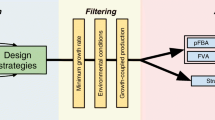
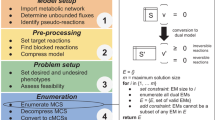
Abstract
Genome-scale metabolic models (GEMs) have been widely used to design cell factories in silico. However, initial flux balance analysis only considers stoichiometry and reaction direction constraints, so it cannot accurately describe the distribution of metabolic flux under the control of various regulatory mechanisms. In the recent years, by introducing enzymology, thermodynamics, and other multiomics-based constraints into GEMs, the metabolic state of cells under different conditions was more accurately simulated and a series of algorithms have been presented for microbial phenotypic analysis. Herein, the development of multiconstrained GEMs was reviewed by taking the constraints of enzyme kinetics, thermodynamics, and transcriptional regulatory mechanisms as examples. This review focused on introducing and summarizing GEMs application tools and cases in cell factory design. The challenges and prospects of GEMs development were also discussed.




Similar content being viewed by others
References
Zeng AP. New bioproduction systems for chemicals and fuels: needs and new development. Biotechnol Adv. 2019;37:508–18. https://doi.org/10.1016/j.biotechadv.2019.01.003.
Lee SY, Lee DY, Kim TY. Systems biotechnology for strain improvement. Trends Biotechnol. 2005;23:349–58. https://doi.org/10.1016/j.tibtech.2005.05.003.
Joyce AR, Palsson BO. The model organism as a system: integrating “omics” data sets. Nat Rev Mol Cell Biol. 2006;7:198–210. https://doi.org/10.1038/nrm1857.
Park JM, Kim TY, Lee SY. Constraints-based genome-scale metabolic simulation for systems metabolic engineering. Biotechnol Adv. 2009;27:979–88. https://doi.org/10.1016/j.biotechadv.2009.05.019.
Price ND, Reed JL, Palsson BO. Genome-scale models of microbial cells: evaluating the consequences of constraints. Nat Rev Microbiol. 2004;2:886–97. https://doi.org/10.1038/nrmicro1023.
Liu J, Qi H, Wang C, Wen J. Model-driven intracellular redox status modulation for increasing isobutanol production in Escherichia coli. Biotechnol Biofuels. 2015;8:108. https://doi.org/10.1186/s13068-015-0291-2.
Mao Z, Zhao X, Yang X, Zhang P, Du J, Yuan Q, Ma H. ECMpy, a simplified workflow for constructing enzymatic constrained metabolic network model. Biomolecules. 2022. https://doi.org/10.3390/biom12010065.
Ding S, Tian Y, Cai P, Zhang D, Cheng X, Sun D, Yuan L, Chen J, Tu W, Wei DQ, Hu QN. novoPathFinder: a webserver of designing novel-pathway with integrating GEM-model. Nucleic Acids Res. 2020;48:W477–87. https://doi.org/10.1093/nar/gkaa230.
Burgard AP, Pharkya P, Maranas CD. Optknock: a bilevel programming framework for identifying gene knockout strategies for microbial strain optimization. Biotechnol Bioeng. 2003;84:647–57. https://doi.org/10.1002/bit.10803.
Jiang S, Otero-Muras I, Banga JR, Wang Y, Kaiser M, Krasnogor N. OptDesign: identifying optimum design strategies in strain engineering for biochemical production. ACS Synth Biol. 2022;11:1531–41. https://doi.org/10.1021/acssynbio.1c00610.
Ye C, Luo Q, Guo L, Gao C, Xu N, Zhang L, Liu L, Chen X. Improving lysine production through construction of an Escherichia coli enzyme-constrained model. Biotechnol Bioeng. 2020;117:3533–44. https://doi.org/10.1002/bit.27485.
Feist AM, Herrgard MJ, Thiele I, Reed JL, Palsson BO. Reconstruction of biochemical networks in microorganisms. Nat Rev Microbiol. 2009;7:129–43. https://doi.org/10.1038/nrmicro1949.
Thiele I, Palsson BO. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat Protoc. 2010;5:93–121. https://doi.org/10.1038/nprot.2009.203.
Edwards JS, Palsson BO. Systems properties of the Haemophilus influenzae Rd metabolic genotype. J Biol Chem. 1999;274:17410–6. https://doi.org/10.1074/jbc.274.25.17410.
Feist AM, Palsson BO. The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nat Biotechnol. 2008;26:659–67. https://doi.org/10.1038/nbt1401.
Forster J, Famili I, Fu P, Palsson BO, Nielsen J. Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network. Genome Res. 2003;13:244–53. https://doi.org/10.1101/gr.234503.
Oh YK, Palsson BO, Park SM, Schilling CH, Mahadevan R. Genome-scale reconstruction of metabolic network in Bacillus subtilis based on high-throughput phenotyping and gene essentiality data. J Biol Chem. 2007;282:28791–9. https://doi.org/10.1074/jbc.M703759200.
King ZA, Lu J, Drager A, Miller P, Federowicz S, Lerman JA, Ebrahim A, Palsson BO, Lewis NE. BiGG Models: a platform for integrating, standardizing and sharing genome-scale models. Nucleic Acids Res. 2016;44:D515-522. https://doi.org/10.1093/nar/gkv1049.
Ye C, Xu N, Dong C, Ye Y, Zou X, Chen X, Guo F, Liu L. IMGMD: A platform for the integration and standardisation of In silico microbial genome-scale metabolic models. Sci Rep. 2017;7:727. https://doi.org/10.1038/s41598-017-00820-6.
Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107:1–8. https://doi.org/10.1016/j.ygeno.2015.11.003.
Ludwig C, Gillet L, Rosenberger G, Amon S, Collins BC, Aebersold R. Data-independent acquisition-based SWATH-MS for quantitative proteomics: a tutorial. Mol Syst Biol. 2018;14:8126. https://doi.org/10.15252/msb.20178126.
Nassar AF, Wu T, Nassar SF, Wisnewski AV. UPLC-MS for metabolomics: a giant step forward in support of pharmaceutical research. Drug Discov Today. 2017;22:463–70. https://doi.org/10.1016/j.drudis.2016.11.020.
Beale DJ, Pinu FR, Kouremenos KA, Poojary MM, Narayana VK, Boughton BA, Kanojia K, Dayalan S, Jones OAH, Dias DA. Review of recent developments in GC-MS approaches to metabolomics-based research. Metabolomics. 2018;14:152. https://doi.org/10.1007/s11306-018-1449-2.
Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457-462. https://doi.org/10.1093/nar/gkv1070.
Sayers EW, Beck J, Brister JR, Bolton EE, Canese K, Comeau DC, Funk K, Ketter A, Kim S, Kimchi A, Kitts PA, Kuznetsov A, Lathrop S, Lu Z, McGarvey K, Madden TL, Murphy TD, O’Leary N, Phan L, Schneider VA, Thibaud-Nissen F, Trawick BW, Pruitt KD, Ostell J. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2020;48:D9–16. https://doi.org/10.1093/nar/gkz899.
Ren Q, Chen K, Paulsen IT. TransportDB: a comprehensive database resource for cytoplasmic membrane transport systems and outer membrane channels. Nucleic Acids Res. 2007;35:D274-279. https://doi.org/10.1093/nar/gkl925.
DeJongh M, Formsma K, Boillot P, Gould J, Rycenga M, Best A. Toward the automated generation of genome-scale metabolic networks in the SEED. BMC Bioinf. 2007;8:139. https://doi.org/10.1186/1471-2105-8-139.
Bordbar A, Monk JM, King ZA, Palsson BO. Constraint-based models predict metabolic and associated cellular functions. Nat Rev Genet. 2014;15:107–20. https://doi.org/10.1038/nrg3643.
Heirendt L, Arreckx S, Pfau T, Mendoza SN, Richelle A, Heinken A, Haraldsdottir HS, Wachowiak J, Keating SM, Vlasov V, Magnusdottir S, Ng CY, Preciat G, Zagare A, Chan SHJ, Aurich MK, Clancy CM, Modamio J, Sauls JT, Noronha A, Bordbar A, Cousins B, El Assal DC, Valcarcel LV, Apaolaza I, Ghaderi S, Ahookhosh M, Ben Guebila M, Kostromins A, Sompairac N, Le HM, Ma D, Sun Y, Wang L, Yurkovich JT, Oliveira MAP, Vuong PT, El Assal LP, Kuperstein I, Zinovyev A, Hinton HS, Bryant WA, Aragon Artacho FJ, Planes FJ, Stalidzans E, Maass A, Vempala S, Hucka M, Saunders MA, Maranas CD, Lewis NE, Sauter T, Palsson BO, Thiele I, Fleming RMT. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat Protoc. 2019;14:639–702. https://doi.org/10.1038/s41596-018-0098-2.
Soh KC, Hatzimanikatis V. Constraining the flux space using thermodynamics and integration of metabolomics data. Methods Mol Biol. 2014;1191:49–63. https://doi.org/10.1007/978-1-4939-1170-7_3.
Lewis NE, Nagarajan H, Palsson BO. Constraining the metabolic genotype-phenotype relationship using a phylogeny of in silico methods. Nat Rev Microbiol. 2012;10:291–305. https://doi.org/10.1038/nrmicro2737.
Kummel A, Panke S, Heinemann M. Putative regulatory sites unraveled by network-embedded thermodynamic analysis of metabolome data. Mol Syst Biol. 2006;2006(2):0034. https://doi.org/10.1038/msb4100074.
Henry CS, Broadbelt LJ, Hatzimanikatis V. Thermodynamics-based metabolic flux analysis. Biophys J. 2007;92:1792–805. https://doi.org/10.1529/biophysj.106.093138.
Kiparissides A, Hatzimanikatis V. Thermodynamics-based metabolite sensitivity analysis in metabolic networks. Metab Eng. 2017;39:117–27. https://doi.org/10.1016/j.ymben.2016.11.006.
Soh KC, Hatzimanikatis V. Network thermodynamics in the post-genomic era. Curr Opin Microbiol. 2010;13:350–7. https://doi.org/10.1016/j.mib.2010.03.001.
Ataman M, Hatzimanikatis V. Heading in the right direction: thermodynamics-based network analysis and pathway engineering. Curr Opin Biotechnol. 2015;36:176–82. https://doi.org/10.1016/j.copbio.2015.08.021.
Salvy P, Fengos G, Ataman M, Pathier T, Soh KC, Hatzimanikatis V. pyTFA and matTFA: a Python package and a Matlab toolbox for thermodynamics-based flux analysis. Bioinformatics. 2019;35:167–9. https://doi.org/10.1093/bioinformatics/bty499.
Mavrovouniotis ML. Group contributions for estimating standard gibbs energies of formation of biochemical compounds in aqueous solution. Biotechnol Bioeng. 1990;36:1070–82. https://doi.org/10.1002/bit.260361013.
Noor E, Haraldsdottir HS, Milo R, Fleming RM. Consistent estimation of Gibbs energy using component contributions. PLoS Comput Biol. 2013;9: e1003098. https://doi.org/10.1371/journal.pcbi.1003098.
Rother K, Hoffmann S, Bulik S, Hoppe A, Gasteiger J, Holzhutter HG. IGERS: inferring Gibbs energy changes of biochemical reactions from reaction similarities. Biophys J. 2010;98:2478–86. https://doi.org/10.1016/j.bpj.2010.02.052.
Noor E, Bar-Even A, Flamholz A, Lubling Y, Davidi D, Milo R. An integrated open framework for thermodynamics of reactions that combines accuracy and coverage. Bioinformatics. 2012;28:2037–44. https://doi.org/10.1093/bioinformatics/bts317.
Jankowski MD, Henry CS, Broadbelt LJ, Hatzimanikatis V. Group contribution method for thermodynamic analysis of complex metabolic networks. Biophys J. 2008;95:1487–99. https://doi.org/10.1529/biophysj.107.124784.
Beg QK, Vazquez A, Ernst J, de Menezes MA, Bar-Joseph Z, Barabasi AL, Oltvai ZN. Intracellular crowding defines the mode and sequence of substrate uptake by Escherichia coli and constrains its metabolic activity. Proc Natl Acad Sci USA. 2007;104:12663–8. https://doi.org/10.1073/pnas.0609845104.
Adadi R, Volkmer B, Milo R, Heinemann M, Shlomi T. Prediction of microbial growth rate versus biomass yield by a metabolic network with kinetic parameters. PLoS Comput Biol. 2012;8:e1002575. https://doi.org/10.1371/journal.pcbi.1002575.
Sanchez BJ, Zhang C, Nilsson A, Lahtvee PJ, Kerkhoven EJ, Nielsen J. Improving the phenotype predictions of a yeast genome-scale metabolic model by incorporating enzymatic constraints. Mol Syst Biol. 2017;13:935. https://doi.org/10.15252/msb.20167411.
Chen Y, Sun Y, Liu Z, Dong F, Li Y, Wang Y. Genome-scale modeling for Bacillus coagulans to understand the metabolic characteristics. Biotechnol Bioeng. 2020;117:3545–58. https://doi.org/10.1002/bit.27488.
Zhou J, Zhuang Y, Xia J. Integration of enzyme constraints in a genome-scale metabolic model of Aspergillus niger improves phenotype predictions. Microb Cell Fact. 2021;20:125. https://doi.org/10.1186/s12934-021-01614-2.
Li G, Hu Y, Jan Z, Luo H, Wang H, Zelezniak A, Ji B, Nielsen J. Bayesian genome scale modelling identifies thermal determinants of yeast metabolism. Nat Commun. 2021;12:190. https://doi.org/10.1038/s41467-020-20338-2.
Wyrick JJ, Young RA. Deciphering gene expression regulatory networks. Curr Opin Genet Dev. 2002;12:130–6. https://doi.org/10.1016/s0959-437x(02)00277-0.
Herrgard MJ, Covert MW, Palsson BO. Reconstruction of microbial transcriptional regulatory networks. Curr Opin Biotechnol. 2004;15:70–7. https://doi.org/10.1016/j.copbio.2003.11.002.
Cruz F, Faria JP, Rocha M, Rocha I, Dias O. A review of methods for the reconstruction and analysis of integrated genome-scale models of metabolism and regulation. Biochem Soc Trans. 2020;48:1889–903. https://doi.org/10.1042/BST20190840.
Covert MW, Knight EM, Reed JL, Herrgard MJ, Palsson BO. Integrating high-throughput and computational data elucidates bacterial networks. Nature. 2004;429:92–6. https://doi.org/10.1038/nature02456.
Shlomi T, Eisenberg Y, Sharan R, Ruppin E. A genome-scale computational study of the interplay between transcriptional regulation and metabolism. Mol Syst Biol. 2007;3:101. https://doi.org/10.1038/msb4100141.
Motamedian E, Mohammadi M, Shojaosadati SA, Heydari M. TRFBA: an algorithm to integrate genome-scale metabolic and transcriptional regulatory networks with incorporation of expression data. Bioinformatics. 2017;33:1057–63. https://doi.org/10.1093/bioinformatics/btw772.
Wang Z, Danziger SA, Heavner BD, Ma S, Smith JJ, Li S, Herricks T, Simeonidis E, Baliga NS, Aitchison JD, Price ND. Combining inferred regulatory and reconstructed metabolic networks enhances phenotype prediction in yeast. PLoS Comput Biol. 2017;13:e1005489. https://doi.org/10.1371/journal.pcbi.1005489.
Chandrasekaran S, Price ND. Probabilistic integrative modeling of genome-scale metabolic and regulatory networks in Escherichia coli and Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2010;107:17845–50. https://doi.org/10.1073/pnas.1005139107.
Banos DT, Trebulle P, Elati M. Integrating transcriptional activity in genome-scale models of metabolism. BMC Syst Biol. 2017;11:134. https://doi.org/10.1186/s12918-017-0507-0.
Jensen PA, Lutz KA, Papin JA. TIGER: Toolbox for integrating genome-scale metabolic models, expression data, and transcriptional regulatory networks. BMC Syst Biol. 2011;5:147. https://doi.org/10.1186/1752-0509-5-147.
Marmiesse L, Peyraud R, Cottret L. FlexFlux: combining metabolic flux and regulatory network analyses. BMC Syst Biol. 2015;9:93. https://doi.org/10.1186/s12918-015-0238-z.
Jensen PA, Papin JA. Functional integration of a metabolic network model and expression data without arbitrary thresholding. Bioinformatics. 2011;27:541–7. https://doi.org/10.1093/bioinformatics/btq702.
Colijn C, Brandes A, Zucker J, Lun DS, Weiner B, Farhat MR, Cheng TY, Moody DB, Murray M, Galagan JE. Interpreting expression data with metabolic flux models: predicting Mycobacterium tuberculosis mycolic acid production. PLoS Comput Biol. 2009;5:e1000489. https://doi.org/10.1371/journal.pcbi.1000489.
Yang X, Mao Z, Zhao X, Wang R, Zhang P, Cai J, Xue C, Ma H. Integrating thermodynamic and enzymatic constraints into genome-scale metabolic models. Metab Eng. 2021;67:133–44. https://doi.org/10.1016/j.ymben.2021.06.005.
O’Brien EJ, Lerman JA, Chang RL, Hyduke DR, Palsson BO. Genome-scale models of metabolism and gene expression extend and refine growth phenotype prediction. Mol Syst Biol. 2013;9:693. https://doi.org/10.1038/msb.2013.52.
Lerman JA, Hyduke DR, Latif H, Portnoy VA, Lewis NE, Orth JD, Schrimpe-Rutledge AC, Smith RD, Adkins JN, Zengler K, Palsson BO. In silico method for modelling metabolism and gene product expression at genome scale. Nat Commun. 2012;3:929. https://doi.org/10.1038/ncomms1928.
Lloyd CJ, Ebrahim A, Yang L, King ZA, Catoiu E, O’Brien EJ, Liu JK, Palsson BO. COBRAme: a computational framework for genome-scale models of metabolism and gene expression. PLoS Comput Biol. 2018;14:e1006302. https://doi.org/10.1371/journal.pcbi.1006302.
Salvy P, Hatzimanikatis V. The ETFL formulation allows multi-omics integration in thermodynamics-compliant metabolism and expression models. Nat Commun. 2020;11:30. https://doi.org/10.1038/s41467-019-13818-7.
Oftadeh O, Salvy P, Masid M, Curvat M, Miskovic L, Hatzimanikatis V. A genome-scale metabolic model of Saccharomyces cerevisiae that integrates expression constraints and reaction thermodynamics. Nat Commun. 2021;12:4790. https://doi.org/10.1038/s41467-021-25158-6.
Lin Z, Zhang Y, Yuan Q, Liu Q, Li Y, Wang Z, Ma H, Chen T, Zhao X. Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass. Microb Cell Fact. 2015;14:185. https://doi.org/10.1186/s12934-015-0369-3.
Ma Z, Ye C, Deng W, Xu M, Wang Q, Liu G, Wang F, Liu L, Xu Z, Shi G, Ding Z. Reconstruction and analysis of a genome-Scale metabolic model of Ganoderma lucidum for improved extracellular polysaccharide production. Front Microbiol. 2018;9:3076. https://doi.org/10.3389/fmicb.2018.03076.
Otero-Muras I, Carbonell P. Automated engineering of synthetic metabolic pathways for efficient biomanufacturing. Metab Eng. 2021;63:61–80. https://doi.org/10.1016/j.ymben.2020.11.012.
Hadadi N, Hafner J, Shajkofci A, Zisaki A, Hatzimanikatis V. ATLAS of Biochemistry: a repository of all possible biochemical reactions for synthetic biology and metabolic engineering studies. ACS Synth Biol. 2016;5:1155–66. https://doi.org/10.1021/acssynbio.6b00054.
Rodrigo G, Carrera J, Prather KJ, Jaramillo A. DESHARKY: automatic design of metabolic pathways for optimal cell growth. Bioinformatics. 2008;24:2554–6. https://doi.org/10.1093/bioinformatics/btn471.
Moriya Y, Shigemizu D, Hattori M, Tokimatsu T, Kotera M, Goto S, Kanehisa M. PathPred: an enzyme-catalyzed metabolic pathway prediction server. Nucleic Acids Res. 2010;38:W138-143. https://doi.org/10.1093/nar/gkq318.
Yim H, Haselbeck R, Niu W, Pujol-Baxley C, Burgard A, Boldt J, Khandurina J, Trawick JD, Osterhout RE, Stephen R, Estadilla J, Teisan S, Schreyer HB, Andrae S, Yang TH, Lee SY, Burk MJ, Van Dien S. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat Chem Biol. 2011;7:445–52. https://doi.org/10.1038/nchembio.580.
Campodonico MA, Andrews BA, Asenjo JA, Palsson BO, Feist AM. Generation of an atlas for commodity chemical production in Escherichia coli and a novel pathway prediction algorithm. GEM-Path Metab Eng. 2014;25:140–58. https://doi.org/10.1016/j.ymben.2014.07.009.
Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006;34:D354-357. https://doi.org/10.1093/nar/gkj102.
Curran KA, Alper HS. Expanding the chemical palate of cells by combining systems biology and metabolic engineering. Metab Eng. 2012;14:289–97. https://doi.org/10.1016/j.ymben.2012.04.006.
Mahadevan R, Schilling CH. The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab Eng. 2003;5:264–76. https://doi.org/10.1016/j.ymben.2003.09.002.
Lee KH, Park JH, Kim TY, Kim HU, Lee SY. Systems metabolic engineering of Escherichia coli for L-threonine production. Mol Syst Biol. 2007;3:149. https://doi.org/10.1038/msb4100196.
Segre D, Vitkup D, Church GM. Analysis of optimality in natural and perturbed metabolic networks. Proc Natl Acad Sci USA. 2002;99:15112–7. https://doi.org/10.1073/pnas.232349399.
Pharkya P, Burgard AP, Maranas CD. OptStrain: a computational framework for redesign of microbial production systems. Genome Res. 2004;14:2367–76. https://doi.org/10.1101/gr.2872004.
Patil KR, Rocha I, Forster J, Nielsen J. Evolutionary programming as a platform for in silico metabolic engineering. BMC Bioinf. 2005;6:308. https://doi.org/10.1186/1471-2105-6-308.
Kim J, Reed JL. OptORF: optimal metabolic and regulatory perturbations for metabolic engineering of microbial strains. BMC Syst Biol. 2010;4:53. https://doi.org/10.1186/1752-0509-4-53.
Lun DS, Rockwell G, Guido NJ, Baym M, Kelner JA, Berger B, Galagan JE, Church GM. Large-scale identification of genetic design strategies using local search. Mol Syst Biol. 2009;5:296. https://doi.org/10.1038/msb.2009.57.
King ZA, Feist AM. Optimizing cofactor specificity of oxidoreductase enzymes for the generation of microbial production strains—OptSwap. Ind Biotechnol. 2013;9:236–46. https://doi.org/10.1089/ind.2013.0005.
Kim K, Choe D, Song Y, Kang M, Lee SG, Lee DH, Cho BK. Engineering Bacteroides thetaiotaomicron to produce non-native butyrate based on a genome-scale metabolic model-guided design. Metab Eng. 2021;68:174–86. https://doi.org/10.1016/j.ymben.2021.10.005.
Massaiu I, Pasotti L, Sonnenschein N, Rama E, Cavaletti M, Magni P, Calvio C, Herrgard MJ. Integration of enzymatic data in Bacillus subtilis genome-scale metabolic model improves phenotype predictions and enables in silico design of poly-gamma-glutamic acid production strains. Microb Cell Fact. 2019;18:3. https://doi.org/10.1186/s12934-018-1052-2.
Fontana J, Dong C, Ham JY, Zalatan JG, Carothers JM. Regulated expression of sgRNAs tunes CRISPRi in E. coli. Biotechnol J. 2018;13:e1800069. https://doi.org/10.1002/biot.201800069.
Patra P, Das M, Kundu P, Ghosh A. Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts. Biotechnol Adv. 2021;47:107695. https://doi.org/10.1016/j.biotechadv.2021.107695.
Ranganathan S, Suthers PF, Maranas CD. OptForce: an optimization procedure for identifying all genetic manipulations leading to targeted overproductions. PLoS Comput Biol. 2010;6:e1000744. https://doi.org/10.1371/journal.pcbi.1000744.
Chowdhury A, Zomorrodi AR, Maranas CD. k-OptForce: integrating kinetics with flux balance analysis for strain design. PLoS Comput Biol. 2014;10:e1003487. https://doi.org/10.1371/journal.pcbi.1003487.
Cheng F, Yu H, Stephanopoulos G. Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid. Metab Eng. 2019;55:276–89. https://doi.org/10.1016/j.ymben.2019.07.003.
Ranganathan S, Tee TW, Chowdhury A, Zomorrodi AR, Yoon JM, Fu Y, Shanks JV, Maranas CD. An integrated computational and experimental study for overproducing fatty acids in Escherichia coli. Metab Eng. 2012;14:687–704. https://doi.org/10.1016/j.ymben.2012.08.008.
Xu P, Ranganathan S, Fowler ZL, Maranas CD, Koffas MA. Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab Eng. 2011;13:578–87. https://doi.org/10.1016/j.ymben.2011.06.008.
Shen F, Sun R, Yao J, Li J, Liu Q, Price ND, Liu C, Wang Z. OptRAM: In-silico strain design via integrative regulatory-metabolic network modeling. PLoS Comput Biol. 2019;15:e1006835. https://doi.org/10.1371/journal.pcbi.1006835.
Han T, Kim GB, Lee SY. Glutaric acid production by systems metabolic engineering of an l-lysine-overproducing Corynebacterium glutamicum. Proc Natl Acad Sci USA. 2020;117:30328–34. https://doi.org/10.1073/pnas.2017483117.
Park JM, Park HM, Kim WJ, Kim HU, Kim TY, Lee SY. Flux variability scanning based on enforced objective flux for identifying gene amplification targets. BMC Syst Biol. 2012;6:106. https://doi.org/10.1186/1752-0509-6-106.
Hari A, Lobo D. Fluxer: a web application to compute, analyze and visualize genome-scale metabolic flux networks. Nucleic Acids Res. 2020;48:W427–35. https://doi.org/10.1093/nar/gkaa409.
Millard P, Enjalbert B, Uttenweiler-Joseph S, Portais JC, Letisse F. Control and regulation of acetate overflow in Escherichia coli. Elife. 2021. https://doi.org/10.7554/eLife.63661.
Monk JM, Lloyd CJ, Brunk E, Mih N, Sastry A, King Z, Takeuchi R, Nomura W, Zhang Z, Mori H, Feist AM, Palsson BO. iML1515, a knowledgebase that computes Escherichia coli traits. Nat Biotechnol. 2017;35:904–8. https://doi.org/10.1038/nbt.3956.
Fan S, Zhang Z, Zou W, Huang Z, Liu J, Liu L. Development of a minimal chemically defined medium for Ketogulonicigenium vulgare WSH001 based on its genome-scale metabolic model. J Biotechnol. 2014;169:15–22. https://doi.org/10.1016/j.jbiotec.2013.10.027.
Andreozzi S, Chakrabarti A, Soh KC, Burgard A, Yang TH, Van Dien S, Miskovic L, Hatzimanikatis V. Identification of metabolic engineering targets for the enhancement of 1,4-butanediol production in recombinant E. coli using large-scale kinetic models. Metab Eng. 2016;35:148–59. https://doi.org/10.1016/j.ymben.2016.01.009.
Dahal S, Zhao J, Yang L. Genome-scale modeling of metabolism and macromolecular expression and their applications. Biotechnol Bioprocess Eng. 2021;25:931–43. https://doi.org/10.1007/s12257-020-0061-2.
Chen K, Gao Y, Mih N, O’Brien EJ, Yang L, Palsson BO. Thermosensitivity of growth is determined by chaperone-mediated proteome reallocation. Proc Natl Acad Sci USA. 2017;114:11548–53. https://doi.org/10.1073/pnas.1705524114.
Yang L, Mih N, Anand A, Park JH, Tan J, Yurkovich JT, Monk JM, Lloyd CJ, Sandberg TE, Seo SW, Kim D, Sastry AV, Phaneuf P, Gao Y, Broddrick JT, Chen K, Heckmann D, Szubin R, Hefner Y, Feist AM, Palsson BO. Cellular responses to reactive oxygen species are predicted from molecular mechanisms. Proc Natl Acad Sci USA. 2019;116:14368–73. https://doi.org/10.1073/pnas.1905039116.
Du B, Yang L, Lloyd CJ, Fang X, Palsson BO. Genome-scale model of metabolism and gene expression provides a multi-scale description of acid stress responses in Escherichia coli. PLoS Comput Biol. 2019;15:e1007525. https://doi.org/10.1371/journal.pcbi.1007525.
Ye C, Xu N, Gao C, Liu G, Xu J, Zhang W, Chen X, Nielsen J, Liu L. Comprehensive understanding of Saccharomyces cerevisiae phenotypes with whole-cell model WM_S288C. Biotechnol Bioeng. 2020;117:1562–74. https://doi.org/10.1002/bit.27298.
Lu H, Kerkhoven EJ, Nielsen J. Multiscale models quantifying yeast physiology: towards a whole-cell model. Trends Biotechnol. 2022;40:291–305. https://doi.org/10.1016/j.tibtech.2021.06.010.
Kim M, Rai N, Zorraquino V, Tagkopoulos I. Multi-omics integration accurately predicts cellular state in unexplored conditions for Escherichia coli. Nat Commun. 2016;7:13090. https://doi.org/10.1038/ncomms13090.
Sahu A, Blatke MA, Szymanski JJ, Topfer N. Advances in flux balance analysis by integrating machine learning and mechanism-based models. Comput Struct Biotechnol J. 2021;19:4626–40. https://doi.org/10.1016/j.csbj.2021.08.004.
Moreno-Paz S, Schmitz J, Martinsos Santos VAP, Suarez-Diez M. Enzyme-constrained models predict the dynamics of Saccharomyces cerevisiae growth in continuous, batch and fed-batch bioreactors. Microb Biotechnol. 2022;15:1434–45. https://doi.org/10.1111/1751-7915.13995.
Campodonico MA, Sukumara S, Feist AM, Herrgard MJ. Computational methods to assess the production potential of bio-based chemicals. Methods Mol Biol. 2018;1671:97–116. https://doi.org/10.1007/978-1-4939-7295-1_7.
Gu D, Zhang C, Zhou S, Wei L, Hua Q. IdealKnock: a framework for efficiently identifying knockout strategies leading to targeted overproduction. Comput Biol Chem. 2016;61:229–37. https://doi.org/10.1016/j.compbiolchem.2016.02.014.
Xu Z, Zheng P, Sun J, Ma Y. ReacKnock: identifying reaction deletion strategies for microbial strain optimization based on genome-scale metabolic network. PLoS ONE. 2013;8:e72150. https://doi.org/10.1371/journal.pone.0072150.
Tepper N, Shlomi T. Predicting metabolic engineering knockout strategies for chemical production: accounting for competing pathways. Bioinformatics. 2010;26:536–43. https://doi.org/10.1093/bioinformatics/btp704.
Jensen K, Broeken V, Hansen ASL, Sonnenschein N, Herrgard MJ. OptCouple: Joint simulation of gene knockouts, insertions and medium modifications for prediction of growth-coupled strain designs. Metab Eng Commun. 2019;8: e00087. https://doi.org/10.1016/j.mec.2019.e00087.
Kim J, Reed JL, Maravelias CT. Large-scale bi-level strain design approaches and mixed-integer programming solution techniques. PLoS ONE. 2011;6: e24162. https://doi.org/10.1371/journal.pone.0024162.
Hatzimanikatis V, Li C, Ionita JA, Henry CS, Jankowski MD, Broadbelt LJ. Exploring the diversity of complex metabolic networks. Bioinformatics. 2005;21:1603–9. https://doi.org/10.1093/bioinformatics/bti213.
Carbonell P, Planson AG, Fichera D, Faulon JL. A retrosynthetic biology approach to metabolic pathway design for therapeutic production. BMC Syst Biol. 2011;5:122. https://doi.org/10.1186/1752-0509-5-122.
Choi HS, Lee SY, Kim TY, Woo HM. In silico identification of gene amplification targets for improvement of lycopene production. Appl Environ Microbiol. 2010;76:3097–105. https://doi.org/10.1128/AEM.00115-10.
Pharkya P, Maranas CD. An optimization framework for identifying reaction activation/inhibition or elimination candidates for overproduction in microbial systems. Metab Eng. 2006;8:1–13. https://doi.org/10.1016/j.ymben.2005.08.003.
Alper H, Jin YS, Moxley JF, Stephanopoulos G. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng. 2005;7:155–64. https://doi.org/10.1016/j.ymben.2004.12.003.
Fong SS, Burgard AP, Herring CD, Knight EM, Blattner FR, Maranas CD, Palsson BO. In silico design and adaptive evolution of Escherichia coli for production of lactic acid. Biotechnol Bioeng. 2005;91:643–8. https://doi.org/10.1002/bit.20542.
Moon SY, Hong SH, Kim TY, Lee SY. Metabolic engineering of Escherichia coli for the production of malic acid. Biochem Eng J. 2008;40:312–20. https://doi.org/10.1016/j.bej.2008.01.001.
Park JH, Lee KH, Kim TY, Lee SY. Metabolic engineering of Escherichia coli for the production of L-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci USA. 2007;104:7797–802. https://doi.org/10.1073/pnas.0702609104.
Boghigian BA, Armando J, Salas D, Pfeifer BA. Computational identification of gene over-expression targets for metabolic engineering of taxadiene production. Appl Microbiol Biotechnol. 2012;93:2063–73. https://doi.org/10.1007/s00253-011-3725-1.
Jung YK, Kim TY, Park SJ, Lee SY. Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol Bioeng. 2010;105:161–71. https://doi.org/10.1002/bit.22548.
Mienda BS, Shamsir MS, Illias RM. Model-guided metabolic gene knockout of gnd for enhanced succinate production in Escherichia coli from glucose and glycerol substrates. Comput Biol Chem. 2016;61:130–7. https://doi.org/10.1016/j.compbiolchem.2016.01.013.
Shah MV, Nazem-Bokaee H, Antoney J, Kang SW, Jackson CJ, Scott C. Improved production of the non-native cofactor F420 in Escherichia coli. Sci Rep. 2021;11:21774. https://doi.org/10.1038/s41598-021-01224-3.
Qu L, Xiu X, Sun G, Zhang C, Yang H, Liu Y, Li J, Du G, Lv X, Liu L. Engineered yeast for efficient de novo synthesis of 7-dehydrocholesterol. Biotechnol Bioeng. 2022;119:1278–89. https://doi.org/10.1002/bit.28055.
Becker J, Zelder O, Hafner S, Schroder H, Wittmann C. From zero to hero–design-based systems metabolic engineering of Corynebacterium glutamicum for l-lysine production. Metab Eng. 2011;13:159–68. https://doi.org/10.1016/j.ymben.2011.01.003.
Bro C, Regenberg B, Forster J, Nielsen J. In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production. Metab Eng. 2006;8:102–11. https://doi.org/10.1016/j.ymben.2005.09.007.
Asadollahi MA, Maury J, Patil KR, Schalk M, Clark A, Nielsen J. Enhancing sesquiterpene production in Saccharomyces cerevisiae through in silico driven metabolic engineering. Metab Eng. 2009;11:328–34. https://doi.org/10.1016/j.ymben.2009.07.001.
Brochado AR, Matos C, Moller BL, Hansen J, Mortensen UH, Patil KR. Improved vanillin production in baker’s yeast through in silico design. Microb Cell Fact. 2010;9:84. https://doi.org/10.1186/1475-2859-9-84.
Huang M, Zhao Y, Li R, Huang W, Chen X. Improvement of l-arginine production by in silico genome-scale metabolic network model guided genetic engineering. 3 Biotech. 2020;10:126. https://doi.org/10.1007/s13205-020-2114-9.
Purdy HM, Pfleger BF, Reed JL. Introduction of NADH-dependent nitrate assimilation in Synechococcus sp PCC 7002 improves photosynthetic production of 2-methyl-1-butanol and isobutanol. Metab Eng. 2022;69:87–97. https://doi.org/10.1016/j.ymben.2021.11.003.
Acknowledgements
This work was financially supported by the Key Research and Development Program of China (2020YFA0908300), the National Natural Science Foundation of China (31870069 and 32021005), and the Fundamental Research Funds for the Central Universities (USRP52019A, JUSRP121010, and JUSRP221013).
Author information
Authors and Affiliations
Contributions
JGL and XYB completed the collection and analysis of relevant literatures and the writing of the first draft. YFL, XQL, JHL, GCD and LL revised the manuscript. XQL and LL designed the manuscript. All authors contributed to the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, J., Bi, X., Liu, Y. et al. In silico cell factory design driven by comprehensive genome-scale metabolic models: development and challenges. Syst Microbiol and Biomanuf 3, 207–222 (2023). https://doi.org/10.1007/s43393-022-00117-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00117-4