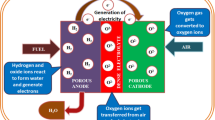
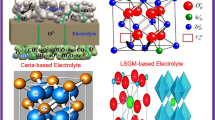
Abstract
Electrolyte supported fuel cells (ESCs) have emerged rapidly as energy conversion technology due to its easy to scale up capacity and low cost of production. The major issues confronting by ESC were its higher ohmic losses compared to other electrode supported fuel cells. These ohmic losses were mostly associated to the thickness of electrolyte (> 200 µm) used in it. Due to the limitations of the mechanical load-bearing capacity, it is very difficult to minimize these ohmic losses conventionally. In this direction, a number of attempt has been made in last few decades, such as use of higher oxide ion conducting electrolyte and minimizing the thickness of electrolyte with mechanical integrity. In case of alteration of electrolyte, doped lanthanum gallate, stabilized zirconia, and doped ceria have been discussed in the prospective of ESC. Moreover, to make a thin electrolyte with mechanical reliability, some engineering applied on the ESC architecture itself, in the last decade, has been discussed. This review covers all the progresses that has been done for the upliftment of ESCs in last 4 decades, and summarize their fundamental aspects.
Graphical abstract









Similar content being viewed by others
Data availability
Not applicable.
References
N.Q. Minh, Solid oxide fuel cells for power generation and hydrogen production. J. Korean Ceram. Soc. 47, 1–7 (2010). https://doi.org/10.4191/KCERS.2010.47.1.001
A. Nazir, H.T.T. Le, C.W. Min, A. Kasbe, J. Kim, C.S. Jin, C.J. Park, Coupling of a conductive Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2 metal-organic framework with silicon nanoparticles for use in high-capacity lithium-ion batteries. Nanoscale 12, 1629–1642 (2020). https://doi.org/10.1039/c9nr08038d
R.M. Ormerod, Solid oxide fuel cells. Chem. Soc. Rev. 32, 17–28 (2003). https://doi.org/10.1039/b105764m
A. Adams, A. Chowdhary, V. Subbaiah, Cost analysis comparison of bloom energy fuel cells with solar energy technology and traditional electric companies, 2011.
X. Ren, H.S. Jung, Recent progress in flexible perovskite solar cell development. J. Korean Ceram. Soc. 55, 325–336 (2018). https://doi.org/10.4191/kcers.2018.55.4.09
A. Kumar, I.-H. Kim, L. Mathur, H. Kim, S.-J. Song, Design of tin polyphosphate for hydrogen evolution reaction and supercapacitor applications. J. Korean Ceram. Soc. 58, 688–699 (2021). https://doi.org/10.1007/s43207-021-00143-3
A. Nazir, H.T.T. Le, A. Kasbe, C.J. Park, Si nanoparticles confined within a conductive 2D porous Cu-based metal–organic framework (Cu3(HITP)2) as potential anodes for high-capacity Li-ion batteries. Chem. Eng. J. 405, 126963 (2021). https://doi.org/10.1016/j.cej.2020.126963
A. Nazir, H.T.T. Le, A.G. Nguyen, J. Kim, C.J. Park, Conductive metal organic framework mediated Sb nanoparticles as high-capacity anodes for rechargeable potassium-ion batteries. Chem. Eng. J. 450, 138408 (2022). https://doi.org/10.1016/j.cej.2022.138408
Y. Yun, A. Kumar, J. Hong, S.-J. Song, Impact of CeO2 nanoparticle morphology: radical scavenging within the polymer electrolyte membrane fuel cell. J. Electrochem. Soc. 168, 114521 (2021). https://doi.org/10.1149/1945-7111/ac3ab4
B. Barik, Y. Yun, A. Kumar, H. Bae, Y. Namgung, J.Y. Park, S.J. Song, Highly enhanced proton conductivity of single-step-functionalized graphene oxide/nafion electrolyte membrane towards improved hydrogen fuel cell performance. Int. J. Hydrogen Energy. (2023). https://doi.org/10.1016/j.ijhydene.2022.12.137
L. Mathur, I.H. Kim, A. Bhardwaj, B. Singh, J.Y. Park, S.J. Song, Structural and electrical properties of novel phosphate based composite electrolyte for low-temperature fuel cells. Compos. Part B Eng. 202, 108405 (2020). https://doi.org/10.1016/j.compositesb.2020.108405
B. Singh, J.-H. Kim, I. Ha-Ni, S.J. Song, Cerium pyrophosphate-based proton-conducting ceramic electrolytes for low temperature fuel cells. J. Korean Ceram. Soc. 51, 248–259 (2014)
S.K. Gautam, A. Singh, L. Mathur, N. Devi, R.K. Singh, S.J. Song, D. Henkensmeier, B. Singh, Sintering and electrical behavior of ZrP2O7–CeP2O7 solid solutions Zr1-xCexP2O7; x = 0–0.2 and (Zr0.92Y0.08)1-yCeyP2O7; y = 0–0.1 for application as electrolyte in intermediate temperature fuel cells. Ionics 25, 155–162 (2019). https://doi.org/10.1007/s11581-018-2563-x
B. Singh, N. Devi, L. Mathur, S.J. Song, A.K. Srivastava, R.K. Singh, M. Ashiq, D.P. Mondal, A new solution phase synthesis of cerium(IV)pyrophosphate compounds of different morphologies using cerium(III)precursor. J. Alloys Compd. 793, 686–694 (2019). https://doi.org/10.1016/j.jallcom.2019.04.221
B. Singh, N. Devi, L. Mathur, R.K. Singh, A. Bhardwaj, S.J. Song, D. Henkensmeier, Fabrication of dense Ce0.9Mg0.1P2O7-PmOn composites by microwave heating for application as electrolyte in intermediate-temperature fuel cells. Ceram. Int. 44, 6170–6175 (2018). https://doi.org/10.1016/j.ceramint.2017.12.252
S. Singhal, Solid oxide fuel cells designs, materials, and applications. J. Korean Ceram. Soc. 42, 777–786 (2005). https://doi.org/10.4191/KCERS.2005.42.12.777
E. Wachsman, T. Ishihara, J. Kilner, Low-temperature solid-oxide fuel cells. MRS Bull. 39, 773–779 (2014). https://doi.org/10.1557/mrs.2014.192
S. Jo, B. Sharma, D.H. Park, J. Ha Myung, Materials and nano-structural processes for use in solid oxide fuel cells: a review. J. Korean Ceram. Soc. 57, 135–151 (2020). https://doi.org/10.1007/s43207-020-00022-3
C. Su, W. Wang, R. Ran, Z. Shao, M.O. Tade, S. Liu, Renewable acetic acid in combination with solid oxide fuel cells for sustainable clean electric power generation. J. Mater. Chem. A. 1, 5620–5627 (2013). https://doi.org/10.1039/c3ta10538e
W. An, X. Sun, Y. Jiao, S. Wang, W. Wang, M.O. Tadé, Z. Shao, S.D. Li, S. Shuang, Inherently catalyzed boudouard reaction of bamboo biochar for solid oxide fuel cells with improved performance. Energy Fuels 32, 4559–4568 (2018). https://doi.org/10.1021/acs.energyfuels.7b03131
S. Hussain, L. Yangping, Review of solid oxide fuel cell materials: cathode, anode, and electrolyte. Energy Transitions. 4, 113–126 (2020). https://doi.org/10.1007/s41825-020-00029-8
S.P.S. Shaikh, A. Muchtar, M.R. Somalu, A review on the selection of anode materials for solid-oxide fuel cells. Renew. Sustain. Energy Rev. 51, 1–8 (2015). https://doi.org/10.1016/j.rser.2015.05.069
C. Sun, R. Hui, J. Roller, Cathode materials for solid oxide fuel cells: a review. J. Solid State Electrochem. 14, 1125–1144 (2010). https://doi.org/10.1007/s10008-009-0932-0
B. Dziurdzia, Z. Magonski, H. Jankowski, Commercialisation of solid oxide fuel cells—opportunities and forecasts. IOP Conf. Ser. Mater. Sci. Eng. (2016). https://doi.org/10.1088/1757-899X/104/1/012020
J.C. Ruiz-Morales, J. Canales-Vázquez, C. Savaniu, D. Marrero-López, W. Zhou, J.T.S. Irvine, Disruption of extended defects in solid oxide fuel cell anodes for methane oxidation. Nature 439, 568–571 (2006). https://doi.org/10.1038/nature04438
W. Wang, C. Su, Y. Wu, R. Ran, Z. Shao, Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 113, 8104–8151 (2013). https://doi.org/10.1021/cr300491e
M. Irshad, K. Siraj, R. Raza, A. Ali, P. Tiwari, B. Zhu, A. Rafique, A. Ali, M.K. Ullah, A. Usman, A brief description of high temperature solid oxide fuel cell’s operation, materials, design, fabrication technologies and performance. Appl. Sci. (2016). https://doi.org/10.3390/app6030075
J. Bae, A novel metal supported SOFC fabrication method developed in kaist: a sinter-joining method. J. Korean Ceram. Soc. 53, 478–482 (2016). https://doi.org/10.4191/kcers.2016.53.5.478
L. Mathur, A. Kumar, I.H. Kim, H. Bae, J.Y. Park, S.J. Song, Novel organic-inorganic polyphosphate based composite material as highly dense and robust electrolyte for low temperature fuel cells. J. Power Sources. 493, 229696 (2021). https://doi.org/10.1016/j.jpowsour.2021.229696
M. Singh, D. Zappa, E. Comini, Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy. 46, 27643–27674 (2021). https://doi.org/10.1016/j.ijhydene.2021.06.020
N.Q. Minh, Solid oxide fuel cell technology—features and applications. Solid State Ionics 174, 271–277 (2004). https://doi.org/10.1016/j.ssi.2004.07.042
F.R. Bianchi, R. Spotorno, P. Piccardo, B. Bosio, Solid oxide fuel cell performance analysis through local modelling. Catalysts 10, 1–14 (2020). https://doi.org/10.3390/catal10050519
M. Tonekabonimoghaddam, A. Shamiri, Simulation and sensitivity analysis for various geometries and optimization of solid oxide fuel cells: a review. Eng. 2, 386–415 (2021). https://doi.org/10.3390/eng2030025
H. Sumi, K. Ukai, Y. Mizutani, H. Mori, C.J. Wen, H. Takahashi, O. Yamamoto, Performance of nickel-scandia-stabilized zirconia cermet anodes for SOFCs in 3% H2O-CH4. Solid State Ionics 174, 151–156 (2004). https://doi.org/10.1016/j.ssi.2004.06.016
H. Bae, Y. Shin, L. Mathur, D. Lee, S.J. Song, Defect chemistry of p-type perovskite oxide La0.2Sr0.8FeO3-δ: a combined experimental and computational study. J. Korean Ceram. Soc. 59, 876–888 (2022). https://doi.org/10.1007/s43207-022-00237-6
A. Bhardwaj, H. Bae, I.-H. Kim, L. Mathur, J.-Y. Park, S.-J. Song, High capacity, rate-capability, and power delivery at high-temperature by an oxygen-deficient perovskite oxide as proton insertion anodes for energy storage devices. J. Electrochem. Soc. 168, 070540 (2021). https://doi.org/10.1149/1945-7111/ac131f
J.-W. Moon, H.-L. Lee, G.-D. Kim, J.-D. Kim, H. Lee, Effect of current collecting layer on the impedance of LSM and LSM-YSZ cathode. J. Korean Ceram. Soc. 35, 1070–1077 (1998)
Y. Yoo, Y. Namgung, A. Bhardwaj, S. Song, A facile combustion synthesis route for performance enhancement of La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF6428) as a robust cathode material for IT-SOFC. J. Korean Ceram. Soc. 56, 497–505 (2019). https://doi.org/10.4191/kcers.2019.56.5.05
D. Sarantaridis, A. Atkinson, Redox cycling of Ni-based solid oxide fuel cell anodes: a review. Fuel Cells. 7, 246–258 (2007). https://doi.org/10.1002/fuce.200600028
J. Laurencin, G. Delette, O. Sicardy, S. Rosini, F. Lefebvre-Joud, Impact of “redox” cycles on performances of solid oxide fuel cells: case of the electrolyte supported cells. J. Power Sources. 195, 2747–2753 (2010). https://doi.org/10.1016/j.jpowsour.2009.10.099
M.E. Chelmehsara, J. Mahmoudimehr, Techno-economic comparison of anode-supported, cathode-supported, and electrolyte-supported SOFCs. Int. J. Hydrogen Energy. 43, 15521–15530 (2018). https://doi.org/10.1016/j.ijhydene.2018.06.114
C. Jin, Y. Mao, D.W. Rooney, N. Zhang, K. Sun, Fabrication and characterization of SSZ tape cast electrolyte-supported solid oxide fuel cells. Ceram. Int. 42, 5523–5529 (2016). https://doi.org/10.1016/j.ceramint.2015.12.110
C. Yuan, Y. Liu, Y. Zhou, Z. Zhan, S. Wang, Fabrication and characterization of a cathode-support solid oxide fuel cell by tape casting and lamination. Int. J. Hydrogen Energy. 38, 16584–16589 (2013). https://doi.org/10.1016/j.ijhydene.2013.08.146
C. Jin, Y. Mao, N. Zhang, K. Sun, Fabrication and characterization of Ni-SSZ/SSZ/LSM-SSZ anode-supported SOFCs by tape casting and single-step co-sintering techniques. Ionics (Kiel). 22, 1145–1152 (2016). https://doi.org/10.1007/s11581-015-1626-5
S. Vafaeenezhad, A.R. Hanifi, M.A. Laguna-Bercero, T.H. Etsell, P. Sarkar, Microstructure and long-term stability of Ni–YSZ anode supported fuel cells: a review. Mater. Futur. 1, 042101 (2022). https://doi.org/10.1088/2752-5724/ac88e7
T. Armstrong, E. EL Batawi, M. Janousek, M. Pillai, Phase stable doped zirconia electrolyte composition with low degradation, US8,580,456 B2, 2013.
K.T. Lim, H.L. Lee, H.C. Shin, Scandia stabilized zirconia electrolyte for solid oxide fuel cell having improved stability in reducing atmosphere, US 10,218,024 B2, 2019.
M. Gottmann, D. Nguyen, E.E. Batawi, T. Armstrong, G. Wang, D. Hickey, Electrolyte supported cell designed for longer life and higher power, US8,999,601B2, 2015.
O. Yamamoto, Y. Arati, Y. Takeda, N. Imanishi, Y. Mizutani, M. Kawai, Y. Nakamura, Electrical conductivity of stabilized zirconia with ytterbia and scandia. Solid State Ionics 79, 137–142 (1995). https://doi.org/10.1016/0167-2738(95)00044-7
H. Shi, C. Su, R. Ran, J. Cao, Z. Shao, Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 30, 764–774 (2020). https://doi.org/10.1016/j.pnsc.2020.09.003
J. Ramírez-González, A.R. West, Electrical properties of calcia-stabilised zirconia ceramics. J. Eur. Ceram. Soc. 40, 5602–5611 (2020). https://doi.org/10.1016/j.jeurceramsoc.2020.06.023
B. Kim, H. Lee, Valence state and ionic conduction in Mn-doped MgO partially stabilized zirconia. J. Am. Ceram. Soc. 101, 1790–1795 (2018). https://doi.org/10.1111/jace.15333
S.P.S. Badwal, F.T. Ciacchi, D. Milosevic, Scandia-zirconia electrolytes for intermediate temperature solid oxide fuel cell operation. Solid State Ionics 136–137, 91–99 (2000). https://doi.org/10.1016/S0167-2738(00)00356-8
M. Lo Faro, A.S. Aricò, Electrochemical behaviour of an all-perovskite-based intermediate temperature solid oxide fuel cell. Int. J. Hydrogen Energy. 38, 14773–14778 (2013). https://doi.org/10.1016/j.ijhydene.2013.08.122
B. Timurkutluk, S. Dokuyucu, The role of tape thickness on mechanical properties and performance of electrolyte supports in solid oxide fuel cells. Ceram. Int. 44, 17399–17406 (2018). https://doi.org/10.1016/j.ceramint.2018.06.205
T. Ishihara, H. Matsuda, Y. Takita, Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J. Am. Chem. Soc. 116, 3801–3803 (1994). https://doi.org/10.1021/ja00088a016
M. Feng, J.B. Goodenough, K. Huang, C. Milliken, Fuel cells with doped lanthanum gallate electrolyte. J. Power Sources. 63, 47–51 (1996). https://doi.org/10.1016/S0378-7753(96)02441-X
D. Marrero-López, J.C. Ruiz-Morales, J. Peña-Martínez, M.C. Martín-Sedeño, J.R. Ramos-Barrado, Influence of phase segregation on the bulk and grain boundary conductivity of LSGM electrolytes. Solid State Ionics 186, 44–52 (2011). https://doi.org/10.1016/j.ssi.2011.01.015
J.W. Stevenson, T.R. Armstrong, D.E. McCready, L.R. Pederson, W.J. Weber, Processing and electrical properties of alkaline earth-doped lanthanum gallate. J. Electrochem. Soc. 144, 3613–3620 (1997). https://doi.org/10.1149/1.1838057
H. Hayashi, H. Inaba, M. Matsuyama, N.G. Lan, M. Dokiya, H. Tagawa, Structural consideration on the ionic conductivity of perovskite-type oxides. Solid State Ionics 122, 1–15 (1999). https://doi.org/10.1016/S0167-2738(99)00066-1
M. Morales, J.J. Roa, J. Tartaj, M. Segarra, A review of doped lanthanum gallates as electrolytes for intermediate temperature solid oxides fuel cells: from materials processing to electrical and thermo-mechanical properties. J. Eur. Ceram. Soc. 36, 1–16 (2016). https://doi.org/10.1016/j.jeurceramsoc.2015.09.025
V.V. Kharton, F.M.B. Marques, A. Atkinson, Transport properties of solid oxide electrolyte ceramics: a brief review. Solid State Ionics 174, 135–149 (2004). https://doi.org/10.1016/j.ssi.2004.06.015
P. Datta, P. Majewski, F. Aldinger, Synthesis and microstructural characterization of Sr- and Mg-substituted LaGaO3 solid electrolyte. Mater. Chem. Phys. 102, 240–244 (2007). https://doi.org/10.1016/j.matchemphys.2006.12.010
R. Polini, A. Pamio, E. Traversa, Effect of synthetic route on sintering behaviour, phase purity and conductivity of Sr- and Mg-doped LaGaO3 perovskites. J. Eur. Ceram. Soc. 24, 1365–1370 (2004). https://doi.org/10.1016/S0955-2219(03)00592-2
S. Li, B. Bergman, Doping effect on secondary phases, microstructure and electrical conductivities of LaGaO3 based perovskites. J. Eur. Ceram. Soc. 29, 1139–1146 (2009). https://doi.org/10.1016/j.jeurceramsoc.2008.08.017
K. Huang, J.-H. Wan, J.B. Goodenough, Increasing power density of LSGM-based solid oxide fuel cells using new anode materials. J. Electrochem. Soc. 148, A788 (2001). https://doi.org/10.1149/1.1378289
T. Fukui, S. Ohara, K. Murata, H. Yoshida, K. Miura, T. Inagaki, Performance of intermediate temperature solid oxide fuel cells with La(Sr)Ga(Mg)O3 electrolyte film. J. Power Sources. 106, 142–145 (2002). https://doi.org/10.1016/S0378-7753(01)01026-6
C. Jin, Z. Yang, H. Zheng, C. Yang, F. Chen, La0.6Sr1.4MnO4 layered perovskite anode material for intermediate temperature solid oxide fuel cells. Electrochem. Commun. 14, 75–77 (2012). https://doi.org/10.1016/j.elecom.2011.11.008
K.N. Kim, B.K. Kim, J.W. Son, J. Kim, H.W. Lee, J.H. Lee, J. Moon, Characterization of the electrode and electrolyte interfaces of LSGM-based SOFCs. Solid State Ionics 177, 2155–2158 (2006). https://doi.org/10.1016/j.ssi.2006.02.011
J.H. Lee, K.N. Kim, J.W.S.J. Kim, B.K. Kim, H.W. Lee, J. Moon, An investigation of the interfacial stability between the anode and electrolyte layer of LSGM-based SOFCs. J. Mater. Sci. 42, 1866–1871 (2007). https://doi.org/10.1007/s10853-006-1315-x
J.H. Wan, J.Q. Yan, J.B. Goodenough, LSGM-based solid oxide fuel cell with 1.4 W/cm2 power density and 30 day long-term stability. J. Electrochem. Soc. 152, A1511 (2005). https://doi.org/10.1149/1.1943587
Z. Bi, Y. Dong, M. Cheng, B. Yi, Behavior of lanthanum-doped ceria and Sr-, Mg-doped LaGaO3 electrolytes in an anode-supported solid oxide fuel cell with a La0.6Sr0.4CoO3 cathode. J. Power Sources. 161, 34–39 (2006). https://doi.org/10.1016/j.jpowsour.2006.03.065
Y. Lin, S.A. Barnett, Co-firing of anode-supported SOFCs with Thin La 0.9Sr 0.1Ga 0.8Mg 0.2O 3-δ electrolytes. Electrochem. Solid-State Lett. 9, 285–288 (2006). https://doi.org/10.1149/1.2191132
Z. Bi, B. Yi, Z. Wang, Y. Dong, H. Wu, Y. She, M. Cheng, A high-performance anode-supported SOFC with LDC-LSGM bilayer electrolytes. Electrochem. Solid-State Lett. 7, 105–107 (2004). https://doi.org/10.1149/1.1667016
W. Guo, J. Liu, Y. Zhang, Electrical and stability performance of anode-supported solid oxide fuel cells with strontium- and magnesium-doped lanthanum gallate thin electrolyte. Electrochim. Acta. 53, 4420–4427 (2008). https://doi.org/10.1016/j.electacta.2008.01.039
X. Zhu, K. Sun, S. Le, N. Zhang, Q. Fu, X. Chen, Y. Yuan, Improved electrochemical performance of NiO-La0.45Ce0.55O2-δ composite anodes for IT-SOFC through the introduction of a La0.45Ce0.55O2-δ interlayer. Electrochim. Acta. 54, 862–867 (2008). https://doi.org/10.1016/j.electacta.2008.03.060
K. Huang, J.B. Goodenough, A solid oxide fuel cell based on Sr- And Mg-doped LaGaO3 electrolyte: the role of a rare-earth oxide buffer. J. Alloys Compd. 303–304, 454–464 (2000). https://doi.org/10.1016/S0925-8388(00)00626-5
K. Huang, R. Tichy, J.B. Goodenough III., Performance tests of single ceramic fuel cells. J. Am. Ceram. Soc. 85, 2581–2585 (1998)
Y.W. Ju, H. Eto, T. Inagaki, S. Ida, T. Ishihara, Preparation of Ni-Fe bimetallic porous anode support for solid oxide fuel cells using LaGaO3 based electrolyte film with high power density. J. Power Sources. 195, 6294–6300 (2010). https://doi.org/10.1016/j.jpowsour.2010.04.068
K.-N. Kim, J. Moon, J.-W. Son, J. Kim, H. Lee, J.-H. Lee, B.-K. Kim, Introduction of a buffering layer for the interfacial stability of LSGM-Based SOFCs. J. Korean Ceram. Soc. 42, 637–644 (2005)
K.J. Hwang, M. Jang, M.K. Kim, S.H. Lee, T.H. Shin, Effective buffer layer thickness of La-doped CeO2 for high durability and performance on La0.9Sr0.1Ga0.8Mg0.2O3-δ electrolyte supported type solid oxide fuel cells. J. Eur. Ceram. Soc. 41, 2674–2681 (2021). https://doi.org/10.1016/j.jeurceramsoc.2020.11.036
T. Ishihara, T. Shibayama, S. Ishikawa, K. Hosoi, H. Nishiguchi, Y. Takita, Novel fast oxide ion conductor and application for the electrolyte of solid oxide fuel cell. J. Eur. Ceram. Soc. 24, 1329–1335 (2004). https://doi.org/10.1016/S0955-2219(03)00508-9
Z. Yang, C. Yang, C. Jin, M. Han, F. Chen, Ba0.9Co0.7Fe0.2Nb0.1O 3-δ as cathode material for intermediate temperature solid oxide fuel cells. Electrochem. Commun. 13, 882–885 (2011). https://doi.org/10.1016/j.elecom.2011.05.029
N. Mahato, A. Banerjee, A. Gupta, S. Omar, K. Balani, Progress in material selection for solid oxide fuel cell technology: a review. Prog. Mater. Sci. 72, 141–337 (2015). https://doi.org/10.1016/j.pmatsci.2015.01.001
S. Hui, J. Roller, S. Yick, X. Zhang, C. Decès-Petit, Y. Xie, R. Maric, D. Ghosh, A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J. Power Sources. 172, 493–502 (2007). https://doi.org/10.1016/j.jpowsour.2007.07.071
V.V. Kharton, A.A. Yaremchenko, E.N. Naumovich, Research on the electrochemistry of oxygen ion conductors in the former Soviet Union. II. Perovskite-related oxides. J. Solid State Electrochem. 3, 303–326 (1999). https://doi.org/10.1007/s100080050161
V.V. Kharton, E.N. Naumovich, A.A. Vecher, Research on the electrochemistry of oxygen ion conductors in the former Soviet Union. I. ZrO2-based ceramic materials. J. Solid State Electrochem. 3, 61–81 (1999). https://doi.org/10.1007/s100080050131
T. Liu, X. Zhang, X. Wang, J. Yu, L. Li, A review of zirconia-based solid electrolytes. Ionics (Kiel). 22, 2249–2262 (2016). https://doi.org/10.1007/s11581-016-1880-1
D.K. Lim, J.G. Guk, H.S. Choi, S.J. Song, Measurement of partial conductivity of 8YSZ by Hebb-Wagner polarization method. J. Korean Ceram. Soc. 52, 299–303 (2015). https://doi.org/10.4191/kcers.2015.52.5.299
J.S. Lee, D.K. Shin, B.Y. Choi, J.K. Jeon, S.H. Jin, K.H. Jung, P.A. An, S.J. Song, Effects of yttria and calcia co-doping on the electrical conductivity of zirconia ceramics. J. Korean Ceram. Soc. 44, 655–659 (2007). https://doi.org/10.4191/KCERS.2007.44.1.655
M.S. Islam, A. Bhardwaj, L. Mathur, I. Kim, J. Park, S. Song, Effects of electrolyte variation on ammonia sensing temperature for BiVO 4 sensing electrode in mixed potential gas sensor. Sens. Actuat. B. Chem. 371, 132504 (2022). https://doi.org/10.1016/j.snb.2022.132504
A. Bhardwaj, I.H. Kim, L. Mathur, J.Y. Park, S.J. Song, Ultrahigh-sensitive mixed-potential ammonia sensor using dual-functional NiWO4 electrocatalyst for exhaust environment monitoring. J. Hazard. Mater. 403, 123797 (2021). https://doi.org/10.1016/j.jhazmat.2020.123797
A. Bhardwaj, H. Bae, L. Mathur, S. Mathur, S.-J. Song, Cubic Bi 2 O 3-based electrochemical nitric oxide sensor using double perovskite oxide electrodes. J. Electrochem. Soc. 169, 117510 (2022). https://doi.org/10.1149/1945-7111/aca2e0
Y. Arachi, H. Sakai, O. Yamamoto, Y. Takeda, N. Imanishai, Electrical conductivity of the ZrO2-Ln2O3 (Ln = lanthanides) system. Solid State Ionics 121, 133–139 (1999). https://doi.org/10.1016/S0167-2738(98)00540-2
F. Wang, Y. Lyu, D. Chu, Z. Jin, G. Zhang, D. Wang, The electrolyte materials for SOFCs of low-intermediate temperature: review. Mater. Sci. Technol. (United Kingdom) 35, 1551–1562 (2019). https://doi.org/10.1080/02670836.2019.1639008
J. Jiang, J.L. Hertz, On the variability of reported ionic conductivity in nanoscale YSZ thin films. J. Electroceramics. 32, 37–46 (2014). https://doi.org/10.1007/s10832-013-9857-1
M. Mogensen, D. Lybye, N. Bonanos, P.V. Hendriksen, F.W. Poulsen, Factors controlling the oxide ion conductivity of fluorite and perovskite structured oxides. Solid State Ionics 174, 279–286 (2004). https://doi.org/10.1016/j.ssi.2004.07.036
J.W. Fergus, Electrolytes for solid oxide fuel cells. J. Power Sources. 162, 30–40 (2006). https://doi.org/10.1016/j.jpowsour.2006.06.062
S.P.S. Badwal, Zirconia-based solid electrolytes: microstructure, stability and ionic conductivity. Solid State Ionics 52, 23–32 (1992). https://doi.org/10.1016/0167-2738(92)90088-7
Z. Zakaria, S.H. Abu Hassan, N. Shaari, A.Z. Yahaya, Y. Boon Kar, A review on recent status and challenges of yttria stabilized zirconia modification to lowering the temperature of solid oxide fuel cells operation. Int. J. Energy Res. 44, 631–650 (2020). https://doi.org/10.1002/er.4944
D. Udomsilp, C. Lenser, O. Guillon, N.H. Menzler, Performance benchmark of planar solid oxide cells based on material development and designs. Energy Technol. 9, 2001062 (2021). https://doi.org/10.1002/ente.202001062
M. Noponen, P. Torri, J. Göös, J. Puranen, H. Kaar, S. Pylypko, M. Roostar, E. Õunpuu, Elcogen—next generation solid oxide cell and stack technology. ECS Trans. 91, 91–97 (2019). https://doi.org/10.1149/09101.0091ecst
Kerafol Keracell III. (2014). https://www.kerafol.com/sofc/komponenten-fuer-brennstoffzellentechnologie/elektrolytgetragene-zellen-esc.
M.A. Buccheri, A. Singh, J.M. Hill, Anode-versus electrolyte-supported Ni-YSZ/YSZ/Pt SOFCs: effect of cell design on OCV, performance and carbon formation for the direct utilization of dry methane. J. Power Sources. 196, 968–976 (2011). https://doi.org/10.1016/j.jpowsour.2010.08.073
Y. Gu, Y. Zhang, L. Ge, Y. Zheng, H. Chen, L. Guo, YSZ electrolyte support with novel symmetric structure by phase inversion process for solid oxide fuel cells. Energy Convers. Manag. 177, 11–18 (2018). https://doi.org/10.1016/j.enconman.2018.09.051
J.H. Joo, G.M. Choi, Thick-film electrolyte (thickness <20 μm)-supported solid oxide fuel cells. J. Power Sources. 180, 195–198 (2008). https://doi.org/10.1016/j.jpowsour.2008.02.013
H. Chang, J. Yan, H. Chen, G. Yang, J. Shi, W. Zhou, F. Cheng, S.-D. Li, Z. Shao, Preparation of thin electrolyte film via dry pressing/heating/quenching/calcining for electrolyte-supported SOFCs. Ceram. Int. 45, 9866–9870 (2019). https://doi.org/10.1016/j.ceramint.2019.02.026
C.K. Ng, S. Ramesh, C.Y. Tan, A. Muchtar, M.R. Somalu, Microwave sintering of ceria-doped scandia stabilized zirconia as electrolyte for solid oxide fuel cell. Int. J. Hydrogen Energy. 41, 14184–14190 (2016). https://doi.org/10.1016/j.ijhydene.2016.06.146
A. Azim Jais, S.A. Muhammed Ali, M. Anwar, M. Rao Somalu, A. Muchtar, W.N.R. WanIsahak, C. Yong Tan, R. Singh, N.P. Brandon, Enhanced ionic conductivity of scandia-ceria-stabilized-zirconia (10Sc1CeSZ) electrolyte synthesized by the microwave-assisted glycine nitrate process. Ceram. Int. 43, 8119–8125 (2017). https://doi.org/10.1016/j.ceramint.2017.03.135
A.O. Zhigachev, V.V. Rodaev, D.V. Zhigacheva, N.V. Lyskov, M.A. Shchukina, Doping of scandia-stabilized zirconia electrolytes for intermediate-temperature solid oxide fuel cell: a review. Ceram. Int. 47, 32490–32504 (2021). https://doi.org/10.1016/j.ceramint.2021.08.285
Z. Zakaria, S.K. Kamarudin, Advanced modification of scandia-stabilized zirconia electrolytes for solid oxide fuel cells application—a review. Int. J. Energy Res. 45, 4871–4887 (2021). https://doi.org/10.1002/er.6206
Q. Wang, H. Fan, Y. Xiao, Y. Zhang, Applications and recent advances of rare earth in solid oxide fuel cells. J. Rare Earths. (2022). https://doi.org/10.1016/j.jre.2021.09.003
V. Shukla, K. Balani, A. Subramaniam, S. Omar, Effect of thermal aging on the phase stability of 1Yb2O3- xSc2O3-(99 - X)ZrO2 (x = 7, 8 mol %). J. Phys. Chem. C. 123, 21982–21992 (2019). https://doi.org/10.1021/acs.jpcc.9b05672
V. Shukla, S. Singh, A. Subramaniam, S. Omar, Long-term conductivity stability of metastable tetragonal phases in 1Yb2O3- xSc2O3-(99 - X)ZrO2(x = 7, 8 mol %). J. Phys. Chem. C. 124, 23490–23500 (2020). https://doi.org/10.1021/acs.jpcc.0c05298
H.C. Shin, J.H. Yu, K.T. Lim, H.L. Lee, K.H. Baik, Effects of partial substitution of CeO2 with M2O3 (M = Yb, Gd, Sm) on electrical degradation of Sc2O3 and CeO2 Co-doped ZrO2. J. Korean Ceram. Soc. 53, 500–505 (2016). https://doi.org/10.4191/kcers.2016.53.5.500
Y. Mizutani, K. Hisada, K. Ukai, H. Sumi, M. Yokoyama, Y. Nakamura, O. Yamamoto, From rare earth doped zirconia to 1 kW solid oxide fuel cell system. J. Alloys Compd. 408–412, 518–524 (2006). https://doi.org/10.1016/j.jallcom.2004.12.177
S. Nakayama, R. Tokunaga, M. Takata, S. Kondo, Y. Nakajima, Crystal phase, electrical properties, and solid oxide fuel cell electrolyte application of scandia-stabilized zirconia doped with rare earth elements. Open Ceram. 6, 100136 (2021). https://doi.org/10.1016/j.oceram.2021.100136
T.I. Politova, J.T.S. Irvine, Investigation of scandia-yttria-zirconia system as an electrolyte material for intermediate temperature fuel cells—influence of yttria content in system (Y2O3)x(Sc2O3) (11–x)(ZrO2)89. Solid State Ionics 168, 153–165 (2004). https://doi.org/10.1016/j.ssi.2004.02.007
C. Haering, A. Roosen, H. Schichl, M. Schnöller, Degradation of the electrical conductivity in stabilised zirconia system Part II: Scandia-stabilised zirconia. Solid State Ionics 176, 261–268 (2005). https://doi.org/10.1016/j.ssi.2004.07.039
S. Omar, N. Bonanos, Ionic conductivity ageing behaviour of 10 mol.% Sc2O 3–1 mol.% CeO2-ZrO2 ceramics. J. Mater. Sci. 45, 6406–6410 (2010). https://doi.org/10.1007/s10853-010-4723-x
S.P.S. Badwal, J. Drennan, Microstructure / conductivity relationship in the scandia-zirconia system. Solid State Ionics 56, 769–776 (1992)
C.N.S. Kumar, R. Bauri, G.S. Reddy, Phase stability and conductivity of rare earth co-doped nanocrystalline zirconia electrolytes for solid oxide fuel cells. J. Alloys Compd. 833, 155100 (2020). https://doi.org/10.1016/j.jallcom.2020.155100
C. Viazzi, J.P. Bonino, F. Ansart, A. Barnabé, Structural study of metastable tetragonal YSZ powders produced via a sol-gel route. J. Alloys Compd. 452, 377–383 (2008). https://doi.org/10.1016/j.jallcom.2006.10.155
F.M. Spiridonov, L.N. Popova, R.Y. Popilskii, On the phase relations and the electrical conductivity in the system ZrO2Sc2O3. J. Solid State Chem. 2, 430–438 (1970). https://doi.org/10.1016/0022-4596(70)90102-7
Q.N. Xue, L.G. Wang, X.W. Huang, J.X. Zhang, H. Zhang, Influence of codoping on the conductivity of Sc-doped zirconia by first-principles calculations and experiments. Mater. Des. 160, 131–137 (2018). https://doi.org/10.1016/j.matdes.2018.09.001
C. Haering, A. Roosen, H. Schichl, Degradation of the electrical conductivity in stabilised zirconia systems Part I: Yttria-stabilised zirconia. Solid State Ionics 176, 253–259 (2005). https://doi.org/10.1016/j.ssi.2004.07.038
H.A. Abbas, C. Argirusis, M. Kilo, H.D. Wiemhöfer, F.F. Hammad, Z.M. Hanafi, Preparation and conductivity of ternary scandia-stabilised zirconia. Solid State Ionics 184, 6–9 (2011). https://doi.org/10.1016/j.ssi.2010.10.012
J.T.S. Irvine, J.W.L. Dobson, T. Politova, S. García Martín, A. Shenouda, Co-doping of scandia-zirconia electrolytes for SOFCs. Faraday Discuss. 134, 41–49 (2007). https://doi.org/10.1039/b604441g
V. Vijaya Lakshmi, R. Bauri, Phase formation and ionic conductivity studies on ytterbia co-doped scandia stabilized zirconia (0.9ZrO2-0.09Sc2O3-0. 01Yb2O3) electrolyte for SOFCs. Solid State Sci. 13, 1520–1525 (2011). https://doi.org/10.1016/j.solidstatesciences.2011.05.014
D.A. Agarkov, M.A. Borik, S.I. Bredikhin, I.N. Burmistrov, G.M. Eliseeva, A.V. Kulebyakin, I.E. Kuritsyna, E.E. Lomonova, F.O. Milovich, V.A. Myzina, N.Y. Tabachkova, Phase compositions, structures and properties of scandia-stabilized zirconia solid solution crystals co-doped with yttria or ytterbia and grown by directional melt crystallization. Solid State Ionics 346, 115218 (2020). https://doi.org/10.1016/j.ssi.2019.115218
A.O. Zhigachev, D.V. Zhigacheva, N.V. Lyskov, Influence of yttria and ytterbia doping on phase stability and ionic conductivity of ScSZ solid electrolytes. Mater. Res. Express. 6, 1–8 (2019). https://doi.org/10.1088/2053-1591/ab3ed0
A. Spirin, V. Ivanov, A. Nikonov, A. Lipilin, S. Paranin, V. Khrustov, A. Spirina, Scandia-stabilized zirconia doped with yttria: synthesis, properties, and ageing behavior. Solid State Ionics 225, 448–452 (2012). https://doi.org/10.1016/j.ssi.2012.02.022
Y. Mizutani, M. Tamura, M. Kawai, O. Yamamoto, Development of high-performance electrolyte in SOFC. Solid State Ionics 72, 271–275 (1994). https://doi.org/10.1016/0167-2738(94)90158-9
R. Slilaty, F. Marques, Electrical conductivity of Yttria Stabilized Zirconia (YSC) doped with transition metals, Boletín La Soc. Española Cerámica y Vidr. 35, 109–115 (1996)
A. Kumar, R.P. Singh, S. Singh, A. Jaiswal, S. Omar, Phase stability and ionic conductivity of cubic xNb2O5-(11–x)Sc2O3-ZrO2(0 ≤ x ≤4). J. Alloys Compd. 703, 643–651 (2017). https://doi.org/10.1016/j.jallcom.2017.01.301
V.S. Singh, A. Jaiswal, K. Balani, A. Subramaniam, S. Omar, Temporal stability of oxygen-ion conductivity in 1Nb2O5-10Sc2O3-89ZrO2. J. Eur. Ceram. Soc. 38, 1688–1694 (2018). https://doi.org/10.1016/j.jeurceramsoc.2017.11.008
Z. Lei, Q. Zhu, Phase transformation and low temperature sintering of manganese oxide and scandia co-doped zirconia. Mater. Lett. 61, 1311–1314 (2007). https://doi.org/10.1016/j.matlet.2006.07.020
O. Bohnke, V. Gunes, K.V. Kravchyk, A.G. Belous, O.Z. Yanchevskii, O.I. V’Yunov, Ionic and electronic conductivity of 3 mol% Fe2O 3-substituted cubic yttria-stabilized ZrO2 (YSZ) and scandia-stabilized ZrO2 (ScSZ). Solid State Ionics 262, 517–521 (2014). https://doi.org/10.1016/j.ssi.2013.11.003
D.A. Agarkov, M.A. Borik, S.I. Bredikhin, I.N. Burmistrov, G.M. Eliseeva, V.A. Kolotygin, A.V. Kulebyakin, I.E. Kuritsyna, E.E. Lomonova, F.O. Milovich, V.A. Myzina, P.A. Ryabochkina, N.Y. Tabachkova, T.V. Volkova, Structure and transport properties of zirconia crystals co-doped by scandia, ceria and yttria. J. Mater. 5, 273–279 (2019). https://doi.org/10.1016/j.jmat.2019.02.004
P. Li, I.W. Chen, J.E. Penner-Hahn, Effect of dopants on zirconia stabilization—an X-ray absorption study: II, tetravalent dopants. J. Am. Ceram. Soc. 77, 118–128 (1994). https://doi.org/10.1111/j.1151-2916.1994.tb05403.x
S. Omar, W. Bin Najib, W. Chen, N. Bonanos, Electrical conductivity of 10 mol% Sc 2 O 3–1 mol% M 2 O 3- ZrO 2 ceramics. J. Am. Ceram. Soc. 95, 1965–1972 (2012). https://doi.org/10.1111/j.1551-2916.2012.05126.x
Y. Arachi, T. Asai, O. Yamamoto, Y. Takeda, N. Imanishi, K. Kawate, C. Tamakoshi, Electrical conductivity of ZrO2-Sc2O3 doped with HfO2, CeO2, and Ga2O3. J. Electrochem. Soc. 148, A520 (2001). https://doi.org/10.1149/1.1366622
C.N. Shyam Kumar, R. Bauri, Enhancing the phase stability and ionic conductivity of scandia stabilized zirconia by rare earth co-doping. J. Phys. Chem. Solids. 75, 642–650 (2014). https://doi.org/10.1016/j.jpcs.2014.01.014
Z. Wang, M. Cheng, Z. Bi, Y. Dong, H. Zhang, J. Zhang, Z. Feng, C. Li, Structure and impedance of ZrO2 doped with Sc2O3 and CeO2. Mater. Lett. 59, 2579–2582 (2005). https://doi.org/10.1016/j.matlet.2004.07.065
M. Liu, C. He, J. Wang, W.G. Wang, Z. Wang, Investigation of (CeO2)x(Sc2O 3)(0.11–x)(ZrO2)0.89 (x = 0.01–0.10) electrolyte materials for intermediate-temperature solid oxide fuel cell. J. Alloys Compd. 502, 319–323 (2010). https://doi.org/10.1016/j.jallcom.2009.12.134
A. Kumar, A. Jaiswal, M. Sanbui, S. Omar, Oxygen-ion conduction in scandia-stabilized zirconia-ceria solid electrolyte (xSc2O3–1CeO2–(99–x)ZrO2, 5 ≤ x ≤ 11). J. Am. Ceram. Soc. 100, 659–668 (2017). https://doi.org/10.1111/jace.14595
A. Escardino, A. Belda, M.J. Orts, A. Gozalbo, Ceria-doped scandia-stabilized zirconia (10Sc2O3·1CeO2·89ZrO2) as electrolyte for SOFCs: Sintering and ionic conductivity of thin, flat sheets. Int. J. Appl. Ceram. Technol. 14, 532–542 (2017). https://doi.org/10.1111/ijac.12675
H. Tu, X. Liu, Q. Yu, Synthesis and characterization of scandia ceria stabilized zirconia powders prepared by polymeric precursor method for integration into anode-supported solid oxide fuel cells. J. Power Sources. 196, 3109–3113 (2011). https://doi.org/10.1016/j.jpowsour.2010.11.108
I.V. Brodnikovska, Y.M. Brodnikovskyi, M.M. Brychevskyi, O.D. Vasylyev, Joint impedance spectroscopy analysis of 10Sc1CeSZ and 8YSZ solid electrolytes for SOFC. Powder Metall. Met. Ceram. 57, 723–730 (2019). https://doi.org/10.1007/s11106-019-00037-4
Y. Brodnikovskyi, N. McDonald, I. Polishko, D. Brodnikovskyi, I. Brodnikovska, M. Brychevskyi, L. Kovalenko, O. Vasylyev, A. Belous, R. Steinberger-Wilckens, Properties of 10Sc1CeSZ-35YSZ(33-, 40-, 50-wt.%) composite ceramics for SOFC application. Mater. Today Proc. 6, 26–35 (2019). https://doi.org/10.1016/j.matpr.2018.10.071
Q. Xue, X. Huang, J. Zhang, H. Zhang, Z. Feng, Grain boundary segregation and its influences on ionic conduction properties of scandia doped zirconia electrolytes. J. Rare Earths. 37, 645–651 (2019). https://doi.org/10.1016/j.jre.2018.11.006
K.S. Yun, Y. Il Kwon, J.H. Kim, S. Jo, C.Y. Yoo, J.H. Yu, J.H. Joo, Effects of Ni diffusion on the accelerated conductivity degradation of scandia-stabilized zirconia films under a reducing atmosphere. J. Eur. Ceram. Soc. 36, 1835–1839 (2016). https://doi.org/10.1016/j.jeurceramsoc.2016.02.007
Z.-P. Li, T. Mori, J. Zou, J. Drennan, Defects clustering and ordering in di- and trivalently doped ceria. Mater. Res. Bull. 48, 807–812 (2013). https://doi.org/10.1016/j.materresbull.2012.11.073
C. Madhusudan, K. Venkataramana, C. Madhuri, C. Vishnuvardhan Reddy, Structural, electrical and thermal studies on microwave sintered Dy and Pr co-doped ceria ceramics as electrolytes for intermediate temperature solid oxide fuel cells. J. Mater. Sci. Mater. Electron. 29, 17067–17077 (2018). https://doi.org/10.1007/s10854-018-9803-8
J.A. Kilner, R.J. Brook, A study of oxygen ion conductivity in doped non-stoichiometric oxides. Solid State Ionics 6, 237–252 (1982). https://doi.org/10.1016/0167-2738(82)90045-5
N. Kim, B.H. Kim, D. Lee, Effect of co-dopant addition on properties of gadolinia-doped ceria electrolyte. J. Power Sources. 90, 139–143 (2000). https://doi.org/10.1016/S0378-7753(00)00389-X
S. Lübke, H.D. Wiemhöfer, Electronic conductivity of Gd-doped ceria with additional Pr-doping. Solid State Ionics 117, 229–243 (1999). https://doi.org/10.1016/s0167-2738(98)00408-1
M.S. Arshad, R. Raza, M.A. Ahmad, G. Abbas, A. Ali, A. Rafique, M.K. Ullah, S. Rauf, M.I. Asghar, N. Mushtaq, S. Atiq, S. Naseem, An efficient Sm and Ge co-doped ceria nanocomposite electrolyte for low temperature solid oxide fuel cells. Ceram. Int. 44, 170–174 (2018). https://doi.org/10.1016/j.ceramint.2017.09.155
F.Y. Wang, S. Chen, Q. Wang, S. Yu, S. Cheng, Study on Gd and Mg co-doped ceria electrolyte for intermediate temperature solid oxide fuel cells. Catal. Today. 97, 189–194 (2004). https://doi.org/10.1016/j.cattod.2004.04.059
E. Suda, B. Pacaud, M. Mori, Sintering characteristics, electrical conductivity and thermal properties of La-doped ceria powders. J. Alloys Compd. 408–412, 1161–1164 (2006). https://doi.org/10.1016/j.jallcom.2004.12.135
X. Sha, Z. Lü, X. Huang, J. Miao, L. Jia, X. Xin, W. Su, Preparation and properties of rare earth co-doped Ce0.8Sm0.2-xYxO1.9 electrolyte materials for SOFC. J. Alloys Compd. 424, 315–321 (2006). https://doi.org/10.1016/j.jallcom.2005.12.061
H. Yahiro, T. Ohuchi, K. Eguchi, H. Arai, Electrical properties and microstructure in the system ceria-alkaline earth oxide. J. Mater. Sci. 23, 1036–1041 (1988). https://doi.org/10.1007/BF01154008
T. Shimonosono, Y. Hirata, S. Sameshima, T. Horita, Electronic conductivity of La-doped ceria ceramics. J. Am. Ceram. Soc. 88, 2114–2120 (2005). https://doi.org/10.1111/j.1551-2916.2005.00401.x
D.W. Joh, M.K. Rath, J.W. Park, J.H. Park, K.H. Cho, S. Lee, K.J. Yoon, J.H. Lee, K.T. Lee, Sintering behavior and electrochemical performances of nano-sized gadolinium-doped ceria via ammonium carbonate assisted co-precipitation for solid oxide fuel cells. J. Alloys Compd. 682, 188–195 (2016). https://doi.org/10.1016/j.jallcom.2016.04.270
J.A. Kilner, Fast anion transport in solids. Solid State Ionics 8, 201–207 (1983)
A. Pandiyan, A. Uthayakumar, C. Lim, V. Ganesan, W. Yu, A. Das, S. Lee, M.N. Tsampas, S. Omar, J.W. Han, S.B. Krishna Moorthy, S.W. Cha, Validation of defect association energy on modulating oxygen ionic conductivity in low temperature solid oxide fuel cell. J. Power Sources. 480, 229106 (2020). https://doi.org/10.1016/j.jpowsour.2020.229106
T. Matsui, M. Inaba, A. Mineshige, Z. Ogumi, Electrochemical properties of ceria-based oxides for use in intermediate-temperature SOFCs. Solid State Ionics 176, 647–654 (2005). https://doi.org/10.1016/j.ssi.2004.10.011
S.M. Haile, Fuel cell materials and components. Acta Mater. 51, 5981–6000 (2003). https://doi.org/10.1016/j.actamat.2003.08.004
T. Mori, J. Drennan, J.H. Lee, J.G. Li, T. Ikegami, Oxide ionic conductivity and microstructures of Sm- or La-doped CeO2-based systems. Solid State Ionics 154–155, 461–466 (2002). https://doi.org/10.1016/S0167-2738(02)00483-6
R. Schmitt, A. Nenning, O. Kraynis, R. Korobko, A.I. Frenkel, I. Lubomirsky, S.M. Haile, J.L.M. Rupp, A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 49, 554–592 (2020). https://doi.org/10.1039/c9cs00588a
J. Koettgen, S. Grieshammer, P. Hein, B.O.H. Grope, M. Nakayama, M. Martin, Understanding the ionic conductivity maximum in doped ceria: trapping and blocking. Phys. Chem. Chem. Phys. 20, 14291–14321 (2018). https://doi.org/10.1039/c7cp08535d
D. Kim, I. Jeong, K.J. Kim, K.T. Bae, D. Kim, J. Koo, H. Yu, K.T. Lee, A brief review of heterostructure electrolytes for high-performance solid oxide fuel cells at reduced temperatures. J. Korean Ceram. Soc. 59, 131–152 (2022). https://doi.org/10.1007/s43207-021-00175-9
W.S. Hsieh, P. Lin, S.F. Wang, Characteristics of electrolyte supported micro-tubular solid oxide fuel cells with GDC-ScSZ bilayer electrolyte. Int. J. Hydrogen Energy. 39, 17267–17274 (2014). https://doi.org/10.1016/j.ijhydene.2014.08.060
D. Hirabayashi, A. Tomita, S. Teranishi, T. Hibino, M. Sano, Improvement of a reduction-resistant Ce0.8Sm0.2O 1.9 electrolyte by optimizing a thin BaCe1-xSm xO3-α layer for intermediate-temperature SOFCs. Solid State Ionics 176, 881–887 (2005). https://doi.org/10.1016/j.ssi.2004.12.007
W. Sun, Z. Shi, Z. Wang, W. Liu, Bilayered BaZr0.1Ce0.7Y0.2O3-δ/Ce0.8Sm0.2O2-δ electrolyte membranes for solid oxide fuel cells with high open circuit voltages. J. Memb. Sci. 476, 394–398 (2015). https://doi.org/10.1016/j.memsci.2014.11.059
Z. Gong, W. Sun, J. Cao, D. Shan, Y. Wu, W. Liu, Ce0.8Sm0.2O1.9 decorated with electron-blocking acceptor-doped BaCeO3 as electrolyte for low-temperature solid oxide fuel cells. Electrochim. Acta. 228, 226–232 (2017). https://doi.org/10.1016/j.electacta.2017.01.065
E.D. Wachsman, K.T. Lee, Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011). https://doi.org/10.1126/science.1204090
Z. Lu, J. Hardy, J. Templeton, J. Stevenson, D. Fisher, N. Wu, A. Ignatiev, Performance of anode-supported solid oxide fuel cell with thin bi-layer electrolyte by pulsed laser deposition. J. Power Sources. 210, 292–296 (2012). https://doi.org/10.1016/j.jpowsour.2012.03.036
S. Zha, A. Moore, H. Abernathy, M. Liu, GDC-based low-temperature SOFCs powered by hydrocarbon fuels. J. Electrochem. Soc. 151, A1128 (2004). https://doi.org/10.1149/1.1764566
S.S. Shin, J.H. Kim, K.T. Bae, K.T. Lee, S.M. Kim, J.W. Son, M. Choi, H. Kim, Multiscale structured low-temperature solid oxide fuel cells with 13 W power at 500 ℃. Energy Environ. Sci. 13, 3459–3468 (2020). https://doi.org/10.1039/d0ee00870b
J. Qian, Z. Tao, J. Xiao, G. Jiang, W. Liu, Performance improvement of ceria-based solid oxide fuel cells with yttria-stabilized zirconia as an electronic blocking layer by pulsed laser deposition. Int. J. Hydrogen Energy. 38, 2407–2412 (2013). https://doi.org/10.1016/j.ijhydene.2012.11.112
J. Qian, Z. Zhu, J. Dang, G. Jiang, W. Liu, Improved performance of solid oxide fuel cell with pulsed laser deposited thin film ceria-zirconia bilayer electrolytes on modified anode substrate. Electrochim. Acta. 92, 243–247 (2013). https://doi.org/10.1016/j.electacta.2013.01.017
D.L. Maricle, T.E. Swarr, S. Karavolis, Enhanced ceria - a low-temperature SOFC electrolyte. Solid State Ionics 52, 173–182 (1992). https://doi.org/10.1016/0167-2738(92)90103-V
W. Huang, P. Shuk, M. Greenblatt, Hydrothermal synthesis and properties of terbium- Or praseodymium-doped Ce1-xSmxO2-x/2 solid solutions. Solid State Ionics 113–115, 305–310 (1998). https://doi.org/10.1016/s0167-2738(98)00402-0
A.S. Babu, R. Bauri, Rare earth co-doped nanocrystalline ceria electrolytes for intermediate temperature solid oxide fuel cells (IT-SOFC). ECS Trans. 57, 1115–1123 (2013). https://doi.org/10.1149/05701.1115ecst
S.G. Bratsch, Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data. 18, 1–21 (1989). https://doi.org/10.1063/1.555839
Y. Liu, M.N. Mushtaq, W. Zhang, A. Teng, X. Liu, Single-phase electronic-ionic conducting Sm3+/Pr3+/Nd3+ triple-doped ceria for new generation fuel cell technology. Int. J. Hydrogen Energy. 43, 12817–12824 (2018). https://doi.org/10.1016/j.ijhydene.2018.04.125
Y. Liu, L. Fan, Y. Cai, W. Zhang, B. Wang, B. Zhu, Superionic conductivity of Sm3+, Pr3+, and Nd3+ triple-doped ceria through bulk and surface two-step doping approach. ACS Appl. Mater. Interfaces. 9, 23614–23623 (2017). https://doi.org/10.1021/acsami.7b02224
X. Fang, J. Zhu, Z. Lin, Effects of electrode composition and thickness on the mechanical performance of a solid oxide fuel cell. Energies (2018). https://doi.org/10.3390/en11071735
A. Nakajo, J. Kuebler, A. Faes, U.F. Vogt, H.J. Schindler, L.K. Chiang, S. Modena, J. Van Herle, T. Hocker, Compilation of mechanical properties for the structural analysis of solid oxide fuel cell stacks. Constitutive materials of anode-supported cells. Ceram. Int. 38, 3907–3927 (2012). https://doi.org/10.1016/j.ceramint.2012.01.043
T. Okamura, S. Shimizu, M. Mogi, M. Tanimura, K. Furuya, F. Munakata, Elastic properties of Sr- and Mg-doped lanthanum gallate at elevated temperature. J. Power Sources. 130, 38–41 (2004). https://doi.org/10.1016/j.jpowsour.2003.12.011
A. Atkinson, A. Selçuk, Mechanical behaviour of ceramic oxygen ion-conducting membranes. Solid State Ionics 134, 59–66 (2000). https://doi.org/10.1016/S0167-2738(00)00714-1
M. Morales, M.Á. Laguna-Bercero, Influence of anode functional layers on electrochemical performance and mechanical strength in microtubular solid oxide fuel cells fabricated by gel-casting. ACS Appl. Energy Mater. 1, 2024–2031 (2018). https://doi.org/10.1021/acsaem.8b00115
A. Nakajo, J. Van Herle, D. Favrat, Sensitivity of stresses and failure mechanisms in SOFCs to the mechanical properties and geometry of the constitutive layers. Fuel Cells. 11, 537–552 (2011). https://doi.org/10.1002/fuce.201000108
F. Fleischhauer, M. Terner, R. Bermejo, R. Danzer, A. Mai, T. Graule, J. Kuebler, Fracture toughness and strength distribution at room temperature of zirconia tapes used for electrolyte supported solid oxide fuel cells. J. Power Sources. 275, 217–226 (2015). https://doi.org/10.1016/j.jpowsour.2014.10.083
F. Fleischhauer, R. Bermejo, R. Danzer, A. Mai, T. Graule, J. Kuebler, High temperature mechanical properties of zirconia tapes used for electrolyte supported solid oxide fuel cells. J. Power Sources. 273, 237–243 (2015). https://doi.org/10.1016/j.jpowsour.2014.09.068
A. Larrea, D. Sola, M.A. Laguna-Bercero, J.I. Peña, R.I. Merino, V.M. Orera, Self-supporting thin Yttria-stabilised zirconia electrolytes for solid oxide fuel cells prepared by laser machining. J. Electrochem. Soc. 158, B1193 (2011). https://doi.org/10.1149/1.3619759
L. Mathur, H. Bae, Y. Namgung, J.Y. Park, S.J. Song, Flow behavior of gadolinium doped ceria under different polymeric and hydrodynamic environment for tape casting application. Korean J. Chem. Eng. 39, 1–13 (2022). https://doi.org/10.1007/s11814-022-1271-4
L. Zhang, S.P. Jiang, W. Wang, Y. Zhang, NiO/YSZ, anode-supported, thin-electrolyte, solid oxide fuel cells fabricated by gel casting. J. Power Sources. 170, 55–60 (2007). https://doi.org/10.1016/j.jpowsour.2007.03.080
Y.J. Leng, S.H. Chan, K.A. Khor, S.P. Jiang, P. Cheang, Effect of characteristics of Y2O3/ZrO2 powders on fabrication of anode-supported solid oxide fuel cells. J. Power Sources. 117, 26–34 (2003). https://doi.org/10.1016/S0378-7753(03)00350-1
P. Tiwari, S. Basu, Performance studies of electrolyte-supported solid oxide fuel cell with Ni-YSZ and Ni-TiO2-YSZ as anodes. J. Solid State Electrochem. 18, 805–812 (2014). https://doi.org/10.1007/s10008-013-2326-6
R. Muccillo, E.N.S. Muccillo, F.C. Fonseca, D.Z. de Florio, Characteristics and performance of electrolyte-supported solid oxide fuel cells under ethanol and hydrogen. J. Electrochem. Soc. 155, B232 (2008). https://doi.org/10.1149/1.2828024
S.G. Kim, S.P. Yoon, S.W. Nam, S.H. Hyun, S.A. Hong, Fabrication and characterization of a YSZ/YDC composite electrolyte by a sol-gel coating method. J. Power Sources. 110, 222–228 (2002). https://doi.org/10.1016/S0378-7753(02)00270-7
Acknowledgements
This research was supported by Korea Electric Power Corporation (Grant No. CX71220030).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mathur, L., Namgung, Y., Kim, H. et al. Recent progress in electrolyte-supported solid oxide fuel cells: a review. J. Korean Ceram. Soc. 60, 614–636 (2023). https://doi.org/10.1007/s43207-023-00296-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-023-00296-3