Abstract
Inflammatory bowel disease (IBD) is a collection of inflammatory conditions of colon and small intestine which affect millions of individuals worldwide and the prevalence amount is on the rise. The organ failure as well as loss of tissue function is because of the inflammatory reaction which is the major contributor of tissue healing leading to lifelong debilitation. To stop the tough consequences of inflammation every patient pursues alternative therapy to relieve symptoms. Green tea polyphenols (GTPs) play significant roles in down regulating signaling pathways because GTPs exert effective antioxidant properties and regulate Toll-like receptor 4 (TLR4) expression via certain receptor, inhibited endotoxin-mediated tumor necrosis factor alpha (TNF-α) production by blocking transcription nuclear factor-kappa B (NF-kB) activation and upstream of mediated I kappa B kinase complex pathway activities, as well as intrusion with the flow of cytokines and synthesis of cyclooxygenase-2 (COX-2). This article highlights the green approach regarding the defensive effects of GTP review-related studies concerning the contrary effects and the key therapeutic targets application of GTPs in biomedical field to treat inflammatory bowel disease (IBD) and its complications.
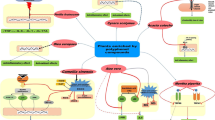
Graphical abstract

.



Similar content being viewed by others
Abbreviations
- GTPs:
-
Green tea polyphenols
- TLR4:
-
Toll-like receptor 4
- COX-2:
-
Cyclooxygenase-2
- IBD:
-
Inflammatory bowel disease
- CED:
-
Celiac disease
- CAM:
-
Complementary and alternative medicine
- UC:
-
Ulcerative colitis
- CD:
-
Crohn’s disease
- TNF-α:
-
Tumor necrosis factor alpha
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- IKK:
-
I kappa B kinase complex
- NF-kB:
-
Nuclear factor-kappa B
- NPCs:
-
Neural progenitor cells
- EGCG (−):
-
Epigallocatechin gallate
- EGC (−):
-
Epigallocatechin
- ECG (−):
-
Epicatechin gallate
- EC (−):
-
Epicatechin
- LPL:
-
Lamina propria lymphocytes
- APAP:
-
Acetaminophen [N-acetyl-p-aminophenol]
- C. diff:
-
Clostridium difficile
- Nrf2:
-
Nuclear factor erythroid 2-related factor
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthases
- iNOS:
-
Inducible nitric oxide synthase
- cNOS:
-
Catalytic nitric oxide synthase
- MMP-9:
-
Matrix metalloproteinase-9
References
Anker SD, von Haehling S (2004) Inflammatory mediators in chronic heart failure: an overview. Heart 90(4):464–470
Avdagic N, Zaciragic A, Babic N, Hukic M, Seremet M, Lepara O, Nakac IE (2013) Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn J Basic Med Sci 13(1):5–9
Bandyopadhyay D, Chatterjee TK, Dasgupta A, Lourduraja J, Dastidar SG (2005) In vitro and in vivo antimicrobial action of tea: the commonest beverage of Asia. Biol Pharm Bull 28(11):2125–2127
Banji D, Banji OJ, Abagoni S, Hayat MS, Kambam VL (2011) Amelioration of behavioral aberrations and oxidative markers by green tea extract in valproate induced autism in animals. Brain Res 1410:141–151
Beers MH, Porter RS, Jones TV, Kaplan JL, Berkwits M (2006) The Merck manual. Merck & Co, Whitehouse Station
Bitzer ZT, Elias RJ, Vijay-KM Lambert JD (2016) (□)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol Nutr Food Res 60:2267–2274
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2(10):907–916
Bruneton J (2001) “Pharmacognosie. Phytochimie. Plantes Me´dicinales”. Paris: Technique documentation. droit prospectif de la Recherche Juridique 31(6):337–360
Cano P, Massot C, Rodriguez L, Castell M (2014) Flavonoids affect host-microbiota crosstalk through TLR modulation. Antioxid 3(4):649–670
Cho JH, Brant SR (2011) Recent insights into the genetics of inflammatory bowel disease. Gastroenterol 140(6):1704–1712
Chung MY, Mah E, Masterjohn C, Noh SK, Park YK, Clark RM et al (2015) Green tea lowers hepatic Cox-2 and prostaglandin E2 in rats with dietary fat-induced nonalcoholic steatohepatitis. J Med Food 18(6):648–655
Conney AH, Zhou S, Lee MJ, Xie JG, Yang CS, Lou YR et al (2007) Stimulatory effect of oral administration of tea, coffee or caffeine on UVB-induced apoptosis in the epidermis of SKH-1 mice. Toxicol Appl Pharmacol 224:209–213
Cross RK, Wilson KT (2003) Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis 9:179–189
Dhillon SS, Mastropaolo LA, Murchie R, Griffiths C, Thoni C, Elkadri A et al (2014) Higher activity of the inducible nitric oxide synthase contributes to very early onset inflammatory bowel disease. Clin Transl Gastroenterol 5(5):e46
Dobutovic B, Smiljanic K, Soskic S, Hans DD, Isenovic ER (2011) Nitric oxide and its role in cardiovascular disease. Open Nitric oxide J 3(1):65–71
Doering J, Begue B, Lentze MJ, Rieux LF, Goulet O, Schmitz J et al (2004) Induction of T lymphocyte apoptosis by sulphasalazine in patients with Crohn’s disease. Gut 53:1632–1638
Evans MO, Starley B, Galagan JC, Yabes JM, Evans S, Salama JJ (2016) Tea and recurrent clostridium difficile infection. Gastroenterol Res Pract 3:451–468
Fan FY, Sang LX, Jiang M (2017) Catechins and their therapeutic benefits to inflammatory bowel disease. Mol 22(3):484
Frobes A, Escher J, Hebuterne X, Klek S, Krznaric Z, Schneider S et al (2016) ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clinic Nutr 36(2017):321–347
Frolkis A, Dieleman LA, Barkema HW, Remo P, Ghosh S, Fedorak RN et al (2013) Environment and the inflammatory bowel diseases. Can J Gastroenterol 27(3):18–24
Gawronska B, Karna JM, Kralisz M, Kemona H (2017) Markers of inflammation and influence of nitric oxide on platelet activation in the course of ulcerative colitis. Oncotarget 8(40):68108–68114
Gracia RLA, Ruigomez A, Panes J (2006) Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterol 130(6):1588–1594
Gradel KO, Nielsen HL, Schonheyder HC, Tove E, Brian K, Nielsen H et al (2009) Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterol 137(2):495–501
Granja A, Frias I, Neves AR, Pinheiro M (2017) Reis S (2017) Therapeutic potential of epigallocatechin gallate nano delivery systems. Biomed Res Int 4:1–15
Gregersen R, Lambertsen K, Finsen B (2000) Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 20(1):53–65
Guandalini S (2014) Are probiotics or prebiotics useful in pediatric irritable bowel syndrome or inflammatory bowel disease? Front Med 1(1):23
Hirsch N, Konstantinov A, Anavi S, Aronis A, Hagay Z, Madar Z et al (2016) Prolonged feeding with green tea polyphenols exacerbates cholesterol-induced fatty liver disease in mice. Mol Nutr Food Res 60:2542–2553
Hsu SW, Chang TC, Wu YK, Lin KT, Shi LS, Lee SY (2017) Rhodiola crenulata extract counteracts the effect of hypobaric hypoxia in rat heart via redirection of the nitric oxide and arginase1 pathway. BMC Complement Altern Med 17(1):29
Ide K, Yamada H, Takuma N, Kawasaki Y, Harada S, Nakase J et al (2016) Effects of green tea consumption on cognitive dysfunction in an elderly population: a randomized placebo-controlled study. Nutr J 15(1):49
Janssens PLRH, Hursel R, Plantenga MSW (2016) Nutraceuticals for body-weight management: the role of green tea catechins. Physiol Behav 162:83–87
Jostins L, Ripke S, Weersma RK, Duerr RH, McgGovern DP, Hui KY et al (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491(7422):119–124
Ju J, Lu G, Lambert JD, Yang CS (2007) Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol 17(5):395–402
Kleinman RE, Baldassano RN, Caplan A, Griffith AM, Heyman MB, Lake AM et al (2004) Nutrition support for pediatric patients with inflammatory bowel disease: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 39(1):15–27
Kostic AD, Xavier RJ, Gevers D (2014) The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterol 146(6):1489–1499
Kubes P, Reinhardt PH, Payne D, Woodman RC (1995) Excess nitric oxide does not cause cellular, vascular, or mucosal dysfunction in the cat small intestine. Am J Physiol 269(G):34–41
Larussa T, Imeneo M, Luzza F (2017) Potential role of nutraceutical compounds in inflammatory bowel disease. World J Gastroenterol 23(14):2483–2492
Lin YS, Tsai YJ, Tsay JS, Lin JK (2003) Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem 51(7):1864–1873
Little CH, Combet E, Mcmillan DC, Horgan PG, Roxburgh CSD (2017) The role of dietary polyphenol in the moderation of the inflammatory responses in early stage colorectal cancer. Crit Rev Food Sci Nutr 57(11):2310–2320
Liu L, Wu X, Zhang B, Yang W, Li D, Dong Y et al (2017) Protective effects of tea polyphenols on exhaustive exercise-induced fatigue, inflammation and tissue damage. Food Nutr Res 61(1):1333390
Ljung T, Lundberg S, Varsanyi M, Johansson C, Schmidt PT, Herulf M et al (2006) Rectal nitric oxide as biomarker in the treatment of inflammatory bowel disease: responders versus non responders. World J Gastroenterol 12(21):3386–3392
Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS (2006) Inhibition of adenoma progression to adenocarcinoma in a 4-methylnitrosamino-1-3-pyridyl-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res 66:11494–11501
Lyons CL, Kennedy EB, Roche HM (2016) Metabolic inflammation—differential modulation by dietary constituents. Nutrients 8(5):247
Mazzon E, Muia C, Paola RD, Genvese T, Sarro AD, Suzuki H et al (2005) Green tea polyphenol extract attenuates colon injury induced by experimental colitis. Free Rad Res 39(9):1017–1025
Mbuthia KS, Mreji PO, Ngure RM, Stomeo F, Kyallo M, Muoki C (2017) Tea (Camellia sinensis) infusions ameliorate cancer in 4TI metastatic breast cancer model. BMC Complement Altern Med 17(1):202
McKay DL, Blumberg JB (2002) The role of tea in human health: an update. J Am Coll Nutr 21:1–13
Michielan A, Inca RD (2015) Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm 5:1–10
Mizoguchi A (2012) Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci 105:263–320
Murakami T, Oshato K (2003) Dietary green tea intake preserves and improves arterial compliance and endothelial function. J Am Coll of Cardiol 41(6):271–274
Naghma K, Mukhtar H (2007) Tea polyphenols for health promotion. Life Sci 81(7):519–533
Naseem KM (2005) The role of nitric oxide in cardiovascular disease. Mol Aspects Med 26(1–2):33–65
Odenwald MA, Turner JR (2013) Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 11(9):1075–1083
Orner GA, Dashwood WM, Blum CA, Diaz GD, Li Q, Al-Fageeh M et al (2002) Response of Apc(min) and A33 (delta N beta-cat) mutant mice to treatment with tea, sulindac, and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Mutat Res 506–507:121–127
Oz HS, Chen TS (2008) Green-tea polyphenols down regulate cyclooxygenase and Bcl-2 activity in acetaminophen-induced hepatotoxicity. Dig Dis Sci 53(11):2980–2988
Oz HS, Ebersole JL (2008) Application of pro-drugs to inflammatory diseases of the gut. Mol 13:452–474
Oz HS, Ebersole J (2010) Green tea polyphenols mediate apoptosis in Intestinal Epithelial Cells. J. Cancer Ther 1:105–113
Oz HS, McClain CJ, Nagasawa HT, deVilliers WJ, Chen TS (2004) Diverse antioxidant protect against acetaminophen hepatotoxicity. J Mol Toxicol 18(6):361–368
Oz HS, Chen TS, deVilliers WJ (2005) Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem 16(5):297–304
Oz HS, Chen TS, de Villiers WJ (2013) Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front Immunol 4(5):132
Pascual V, Pascual Dieli CR, Lopez-PN Bodas A, Medrano LM et al (2014) Inflammatory bowel disease and celiac disease: overlaps and differences. World J Gastroenterol 20(17):4846–4856
Peters U, Poole C, Arab L (2001) Does tea affect cardiovascular disease? A meta-analysis. Am J Epidemiol 154(6):495–503
Porath D, Riegger D, Drewe J, Schwager J (2005) Epigallocatechin-3-gallate impairs chemokine production in human colon epithelial cell lines. J Pharmacol Exp Ther 315(3):1172–1180
Predonzani A, Calì B, Agnellini AH, Molon B (2015) Spotlights on immunological effects of reactive nitrogen species: when inflammation says nitric oxide. World J Exp Med 5(2):64
Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK (1995) Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut 36(5):718–723
Rachmilewitz D, Eliakim R, Ackerman Z, Karmeli F (1998) Direct determination of colonic nitric oxide level–a sensitive marker of disease activity in ulcerative colitis. Am J Gastroenterol 93(3):409–412
Raederstorff DG, Schlachter MF, Elste V, Weber P (2003) Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nut Biochem 14(6):326–332
Rafa H, Saoula H, Belkhelfa M, Medjeber O, Soufli I, Toumi R, de Launoit Y, Morales O, Nakmouche M, Delhem N, Touil BC (2013) IL-23/IL-17A axis correlates with the nitric oxide pathway in inflammatory bowel disease: immuno-modulatory effect of retinoic acid. J Interferon Cytokine Res 33(7):355–368
Renaud J, Nabavi SF, Daglia M, Nabavi SM, Martinoli MG (2015) Epigallocatechin-3-Gallate, a promising molecule for Parkinson’s disease? Rejuvenation Res 18(3):257–269
Schwartz M, Deczkowska A (2016) Neurological disease as a failure of brain-immune crosstalk: the multiple faces of neuroinflammation. Trends Immunol 37(10):668–679
Shaparoodi H, Hashemi M, Sharif ZN, Moezi L, Janahmadi Z, Dehpour AR (2016) The possible role of nitric oxide and oxidative stress in the enhanced apoptosis of cardiac cells in cirrhotic rats. Acta Med Iran 55(1):29–34
Shimizu A, Deguch AK, Joe JF, Mckoy H, Moriwaki IB, Weinstein IB (2005) EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J Exp Ther Oncol 5(1):69–78
Shimizu MO, Shirakai Y, Sakai H, Adachi A, Hata K, Hirose Y et al (2008) The EGCG administration caused a significant decrease in the serum levels of triglyceride mice (-)-epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila) 1(4):298–304
Shirakami Y, Shimizu M, Tsurumi H, Hara Y, Tanaka T, Moriwaki H (2008) EGCG and polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol Med Rep 1(3):355–361
Silagy CA, McNeil JJ, Donnan Tonkin AM, Worsam K (1993) Adverse effects of low-dose aspirin in a healthy elderly population. Clin Pharmacol Ther 54(1):84–89
Sosnowska B, Penson P, Banach M (2017) The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc Diagn Ther 7(1):S21
Soufli I, Rafa H, Boukoffa CT (2016) Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther 7(3):353–360
Steinmann J, Buer J, Pietschmann T, Steinmann E (2013) Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol 168(5):1059–1073
Stephani BCM, Harrison SL, Keage HAD, Babateen A, Robinson L, Siervo M (2017) Cardiovascular disease, the nitric oxide pathway and risk of cognitive impairment and dementia. Curr Cardiol Rep 19(9):87
Strober W, Fuss IJ, Blumberg RS (2002) The immunology of mucosal models of inflammation. Annu Rev Immunol 20(1):495–549
Szypuła J, Iwulski P, Kędziora J (2009) Blood platelets the hope for the future. Pol Merk Lek 26(156):587–590
Ting A, Chow Y, Tan W (2013) Microbial and heavy metal contamination in commonly consumed traditional Chinese herbal medicines. J Tradit Chin Med 33:119–124
Tsutsui M, Shimokawa H, Otsuji Y, Yanagihara N (2010) Pathophysiological relevance of NO signaling in the cardiovascular system: novel insight from mice lacking all NO synthases. Pharmacol Ther 128(3):499–508
Uceyler N, Schafers M, Sommer C (2009) Mode of action of cytokines on nociceptive neurons. Exp Brain Res 196(1):67–78
Varilek GW, Yang F, deVillers WJ, Zhong J, Oz HS, Westberry KF et al (2001) Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J Nutr 131(7):2034–2039
Verhoef MJ, Sutherland LR (1990) Outpatient healthcare utilization of patients with inflammatory bowel disease. Dig Dis Sci 35(10):1276–1280
Vuong QV, Golding JB, Nguyen M, Roach PD (2010) Extraction and isolation of catechins from tea. J Sep Sci 33(21):3415–3428
Webber C, Andy C, Darren T, Christian S, Rupert L (2017) Medication adherence in inflammatory bowel disease. Intest Res 15(4):434–445
Wędrychowicz A, Zajac A, Omasik P (2016) Advances in nutritional therapy in inflammatory bowel diseases: review. World J Gastroenterol 22(3):1045–1066
Weisburger JH (1999) Tea and health: the underlying mechanisms. Exp Biol Med 220(4):271–275
Wu P, Jia F, Zhang B, Zhang P (2017) Risk of cardiovascular disease in inflammatory bowel disease (review). Exp Therap Med 13:395–400
Yamamoto T, Juneja LR, Chu DC, Kim M (1997) Chemistry and applications of green tea. CRC Press LLC, USA
Yang CS, Wang H (2011) Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res 55(6):819–831
Yang F, Oz HS, Barve S, de Villers WJ, McClain CJ, Varilek GW (2001) The green tea polyphenols (_)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol 60(3):528–533
Yang Y, Qin YJ, Chan KP, Chu KO, Chu WK, Kin TN et al (2016) Green tea catechins are potent anti-oxidants that ameliorate sodium iodate-induced retinal degeneration in rats. Sci. Rep 6:29546
Yoon E, Babar A, Choudhary M, Kutner Pyrsopoulos (2016) Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol 4(2):131–142
Zhang JC, Xu H, Yuan Y, Chen YJ, Lin Y, Yuan SY (2017) Delayed treatment with green tea polyphenol EGCG promotes neurogenesis after ischemic stroke in adult mice. Mol Neurobiol 54(5):3652–3664
Acknowledgements
The work was jointly supported by the National Natural Science Foundation of China (No. 31472250), Postdoctoral Science Fund of Anhui Province (2016B117) and the Project of Modern Agricultural Industry and Technology System of Anhui Province (2016–2020). We wish to thank anonymous reviewers for their kind advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Rights and permissions
About this article
Cite this article
Rahman, S.U., Li, Y., Huang, Y. et al. Treatment of inflammatory bowel disease via green tea polyphenols: possible application and protective approaches. Inflammopharmacol 26, 319–330 (2018). https://doi.org/10.1007/s10787-018-0462-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0462-4