERK phosphorylation by regulation of the Nrf2 and NF-κB pathways. J Pain. 2016;17:282–97.
Wright S, Altman E. Inhibition of herpes simplex viruses, Types 1 and 2, by Ginsenoside 20(S)-Rg3. J Microbiol Biotechnol. 2020;30:101–8.
Mei Y, Fang C, Ding S, Liu X, Hu J, Xu J, et al. PAP-1 ameliorates DSS-induced colitis with involvement of NLRP3 inflammasome pathway. Int Immunopharmacol. 2019;75:105776.
Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262.
Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70–89.
Lu H, Li H, Fan C, Qi Q, Yan Y, Wu Y, et al. RIPK1 inhibitor ameliorates colitis by directly maintaining intestinal barrier homeostasis and regulating following IECs-immuno crosstalk. Biochem Pharmacol. 2020;172:113751.
Sánchez de Medina F, Romero-Calvo I, Mascaraque C, Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis. 2014;20:2394–404.
Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10.
Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity (Trends in Immunology 39, 677-696, 2018). Trends Immunol. 2019;40:174.
Davies JM, Abreu MT. The innate immune system and inflammatory bowel disease. Scand J Gastroenterol. 2015;50:24–33.
Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345:1254009.
Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 2015;348:1031–5.
Wang G, Joel MDM, Yuan J, Wang J, Cai X, Ocansey DKW, et al. Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease by inhibiting ERK phosphorylation in neutrophils. Inflammopharmacology. 2020;28:603–16.
Huang G, Wang Y, Chi H. Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol. 2012;9:287–95.
Zanello G, Kevans D, Goethel A, Silverberg M, Tyler A, Croitoru K. Genetics and innate and adaptive immunity in IBD. Nestle Nutr Inst Workshop Ser. 2014;79:41–55.
Zhu C, Song K, Shen Z, Quan Y, Tan B, Luo W, et al. Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T cells differentiation in colitis. Mol Med Rep. 2018;17:7567–74.
Zheng Y, Huang L, Ge W, Yang M, Ma Y, Xie G, et al. Protein arginine methyltransferase 5 inhibition upregulates Foxp3(+) regulatory T cells frequency and function during the ulcerative colitis. Front Immunol. 2017;8:596.
Shohan M, Elahi S, Shirzad H, Rafieian-Kopaei M, Bagheri N, Soltani E. Th9 cells: probable players in ulcerative colitis pathogenesis. Int Rev Immunol. 2018;37:192–205.
Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, et al. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009;86:1007–16.
Sattler S, Ling G, Xu D, Hussaarts L, Romaine A, Zhao H, et al. IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J Autoimmun. 2014;50:107–22.
Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189.
Luzardo-Ocampo I, Campos-Vega R, Gonzalez de Mejia E, Loarca-Piña G. Consumption of a baked corn and bean snack reduced chronic colitis inflammation in CD-1 mice via downregulation of IL-1 receptor, TLR, and TNF-α associated pathways. Food Res Int. 2020;132:109097.
Rampal R, Wari N, Singh AK, Das U, Bopanna S, Gupta V, et al. Retinoic acid is elevated in the mucosa of patients with active ulcerative colitis and displays a proinflammatory role by augmenting IL-17 and IFNγ production. Inflamm Bowel Dis. 2021;27(1):74–83.
Huber S, Gagliani N, Esplugues E, O’Connor W Jr, Huber FJ, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3- and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–65.
Sedda S, Marafini I, Dinallo V, Di Fusco D, Monteleone G. The TGF-β/Smad system in IBD pathogenesis. Inflamm Bowel Dis. 2015;21:2921–5.
Lopetuso LR, De Salvo C, Pastorelli L, Rana N, Senkfor HN, Petito V, et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A. 2018;115:E9362-e9370.
Zhu J, Xu Y, Zhu C, Zhao J, Meng X, Chen S, et al. IL-33 induces both regulatory B cells and regulatory T cells in dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2017;46:38–47.
Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322:127–35.
Wang CZ, Yao H, Zhang CF, Chen L, Wan JY, Huang WH, et al. American ginseng microbial metabolites attenuate DSS-induced colitis and abdominal pain. Int Immunopharmacol. 2018;64:246–51.
Yang N, Liang G, Lin J, Zhang S, Lin Q, Ji X, et al. Ginsenoside Rd therapy improves histological and functional recovery in a rat model of inflammatory bowel disease. Phytother Res. 2020;34(11):3019–28.
Yang XL, Guo TK, Wang YH, Gao MT, Qin H, Wu YJ. Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch Pharm Res. 2012;35:1231–9.
Lee SY, Jeong JJ, Eun SH, Kim DH. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol. 2015;762:333–43.
Lee IA, Hyam SR, Jang SE, Han MJ, Kim DH. Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food Chem. 2012;60:9595–602.
Zhang W, Xu L, Cho SY, Min KJ, Oda T, Zhang L, et al. Ginseng berry extract attenuates dextran sodium sulfate-induced acute and chronic colitis. Nutrients. 2016;8:199.
Jeon-Kyung K, Jae-Young K, Se-Eun J, Min-Sun C, Hyo-Min J, Hae-Hyun Y, et al. Fermented red ginseng alleviates cyclophosphamide-induced immunosuppression and 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by regulating macrophage activation and T cell differentiation. Am J Chin Med. 2018;46(8):1879–97.
Keates AC, Castagliuolo I, Cruickshank WW, Qiu B, Arseneau KO, Brazer W, et al. Interleukin 16 is up-regulated in Crohn’s disease and participates in TNBS colitis in mice. Gastroenterology. 2000;119:972–82.
Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, McDermott MJF. Tumour necrosis factor signalling in health and disease. F1000Res. 2019;8:F1000 Faculty Rev-111.
Zhu G, Wang H, Wang T, Shi F. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int Immunopharmacol. 2017;50:1–5.
Joh EH, Lee IA, Jung IH, Kim DH. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation—the key step of inflammation. Biochem Pharmacol. 2011;82:278–86.
Li J, Zhong W, Wang W, Hu S, Yuan J, Zhang B, et al. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PLoS ONE. 2014;9(2):e87810.
Gao Y, Huang Y, Zhao Y, Hu Y, Li Z, Guo Q, et al. LL202 protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting MAPK/AP-1 signaling. Oncotarget. 2016;7:63981–94.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023.
Zhang J, Cao L, Wang H, Cheng X, Wang L, Zhu L, et al. Ginsenosides regulate PXR/NF-κB signaling and attenuate dextran sulfate sodium-induced colitis. Drug Metab Dispos. 2015;43:1181–9.
Juan L, Wei Z, Weiwei W, Shaoping H, Jiahui Y, Bing Z, et al. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0087810.
Liu C, Wang J, Yang Y, Liu X, Zhu Y, Zou J, et al. Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochem Pharmacol. 2018;155:366–79.
Yang XL, Guo TK, Wang YH, Huang YH, Liu X, Wang XX, et al. Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int Immunopharmacol. 2012;12:408–14.
Ye H, Wu Q, Zhu Y, Guo C, Zheng X. Ginsenoside Rh2 alleviates dextran sulfate sodium-induced colitis via augmenting TGFβ signaling. Mol Biol Rep. 2014;41:5485–90.
Sanada S, Kondo N, Shoji J, Tanaka O, Shibata S. Studies on the Saponins of Ginseng. I. Structures of Ginsenoside-Ro, -Rb1, -Rb2, -Rc and -Rd. Chem Pharm Bull. 1974. https://doi.org/10.1248/cpb.22.421.
Sanada S, Shoji J. Studies on the Saponins of Ginseng. III. Structures of Ginsenoside-Rb3 and 20-Glucoginsenoside-Rf. Chem Pharm Bull. 1978. https://doi.org/10.1248/cpb.26.1694.
Yang Z. Chemical studies on the stems of Panax ginseng C.A. Meyer: (1). Isolation and identification of ginseng stem saponins I, II and III. Zhong Yao Tong Bao (Beijing, China: 1981). 1987;12:36–40.
Kasai R, Besso H, Tanaka O, Saruwatari Y, Fuwa TJC, Bulletin P. Saponins of red ginseng. Chem Pharm Bull. 1983;31:2120–5.
Kitagawa I, Taniyama T, Shibuya H, Noda T, Yoshikawa M. Chemical studies on crude drug processing. V. On the constituents of ginseng radix rubra (2): comparison of the constituents of white ginseng and red ginseng prepared from the same Panax ginseng root. Yakugaku Zasshi. 1987;107:495–505.
Chen C, Zhang H, Xu H, Zheng Y, Wu T, Lian Y. Ginsenoside Rb1 ameliorates cisplatin-induced learning and memory impairments. J Ginseng Res. 2019;43:499–507.
Gao X, Zhang X, Cui L, Chen R, Zhang C, Xue J, et al. Ginsenoside Rb1 promotes motor functional recovery and axonal regeneration in post-stroke mice through cAMP/PKA/CREB signaling pathway. Brain Res Bull. 2020;154:51–60.
Li CY, Yang P, Jiang YL, Lin Z, Pu YW, Xie LQ, et al. Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia reperfusion injury through mTOR signal pathway. Biomed Pharmacother. 2020;125:109913.
Liu D, Liu T, Teng Y, Chen W, Zhao L, Li X. Ginsenoside Rb1 inhibits hypoxia-induced epithelial–mesenchymal transition in ovarian cancer cells by regulating microRNA-25. Exp Ther Med. 2017;14:2895–902.
Liu Y, Zong X, Huang J, Guan Y, Li Y, Du T, et al. Ginsenoside Rb1 regulates prefrontal cortical GABAergic transmission in MPTP-treated mice. Aging (Albany NY). 2019;11:5008–34.
Liu Z, Song L, Zhang P, Cao Z, Hao J, Tian Y, et al. Ginsenoside Rb1 exerts antiarrhythmic effects by inhibiting I(Na) and I(CaL) in rabbit ventricular myocytes. Sci Rep. 2019;9:20425.
Lou MD, Li J, Cheng Y, Xiao N, Ma G, Li P, et al. Glucagon up-regulates hepatic mitochondrial pyruvate carrier 1 through cAMP-responsive element-binding protein; inhibition of hepatic gluconeogenesis by ginsenoside Rb1. Br J Pharmacol. 2019;176:2962–76.
Park SJ, Park M, Sharma A, Kim K, Lee HJ. Black Ginseng and Ginsenoside Rb1 promote browning by inducing UCP1 expression in 3T3-L1 and primary white adipocytes. Nutrients. 2019. https://doi.org/10.3390/nu11112747.
Zhang J, Wang J, Wu X, Wei Y. Ginsenoside Rb1 inhibits proliferation and promotes apoptosis by regulating HMGB1 in uterine fibroid cells. Artif Cells Nanomed Biotechnol. 2019;47:2967–71.
Zhou P, Xie W, He S, Sun Y, Meng X, Sun G, et al. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. 2019. https://doi.org/10.3390/cells8030204.
Zhou P, Zhang X, Guo M, Guo R, Wang L, Zhang Z, et al. Ginsenoside Rb1 ameliorates CKD-associated vascular calcification by inhibiting the Wnt/β-catenin pathway. J Cell Mol Med. 2019;23:7088–98.
Dai G, Sun B, Gong T, Pan Z, Meng Q, Ju W. Ginsenoside Rb2 inhibits epithelial–mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine. 2019;56:126–35.
Dai S, Hong Y, Xu J, Lin Y, Si Q, Gu X. Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT-dependent mechanisms. Biomed Pharmacother. 2018;100:93–100.
Hong Y, Lin Y, Si Q, Yang L, Dong W, Gu X. Ginsenoside Rb2 alleviates obesity by activation of brown fat and induction of browning of white fat. Front Endocrinol (Lausanne). 2019;10:153.
Kim DH, Kim DW, Jung BH, Lee JH, Lee H, Hwang GS, et al. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J Ginseng Res. 2019;43:326–34.
Phi LTH, Wijaya YT, Sari IN, Yang YG, Lee YK, Kwon HY. The anti-metastatic effect of ginsenoside Rb2 in colorectal cancer in an EGFR/SOX2-dependent manner. Cancer Med. 2018;7:5621–31.
Chen X, Wang Q, Shao M, Ma L, Guo D, Wu Y, et al. Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomed Pharmacother. 2019;120:109487.
Liu X, Jiang Y, Yu X, Fu W, Zhang H, Sui D. Ginsenoside-Rb3 protects the myocardium from ischemia–reperfusion injury via the inhibition of apoptosis in rats. Exp Ther Med. 2014;8:1751–6.
Meng F, Su X, Li W, Zheng Y. Ginsenoside Rb3 strengthens the hypoglycemic effect through AMPK for inhibition of hepatic gluconeogenesis. Exp Ther Med. 2017;13:2551–7.
Wang Y, Li Y, Zhang Y, Feng G, Yang Z, Guan Q, et al. Multi-dimensional spectrum-effect relationship of the impact of Chinese herbal formula Lichong Shengsui Yin on ovarian cancer. Molecules. 2017. https://doi.org/10.3390/molecules22060979.
Yang JW, Kim SS. Ginsenoside Rc promotes anti-adipogenic activity on 3T3-L1 adipocytes by down-regulating C/EBPα and PPARγ. Molecules. 2015;20:1293–303.
Chian S, Zhao Y, Xu M, Yu X, Ke X, Gao R, et al. Ginsenoside Rd reverses cisplatin resistance in non-small-cell lung cancer A549 cells by downregulating the nuclear factor erythroid 2-related factor 2 pathway. Anticancer Drugs. 2019;30:838–45.
Yao L, Han Z, Zhao G, Xiao Y, Zhou X, Dai R, et al. Ginsenoside Rd ameliorates high fat diet-induced obesity by enhancing adaptive thermogenesis in a cAMP-dependent manner. Obesity (Silver Spring). 2020;28:783–92.
Zhang N, An X, Lang P, Wang F, Xie Y. Ginsenoside Rd contributes the attenuation of cardiac hypertrophy in vivo and in vitro. Biomed Pharmacother. 2019;109:1016–23.
Zhang X, Liu X, Hu G, Zhang G, Zhao G, Shi M. Ginsenoside Rd attenuates blood–brain barrier damage by suppressing proteasome-mediated signaling after transient forebrain ischemia. NeuroReport. 2020;31:466–72.
Kim K, Nam KH, Yi SA, Park JW, Han JW, Lee J. Ginsenoside Rg3 induces browning of 3T3-L1 adipocytes by activating AMPK signaling. Nutrients. 2020. https://doi.org/10.3390/nu12020427.
Lee A, Yun E, Chang W, Kim J. Ginsenoside Rg3 protects against iE-DAP-induced endothelial-to-mesenchymal transition by regulating the miR-139-5p-NF-κB axis. J Ginseng Res. 2020;44:300–7.
Lee H, Kong G, Tran Q, Kim C, Park J, Park J. Relationship between ginsenoside Rg3 and metabolic syndrome. Front Pharmacol. 2020;11:130.
Li B, Qu G. Inhibition of the hypoxia-induced factor-1α and vascular endothelial growth factor expression through ginsenoside Rg3 in human gastric cancer cells. J Cancer Res Ther. 2019;15:1642–6.
Li L, Wang Y, Guo R, Li S, Ni J, Gao S, et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Control Release. 2020;317:259–72.
Oh J, Yoon HJ, Jang JH, Kim DH, Surh YJ. The standardized Korean Red Ginseng extract and its ingredient ginsenoside Rg3 inhibit manifestation of breast cancer stem cell-like properties through modulation of self-renewal signaling. J Ginseng Res. 2019;43:421–30.
Zhang Y, Yang X, Wang S, Song S. Ginsenoside Rg3 prevents cognitive impairment by improving mitochondrial dysfunction in the rat model of Alzheimer’s disease. J Agric Food Chem. 2019;67:10048–58.
Zou J, Su H, Zou C, Liang X, Fei Z. Ginsenoside Rg3 suppresses the growth of gemcitabine-resistant pancreatic cancer cells by upregulating lncRNA-CASC2 and activating PTEN signaling. J Biochem Mol Toxicol. 2020;34:e22480.
Hou J, Yun Y, Xue J, Jeon B, Kim S. Doxorubicin-induced normal breast epithelial cellular aging and its related breast cancer growth through mitochondrial autophagy and oxidative stress mitigated by ginsenoside Rh2. Phytother Res. 2020. https://doi.org/10.1002/ptr.6636.
Jin X, Yang Q, Cai N, Zhang Z. A cocktail of betulinic acid, parthenolide, honokiol and ginsenoside Rh2 in liposome systems for lung cancer treatment. Nanomedicine (Lond). 2020;15:41–54.
Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT, Chen ZC. Ginsenoside Rh2 improves cardiac fibrosis via PPARδ-STAT3 signaling in Type 1-like diabetic rats. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18071364.
Lu C, Wang Y, Lv J, Jiang N, Fan B, Qu L, et al. Ginsenoside Rh2 reverses sleep deprivation-induced cognitive deficit in mice. Behav Brain Res. 2018;349:109–15.
Zhang XP, Li KR, Yu Q, Yao MD, Ge HM, Li XM, et al. Ginsenoside Rh2 inhibits vascular endothelial growth factor-induced corneal neovascularization. FASEB J. 2018;32:3782–91.
Gao Y, Zhu P, Xu SF, Li YQ, Deng J, Yang DL. Ginsenoside Re inhibits PDGF-BB-induced VSMC proliferation via the eNOS/NO/cGMP pathway. Biomed Pharmacother. 2019;115:108934.
Jang HJ, Han IH, Kim YJ, Yamabe N, Lee D, Hwang GS, et al. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem. 2014;62:2830–6.
Kim JM, Park CH, Park SK, Seung TW, Kang JY, Ha JS, et al. Ginsenoside Re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet-induced C57BL/6 mice. J Agric Food Chem. 2017;65:2719–29.
Li J, Liu Y, Li W, Wang Z, Guo P, Li L, et al. Metabolic profiling of the effects of ginsenoside Re in an Alzheimer’s disease mouse model. Behav Brain Res. 2018;337:160–72.
Yu Y, Sun J, Liu J, Wang P, Wang C. Ginsenoside Re preserves cardiac function and ameliorates left ventricular remodeling in a rat model of myocardial infarction. J Cardiovasc Pharmacol. 2020;75:91–7.
Du Y, Fu M, Wang YT, Dong Z. Neuroprotective effects of ginsenoside Rf on amyloid-β-induced neurotoxicity in vitro and in vivo. J Alzheimers Dis. 2018;64:309–22.
Li Y, Chen C, Li S, Jiang C. Ginsenoside Rf relieves mechanical hypersensitivity, depression-like behavior, and inflammatory reactions in chronic constriction injury rats. Phytother Res. 2019;33:1095–103.
Siraj FM, Natarajan S, Huq MA, Kim YJ, Yang DC. Structural investigation of ginsenoside Rf with PPARγ major transcriptional factor of adipogenesis and its impact on adipocyte. J Ginseng Res. 2015;39:141–7.
Alolga RN, Nuer-Allornuvor GF, Kuugbee ED, Yin X, Ma G. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: a review of scientific findings and call for further research. Pharmacol Res. 2020;152:104630.
Gao J, Bai P, Li Y, Li J, Jia C, Wang T, et al. Metabolomic profiling of the synergistic effects of ginsenoside Rg1 in combination with neural stem cell transplantation in ischemic stroke rats. J Proteome Res. 2020;19(7):2676–88.
Luo M, Yan D, Sun Q, Tao J, Xu L, Sun H, et al. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem. 2020;121:2994–3004.
Mo J, Zhou Y, Yang R, Zhang P, He B, Yang J, et al. Ginsenoside Rg1 ameliorates palmitic acid-induced insulin resistance in HepG2 cells in association with modulating Akt and JNK activity. Pharmacol Rep. 2019;71:1160–7.
Wang L, Lu J, Zeng Y, Guo Y, Wu C, Zhao H, et al. Improving Alzheimer’s disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiol Lett. 2020. https://doi.org/10.1093/femsle/fnaa011.
Wang YM, Ma YQ, Bi SC, Ma XD, Guan R, Wang SH, et al. Therapeutic effect of ginsenoside Rg1 on mastitis experimentally induced by lipopolysaccharide in lactating goats. J Dairy Sci. 2019;102:2443–52.
Liu H, Liu M, Jin Z, Yaqoob S, Zheng M, Cai D, et al. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019;10:3603–14.
Pi MS, Ru Q, Gong XK, Wu RH, Tian X, Xiong Q, et al. Effect of ginsenoside Rg2 and its stereoisomers on oxygen-glucose deprivation and reperfusion induced cortical neuronal injury model. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36:333–8.
Lu C, Shi Z, Dong L, Lv J, Xu P, Li Y, et al. Exploring the effect of ginsenoside Rh1 in a sleep deprivation-induced mouse memory impairment model. Phytother Res. 2017;31:763–70.
Lyu X, Xu X, Song A, Guo J, Zhang Y, Zhang Y. Ginsenoside Rh1 inhibits colorectal cancer cell migration and invasion in vitro and tumor growth in vivo. Oncol Lett. 2019;18:4160–6.
Shin JH, Kwon HW, Cho HJ, Rhee MH, Park HJ. Vasodilator-stimulated phosphoprotein–phosphorylation by ginsenoside Ro inhibits fibrinogen binding to αIIb/β (3) in thrombin-induced human platelets. J Ginseng Res. 2016;40:359–65.
Zheng SW, Xiao SY, Wang J, Hou W, Wang YP. Inhibitory effects of ginsenoside Ro on the growth of B16F10 melanoma via its metabolites. Molecules. 2019. https://doi.org/10.3390/molecules24162985.
Guan X, Yuan Y, Wang G, Zheng R, Zhang J, Dong B, et al. Ginsenoside Rg3 ameliorates acute exacerbation of COPD by suppressing neutrophil migration. Int Immunopharmacol. 2020;83:106449.
Son KJ, Choi KR, Lee SJ, Lee H. Immunogenic cell death induced by ginsenoside Rg3: significance in dendritic cell-based anti-tumor immunotherapy. Immune Netw. 2016;16:75–84.
Xin C, Kim J, Quan H, Yin M, Jeong S, Choi JI, et al. Ginsenoside Rg3 promotes Fc gamma receptor-mediated phagocytosis of bacteria by macrophages via an extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent mechanism. Int Immunopharmacol. 2019;77:105945.
Zhang Y, Wang S, Song S, Yang X, Jin G. Ginsenoside Rg3 alleviates complete Freund’s adjuvant-induced rheumatoid arthritis in mice by regulating CD4(+)CD25(+)Foxp3(+)Treg cells. J Agric Food Chem. 2020;68:4893–902.
Huang Y, Zou Y, Lin L, Zheng R. Ginsenoside Rg1 activates dendritic cells and acts as a vaccine adjuvant inducing protective cellular responses against lymphomas. DNA Cell Biol. 2017;36:1168–77.
Lee JH, Han Y. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int Immunopharmacol. 2006;6:1424–30.
Park HY, Lee SH, Lee KS, Yoon HK, Yoo YC, Lee J, et al. Ginsenoside Rg1 and 20(S)-Rg3 induce IgA production by mouse B cells. Immune Netw. 2015;15:331–6.
Wang Y, Liu Y, Zhang XY, Xu LH, Ouyang DY, Liu KP, et al. Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-κB and PI3K/Akt/mTOR pathways. Int Immunopharmacol. 2014;23:77–84.
Lee MJ, Jang M, Choi J, Chang BS, Kim DY, Kim SH, et al. Korean Red Ginseng and ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Mol Neurobiol. 2016;53:1977–2002.
Liu HY, Shi DZ, Ge JB. Effect of ginsenoside Rb1 on immune maturation of human monocyte-derived dendritic cells induced by oxidized low-density lipoprotein. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31:350–4.
Xin C, Quan H, Kim JM, Hur YH, Shin JY, Bae HB, et al. Ginsenoside Rb1 increases macrophage phagocytosis through p38 mitogen-activated protein kinase/Akt pathway. J Ginseng Res. 2019;43:394–401.
Chen J, Wang Q, Wu H, Liu K, Wu Y, Chang Y, et al. The ginsenoside metabolite compound K exerts its anti-inflammatory activity by downregulating memory B cell in adjuvant-induced arthritis. Pharm Biol. 2016;54:1280–8.
Chen J, Wu H, Wang Q, Chang Y, Liu K, Song S, et al. Ginsenoside metabolite compound k alleviates adjuvant-induced arthritis by suppressing T cell activation. Inflammation. 2014;37:1608–15.
Chen J, Wu H, Wang Q, Chang Y, Liu K, Wei W. Ginsenoside metabolite compound K suppresses T-cell priming via modulation of dendritic cell trafficking and costimulatory signals, resulting in alleviation of collagen-induced arthritis. J Pharmacol Exp Ther. 2015;353:71–9.
Wang R, Zhang M, Hu S, Liu K, Tai Y, Tao J, et al. Ginsenoside metabolite compound-K regulates macrophage function through inhibition of β-arrestin2. Biomed Pharmacother. 2019;115:108909.
Funding
This study was supported in part by the National Natural Science Foundation of China (No. 8180792 and 81760808), the Natural Science Foundation of Jiangxi Province (No. 20192ACB20015, 20192BAB215050, and 20202ACBL206026) and the 1050 Young talents project (No. 1141900603) and first-class subjects starting funds (No. JXSYLXK-ZHYI022, JXSYLXK-ZHYAO132, and JXSYLXK-ZHYAO108) of Jiangxi University of Traditional Chinese Medicine.
Author information
Authors and Affiliations
Contributions
Zhao HM and Liu DY conceived and designed the manuscript. Kang ZP, Zhong YB, and Liu DY wrote the manuscript. Kang ZP, Zhong YB, Huang JQ, and Wu TT collected and analyzed the references. Zhao HM, Liu DY, Zhong YB, and Huang JQ checked, proofread, and polished the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The author declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, Z., Zhonga, Y., Wu, T. et al. Ginsenoside from ginseng: a promising treatment for inflammatory bowel disease. Pharmacol. Rep 73, 700–711 (2021). https://doi.org/10.1007/s43440-020-00213-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00213-z