Abstract
A large body of evidence suggests that processing of affective information is typically disrupted in anxiety. It has also been hypothesized that anxious individuals are less able to evaluate contextual cues and to respond in an adaptive way to stress. In the present study, 25 participants (16 females; 9 males) scoring high (scores of 45 or above) and 26 participants (13 females; 13 males) scoring low (scores of 35 and below) on a standardized measure of trait anxiety performed an emotion search task to investigate attentional biases when the task provides an explicit emotional context. An emotional context was set in each block by asking participants to look as quickly as possible at a face expressing a specific emotion, while eye movements were being recorded. On each trial, two faces appeared, one of them expressing the target emotion and the other one expressing a distractor emotion. High trait-anxious participants showed slower response times (time to look at the instructed emotion), regardless of the affective context, compared to the control group. Additionally, we found slower responses to happy faces (positive context) in the anxious group in the presence of neutral and fearful distractors. Cognitive control may therefore be disrupted in anxiety, as anxious people take longer to process (search for) happy faces, presumably because attentional resources are drawn by neutral and fearful distractors. Those differences were not observed in a simple reaction times task, which suggests that attentional biases, and not differential processing of low-level facial features, are responsible for those differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anxiety-related attentional biases towards threat-related stimuli are often reported (e.g. Fox, 2002; MacLeod & Mathews, 1988), typically using a version of a dot-probe task (MacLeod et al., 1986). Briefly, in this task, two images (e.g. words or faces) appear simultaneously and differ in the emotion they express (e.g. an angry facial expression alongside a neutral expression). These images are then replaced by a neutral probe that the participants must detect. The trial is congruent if the probe replaces the emotional face and incongruent if it appears at the location of the neutral face. The difference in response times between congruent and incongruent trials is taken to reflect attentional bias towards negative emotions in anxious individuals (e.g. Cannito et al., 2020; Clauss et al., 2022; Egloff & Hock, 2001; Eysenck et al., 2007; Fox et al., 2007; Mathews & MacLeod, 1985; Okon-Singer, 2018). Importantly, the mechanisms underlying the effects of state and trait anxiety on attention differ. While state anxiety refers to a transitory state, trait anxiety is a more stable measure of emotional response to different types of situations or events (Spielberger, 1983). Pacheco-Unguetti et al. (2010) showed a double dissociation of the effect of anxiety on attention: while state anxiety was linked to an over-functioning of the alerting and orienting components of attention, trait anxiety was characterized by decreased executive control. In trait anxiety, this decrease in executive control is associated with a reduction in the recruitment of attentional control mechanisms in prefrontal brain regions (Bishop, 2008; Modi et al., 2015). Using EEG methods, it has been reported that high trait anxiety modulates electrophysiological markers of attention in the dot-probe paradigm (Fox et al., 2008). However, some studies have failed to find evidence for anxiety-related biases in attention using the dot-probe task (Kruijt et al., 2019). The measurement of attentional biases by means of response times has been questioned recently given that reaction time-based difference scores have been shown to lack reliability (Kappenman et al., 2014; Parsons et al., 2019).
These findings raise concerns about an overreliance on reaction time measures to assess biased attention. Some possible ways to provide more reliable methods to evaluate anxiety-related attentional biases are to use eye-tracking (e.g. Skinner et al., 2018) or electrophysiological (Fox et al, 2008; Tipura & Fox, 2020) methodology. Not only may such methods provide a more accurate assessment of attentional bias, but eye-tracking and electroencephalography also provide a window into the dynamic processes that underpin visual attention across an entire task (e.g. Sharpe et al., 2017). To illustrate, a recent meta-analysis of eye-tracking studies showed attentional biases for pain-related words and pictures in terms of both probability of first fixation and overall dwell time on pain-related images (Jones et al., 2020). Anxiety-related attentional biases towards threat using eye-tracking methodology have also been reported in generalized anxiety disorder (e.g. Mogg et al., 2000) and show an increase in viewing time on threatening images in state (Quigley et al., 2012) and trait anxiety (Veerapa et al., 2020) as well as a difficulty in disengaging from threatening body postures in trait anxiety (Azarian et al., 2016). Biases in the attentional processing of threat-related information can be understood in the context of attentional control theory (ACT; Derakshan & Eysenck, 2009), which proposes that anxiety tips the balance to stimulus-driven processes so that highly salient affective cues will override top-down control mechanisms, which would explain enhanced attentional orienting towards emotional faces in anxious individuals.
Attentional processes are also of interest in anxiety and related states as they are likely to underlie the emotion regulation impairments often reported in high-anxious individuals (Campbell-Sills & Barlow, 2007; Mennin et al., 2007). For example, a study that attempted to reduce the degree of attentional biases using a cognitive training technique led to changes in reappraisal processes which in turn led to reduced negative emotions (Sanchez et al., 2016). The potential of cognitive training techniques, such as ‘attention bias modification’ (MacLeod et al., 2002; Van Bockstaele et al., 2019), to modify emotion regulation processes has obvious therapeutic and preventative potential. However, it is important to note that contextual factors can have profound impact on the efficacy of any particular emotion regulation strategy (e.g. Aldao, 2013; Bonanno & Burton, 2013; Troy et al., 2013). Rather than assuming that specific strategies are always adaptive or maladaptive, it is now widely accepted that the flexible use of different strategies and their relevance to a specific context is optimal (Aldao, 2013; Bonanno & Burton, 2013). It is therefore vital to take contextual factors into account when evaluating anxiety-related biases in attention and deciding whether the modification of such biases might be of benefit.
We therefore think that it is useful to consider the nature of anxiety-related attentional biases within a framework of regulatory flexibility (e.g. Bonanno & Burton, 2013; Parsons et al., 2016). Bonanno and Burton (2013) have provided a useful framework and proposed that ‘regulatory flexibility’ is characterized by three sequential components: context sensitivity, repertoire and feedback. When a stressful event occurs and people must choose between different regulation strategies, they will first evaluate demands and opportunities in the environment (context sensitivity). This first component relies on an ability to read and decode contextual cues. Once contextual cues are decoded, the next step is to select the regulatory strategy among all the strategies available in a person’s repertoire. Finally, through a feedback process, the regulation strategy is monitored and modified if needed, resulting in either adjustment, maintenance or cessation. At the end of the process, the re-evaluation of demands and opportunities might be necessary so that optimal regulation will be the one engaging the most flexible pattern. It has been suggested that psychopathological states such as depression or anxiety may reflect an insensitivity in the processing of context (Bylsma et al., 2008; Larson et al., 2007; Lenzo et al., 2021). It is clear that if attentional processes interfere with the processing of context right at the outset, the entire regulatory process will be disrupted.
If the emotional context is incorrectly decoded, then this will affect the other components of regulatory flexibility, and therefore, emotion regulation generally will be affected. Given the close association between the use of emotion regulation strategies and psychopathology (Dixon-Gordon et al., 2015), we propose that it may be informative to investigate negative attentional biases within a framework of regulatory flexibility. Indeed, one of the challenges of investigating emotion perception in laboratory settings is the absence of contextual features that allow participants to evaluate the extent to which an emotional stimulus (like a face) is relevant to the task or not. While different types of stimuli (scenes, bodies, faces or words) have been used to indicate specific emotions, these stimuli are quite often taken out of their usual context, making it difficult to translate the findings to more realistic situations (Barrett et al., 2011). In line with this idea, the constructed emotion approach (Barrett et al., 2011) assumes that context is vital to build an emotion. Without context, it is difficult to assess emotions, especially in laboratory settings. For instance, structural features of a face might not be sufficient for decoding emotions. Therefore, it is important to understand the effect of emotional context on attentional biases in the anxious population.
The current study was inspired by the effects of threat-related stimuli on attentional processes in trait-anxious individuals. We sought to test whether these processes are affected by the decoding of emotional contextual cues by using eye movement measures. We operationalized the notion of emotional context by asking participants to search for specific emotions over several trials. Specifically, participants were instructed to search for, and fixate as fast as possible, a face displaying a given emotion in the presence of a distractor emotion. An instruction that monetary compensation will depend on their performance added pressure to perform and acted as a stressor in our study. We used gaze as an overt measure of selective attention, with differences in response times (time to fixate the target emotion) indicative of differences in the processing of different emotional faces. The idea is that if anxious people are less sensitive to context—as determined by the ‘target’ facial expression—they would be more prone to distraction from irrelevant facial expressions. In contrast, if lower anxiety is associated with an enhanced ability to decode context, then there should be less interference from an irrelevant distractor.
Method
Participants
Fifty-five participants from the University of Oxford and the local community were preselected based on their scores on the trait scale of the State-Trait Anxiety Inventory (STAI; Spielberger, 1983) which was completed online before the experiment. There was a maximum of 1-week interval between the assessment of anxiety and the participation in the search task. We selected twenty-eight participants scoring high (scores of 45 or above) and twenty-eight participants scoring low (scores of 35 and below) on the STAI (Fox et al., 2007; Georgiou et al., 2005). Following the eye movements artefact rejection procedure and the suppression of incorrect trials, 1 participant was excluded due to a high percentage of incorrect trials (> 50%) and 3 participants were excluded from the analyses due to a high percentage of trial loss during the artefact rejection procedure (> 50%). The final sample consisted of 51 participants. The trait-anxious group consisted of 16 females and 9 males with a mean age of 25.44 years (SD = 5.07) and had a mean of 49.28 (SD = 4.91) on the STAI-T. The control group consisted of 13 females and 13 males with a mean age of 24.62 years (SD = 4.86) and a mean of 28.11 (SD = 4.9) on the STAI-T. In the literature and in our sample the STAI-T shows excellent consistency (Cronbach’s alpha is respectively .94 and .95). Participants had a normal or a corrected-to-normal vision. They were paid £10 per hour for their participation.
Prior to the experiment, participants completed the Context Sensitivity Index (CSI; Bonanno et al., 2020), which is a 20-item questionnaire reflecting cue presence (sensitivity to the presence of contextual cues) and cue absence (sensitivity to the relative absence of cues). The index of cue absence has been inversely associated with anxiety, namely more anxious people are less able to detect the absence of contextual cues (Bonanno et al., 2020). This measure has been proposed to best account for context sensitivity, as experimental measures tend to confound perception and response to context (CSI; Bonanno et al., 2020). We used this measure to test whether anxious participants are less inclined to detect contextual cues and determine cue absence, which would replicate the original findings (Bonanno et al., 2020). In our sample, the correlation between trait anxiety and cue presence was small (r(49) = − 0.0002, p = 0.99) and the correlation between trait anxiety and cue absence was significant (r(49) = − 0.52, p < 0.001), confirming the observations made by Bonanno et al. (2020).
An a priori power analysis was performed using G*Power 3.1.9.4 (Faul et al., 2007) with two groups and three measurements for a medium effect size of 0.25, using ‘F-tests’ and ‘ANOVA: repeated measures, within-between interaction’, and an alpha of 0.05. A total sample of 44 participants was required to achieve a power of 0.95.
Stimuli and Experimental Procedure
Stimuli
Stimuli were photographs of 10-male and 10-female grayscale fearful, happy and neutral facial expressions taken from the Radboud faces database (Langner et al., 2010). The borders of the faces were removed to avoid the influence of features such as the hair or ears. All the stimuli were created using Adobe Photoshop which allowed us to equate gray levels across all categories. Additionally, the SHINE toolbox was used to control for low-level properties of the stimuli across all categories and adjust mean gray level and contrast between the different faces (Willenbockel et al., 2010).
Experimental Procedure
The experiment was divided in 72 blocks of 10 trials, each block representing a specific context. Each block started with the following instruction: ‘In the next block of 10 trials, you will have to look as quickly as possible to the fearful / happy / neutral face to avoid losing points’. This instruction defined the emotional context: when instructed to look at the fearful face the context was ‘fear’, when instructed to look at the happy face the context was ‘happy’, when instructed to look at the neutral face the context was ‘neutral’. Next, two faces were presented side by side. One of the faces was congruent with the emotional context (either fearful, happy or neutral) and the other face expressed one of the two other emotions and acted as a distractor. The task of the participant was to look as quickly as possible towards the face expressing the emotion indicated by the previous instruction (the emotional context). Once the gaze of the participant was on a face, the face disappeared. This acted as a feedback for the participant. If the face expressing the emotion that matched the context disappeared, then it was to be considered a correct response. If the other face disappeared, it was to be considered an incorrect response (see Fig. 1).
The instruction acted as a mild stressor since participants were told that the number of correct responses would define the amount of money they receive. But in fact, all the participants were paid £10 per hour for their participation.
Simple Reaction Time Control with Trait-Anxious and Control Groups Study
Given that the three emotions differ in low-level features due to appearance of some facial features such as the teeth, to make sure that the results were not due to these low-level properties and only reflect orientation to a direction due to these low-level properties, we conducted an additional experiment with one face appearing on the right or left side of the screen and asked the participants to stare at the face as quickly as possible. The face was expressing either happy, neutral or fearful emotion and was composed of 90 trials randomly assigned to one of the emotions. Reaction times were analysed using a repeated measures ANOVA with emotion (fear/happy/neutral) as the within-subject factor and group (trait-anxious/control) as the between-subject factor. We also conducted a Bayesian repeated measures ANOVA to ensure that there were no differences between emotions. For the Bayesian analyses, models were compared to the null model with the default settings implemented in JASP (JASP Team, 2023).
The study was approved by the Central University Research Ethics Committee at the University of Oxford (CUREC Approval Reference: R63832/RE002) and all participants signed an informed consent form before participating. The study was performed in agreement with the Declaration of Helsinki.
Eye-Tracking Apparatus
We used a Tobii Pro TX300 (Stockholm, Sweden) eye-tracking system (screen + eyetracker). Gaze measurements are based on the pupil centroid (dark-pupil tracking) with a sampling frequency of 300 Hz. The TFT screen resolution was set to 1980 × 1024 pixels at 60 Hz. A custom MATLAB script was used to display the stimuli with the Psychtoolbox-3 (Kleiner et al., 2007) and interface with the eyetracker with the Tobii Pro Software Development Kit (SDK, downloaded 12/06/2019). The participants’ head rested on a chinrest at 60 cm from the screen centre.
Analyses
Eye Movements Pre-processing
Saccade and fixation identification used the default settings of the Tobii Pro I-VT filter (Komogortsev et al., 2010). We excluded saccades which direction was opposite to the targets’; we also excluded saccades which duration was below 10 ms and those which amplitude was below a 1.5 degrees of visual angle. Finally, there were some rare trials where a saccade could not be detected within the 5 s of the presentation of the face. This amounted to 2.4% of trials. Response times (time to look at the face displaying the instructed emotion) shorter than 200 ms and longer than 1000 ms were excluded from the analysis.
Statistical Analysis
We did separate analyses for the median time to focus (the time between the appearance of the two faces and the saccade made by the participant) and accuracy (if the participant looked at the emotional expression as instructed in the block the response was correct; incorrect if the participant looked at the distractor) in the three contexts (i.e. instructions to orient towards specific emotional expressions) and for transitions between the contexts. We also did separate analyses for the emotional context and for the distractor (the distractor is the other face displayed during the trial, which always expresses a distinct emotion than the one instructed). We analysed the percentage of incorrect responses and time to focus as a function of emotional context (fear/happy/neutral) and distractor (when the distractor was fear, happy or neutral) in trait-anxious and control (low anxiety) participants.
A repeated measures ANOVA was first performed on the percentage of incorrect responses with emotional context (fear/happy/neutral) as the within-subjects factor and anxiety group (trait-anxious/control) as the between-subjects factor. For the distractor, a repeated measures ANOVA was performed on the percentage of incorrect responses with the distractor valence (fear/happy/neutral) as the within-subjects factor and anxiety group (trait-anxious/control) as the between-subjects factor. The same analyses were performed with time to focus as the dependent variable.
To analyse how transitions between contexts affect response time, we took (individually) the median time to focus within a block of trials for each transition, as defined by the preceding and current emotional context, making six different transitions. A repeated measures ANOVA was performed on time to focus with transitions (happy following fear, happy following neutral, fear following happy, fear following neutral, neutral following fear, neutral following happy) as the within-subjects factor and anxiety group (trait-anxious/control) as the between-subjects factor.
When necessary, adjusted p-values were used to control for sphericity. In the ‘Results’ section, partial eta squared (η2p) and Cohen’s d are reported as effect sizes with η2p = 0.01 indicating a small effect, η2p = 0.06 indicating a medium effect, η2p = 0.14 indicating a large effect, d = 0.2 indicating a small effect, d = 0.5 indicating a medium effect and d ≥ 0.8 indicating a large effect (Cohen, 1988).
Vincent Averaging
To better capture the differences in the distributions of response times (RTs) of our two groups (trait-anxious and control), we performed Vincent averaging (Balota et al., 2008; Jiang et al., 2004). We first ordered each participants’ RTs (time to focus) from slowest to fastest and divided them in five equal-sized quantiles (20% of the data in each quantile). RTs of each quantile and participant were averaged individually and then averaged across participants. We explored the distributions of the data in each group using boxplots, which showed more variability and a slightly positively skewed distribution in anxious participants (Fig. 2a). We performed an ANOVA with anxiety group (control vs trait-anxious) as the between-subjects factor and quantile (quantile 1 to 5) as the within-subjects factor. This approach potentially allows the distinction between early processing (sensory) stages of visual stimuli (faces) and later evaluative stages (linking the face to the context).
Results
Accuracy
The effect of emotional context on accuracy was significant (F(1.96, 96.13) = 50.77, p < 0.001, η2p = 0.51; large effect size). Post hoc HSD Tukey tests showed that accuracy in all the three conditions differed significantly (ps < 0.05; see Table 1 for the percentage of incorrect responses in each condition).
For the distractor, the effect of emotion congruency on accuracy was significant (F(1.85, 90.49) = 100.79, p < 0.001, η2p = 0.67; large effect size). Post hoc HSD Tukey tests revealed that accuracy for both fearful and neutral distractors was significantly different from accuracy for the happy distractor (ps < 0.05; see Table 1 for the percentage of incorrect responses in each condition).
Time to Focus
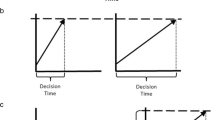
For the emotional context, the effect of anxiety group on RT was significant (F(1, 49) = 6.13, p = 0.016, η2p = 0.11; medium effect size) with longer time to focus for anxious than control participants (Fig. 2a). The effect of emotional context on RT was also significant (F(1.96, 95.85) = 25.17, p < 0.001, η2p = 0.34; large effect size). Post hoc HSD Tukey tests revealed that the RT in both fear and neutral contexts were significantly different from the RT in the happy context (ps < 0.05; see Table 2 for time to focus). Finally, the interaction between anxiety group and emotional context was significant (F(1.96, 95.85) = 6.34, p = 0.002, η2p = 0.12; medium effect size) with post hoc HSD Tukey tests showing that in the anxious group, both fear and neutral contexts were significantly different from the happy context (ps < 0.05).
For the distractor, the effect of distractor valence was significant (F(1.34, 65.83) = 22.74, p < 0.001, η2p = 0.317; large effect size). Post hoc HSD Tukey tests revealed that both fearful and neutral distractors were significantly different from the happy distractor (ps < 0.05; see Table 2 for time to focus).
Vincent Averaging
Figure 2b shows the mean RTs for each quantile in each group. The ANOVA showed a significant effect of anxiety group (F(1, 49) = 7.9, p = 0.007, η2p = 0.139; medium effect size), with longer RTs in the anxious group. The effect of quantile was significant (F(1.26, 61.93) = 708.66, p < 0.001, η2p = 0.935; large effect size). The interaction anxiety group × quantile was also significant (F(1.26, 61.93) = 19.99, p < 0.001, η2p = 0.289; large effect size). We then computed simple effects between groups for each quantile to test if RT differences were specific to longer times to focus, as suggested by the Vincentized distributions. The first two quantiles did not differ significantly between groups (t(49) = 1.18, p = 0.24, d = 0.33 for quantile 1; t(49) = 1.73, p = 0.09, d = 0.48 for quantile 2). The three last quantiles were significantly different between groups (t(49) = 2.43, p = 0.018, d = 0.68 for quantile 3 (medium effect size); t(49) = 3.11, p = 0.003, d = 0.87 for quantile 4 (large effect size); t(49) = 3.93, p < 0.001, d = 1.10 for quantile 5 (large effect size)). These results suggest that the difference in RTs between groups is pronounced for longer RTs.
Reaction Times for the Transitions
For the transitions, the effect of anxiety group on RT was significant (F(1, 49) = 6.12, p = 0.016, η2p = 0.11; medium effect size), as well as the effect of condition on RT (F(3.98, 194.97) = 13.72, p < 0.001, η2p = 0.218; large effect size). The interaction between group and condition on RT was also significant (F(3.98, 194.97) = 3.77, p = 0.005, η2p = 0.07; medium effect size). Post hoc HSD Tukey tests showed that in the trait-anxious group, RTs in a happy context following fearful or neutral contexts were significantly longer than all the other transitions. Also, RTs were significantly longer in these two conditions in the anxious group as compared to the control group (see Fig. 3).
Results of the Simple Response Time Task Control
For the frequentist ANOVA, none of the effects reached significance (all ps > 0.05). For the Bayesian ANOVA, BFincl = 0.075, which indicates strong evidence for H0 for the effect of emotion (Schönbrodt & Wagenmakers, 2018; Table 1), thus confirming that the faces did not differ with regard to their low-level properties, or at least that these differences were not sufficient to attract participant’s attention and bias the outcome measures.
Discussion
The present study investigated the influence of emotional context in a modified version of the dot-probe task. We used eye-gaze as an index of attentional biases and recorded the timing of the first saccade toward emotional faces in three different emotional contexts (fearful, happy and neutral) in groups of high and low trait-anxious participants.
First, we show that effects of anxiety appear in analysis of response times (time to focus on the instructed emotion), and not accuracy. We report two main findings and will interpret our results within the framework of anxiety-related attentional biases and Bonanno and Burton’s model of regulatory flexibility (2013). First, anxious participants exhibited slower RTs than control participants, regardless of emotional context or distractor valence. Vincent averaging confirmed that this effect was restricted to longer RTs: for short RTs, there was no significant difference between groups. Second, anxious participants were slower to focus on happy faces (positive context) than neutral or fearful faces. They were however not influenced by the valence of the distractor. This was confirmed in the analysis of transitions between emotions, where RTs were slower in trait-anxious individuals for the happy context following either neutral or fearful contexts. The control study showed no effects of emotion, confirming that the low-level properties of the faces are unlikely to have impacted our outcome measures.
We compared mean RTs between groups as well as for different quantiles. In general, highly anxious participants displayed longer RTs than low-anxious participants, especially in the last quantiles (corresponding to longer RTs). We did not observe any biases to fearful faces in our study. Previous research has reported a dissociation in neural networks engaged in the processing of emotional distractors in high trait and state anxiety (Bishop et al., 2006). While high state anxious individuals were characterized by amygdala and superior temporal responses during low perceptual load processing, high trait-anxious participants showed a decrease in the prefrontal cortex associated with the processing of threat distractors. This was correlated with lower self-reported scores of attentional control and suggests diminished cognitive control in the presence of threat-related distractors. Importantly, this reduced cognitive control in trait anxiety was confirmed in tasks without emotional material (Bishop, 2008) and is accompanied by delayed RTs in identification tasks. This impoverished control may in part explain why high trait-anxious participants exhibited longer RTs in our task, which could reflect reduced executive control enhanced by the stressor (Pacheco-Unguetti et al., 2010).
The fact that RT differences between the two groups were prominent for later quantiles suggests that the timing of this processing is crucial to understand attentional biases in anxiety. Differences in later quantiles suggest an effect at an evaluative stage, the ability to decide which emotional face fits the context, and not at an earlier stage, such as a sensory stage affecting the ability to detect an emotional face. If that was the case, the earliest responses should be different as well. It is possible that in the earliest quantiles, the context is very salient and is processed without deficit by anxious individuals, whereas in later quantiles, the context is less salient and attentional biases are manifest.
In our study, attentional biases were restricted to the positive faces, as time to focus was longer for the positive context than neutral or negative contexts in the trait-anxious group. Since this effect was not present when the distractor was positive, we hypothesize that this is not an attentional bias away from positive stimuli but is likely a difficulty in attending toward positive information. This was confirmed in the analysis of transitions between emotional contexts, where RTs were longer for the happy context, whether it followed the neutral or the fearful context. These results are consistent with earlier reports in which anxiety vulnerability has been associated with reduced attention allocation to positive cues (Taylor et al., 2011). Taylor et al (2011) reported that attentional training to enhance attention towards positive stimuli resulted in less subsequent stress-related responses. Similarly, participants instructed to direct their attention toward positive stimuli demonstrated less frustration during the completion of a stressful anagram task (Johnson, 2009), again showing the adaptive role of positive emotions in response to stressors. Twivy et al. (2021) have also found in an affective flexibility task that high-anxious participants were slower to shift attention towards the affective aspects of positive material. Crucially, this impaired processing of positive information was associated with increasing levels of self-reported anxiety over a 7-week period. Taken together, these findings suggest that disruptions to the processing of positive information may be an important cognitive aspect of anxiety vulnerability.
From the perspective of decoding contextual cues, the importance of positive emotions in coping with chronic stress has also been highlighted (Folkman and Moskowitz, 2000), and difficulties in experiencing positive emotions are frequently reported in depression (Gruber et al., 2010). As discussed earlier, we know that psychopathological states such as depression or anxiety are often associated with context insensitivity (see Bonanno & Burton, 2013; Larson et al., 2007). Three of our findings provide evidence for context insensitivity in anxious individuals. First, we replicate an inverse association between the CSI-cue absence and measures of trait anxiety. Second, high-anxious individuals displayed slower times to focus on a target facial expression than low-anxious participants. This finding could reflect a difficulty in linking emotional expressions (at the perceptual stage) and responses to contextual demands (actually orienting one’s gaze to the instructed emotion). This interpretation is supported by the difference between anxious and control participants being prominent for longer RTs (later quantiles after Vincent averaging), hence at the evaluation stage and not during the perception stage. Finally, the effect of context insensitivity was restricted to positive emotions, therefore suggesting that anxious participants have difficulty engaging towards positive emotions in a positive context. If the context is not correctly decoded or is delayed (as reflected by longer response times in the happy context), it is likely to impact the other components of emotion regulation.
The present study had some limitations that should be taken into consideration and guide future research on emotional processing in anxiety using measures of context sensitivity. First, it is not clear to which extent our reward structure is a necessary feature of the paradigm or to which extent it was effective in generating mild anxiety. In addition to that, cash rewards may be less of an incentive in wealthier populations, which could have an impact on generalizability. If there is an effect of reward, it could be driven by the stress of losing money and this effect could be modulated by individual needs. Future investigations could consider the effect of reward and how it is modulated by individual factors. Second, we did not consider participants with clinical levels of anxiety, which could be an interesting avenue for future research. As such, we lacked information about participants’ mental health history and whether they had a diagnosis of anxiety. Third, since there is an interval of a maximum of 1 week between the assessment of trait anxiety and the experimental phase, there is a possibility that anxiety levels could have been significantly different between those two points in time. Although trait anxiety has shown stability over time in a 2-year (Usala & Hertzog, 1991) or 12-month (Werner et al., 2022) interval, we cannot exclude that external life events could have differentially impacted participants’ anxiety levels during the experimental phase.
While anxiety-related attentional biases have been widely reported, these have often been based on dot-probe paradigms based on analysis of reaction times where serious questions have been raised about reliability (Parsons et al., 2016). Our use of gaze to assess biased attention is likely to provide a more ecologically valid measure and reliable assessment. Our results support the growing evidence that processing of affectively positive information may be an important part of the development of anxiety vulnerability. We have also attempted in this work to investigate biased attention within a broader framework of sensitivity to context. Further work is required to find better ways of measuring context sensitivity in paradigms that can assess biases in attention and other cognitive processes.
Data and Code Availability
MATLAB scripts and data are freely available on the Open Science Framework at https://osf.io/qjyt9/
References
Aldao, A. (2013). The future of emotion regulation research: Capturing context. Perspectives on Psychological Science, 8(2), 155–172. https://doi.org/10.1177/1745691612459518
Azarian, B., Esser, E. G., & Peterson, M. S. (2016). Evidence from the eyes: Threatening postures hold attention. Psychonomic Bulletin & Review, 23(3), 764–770. https://doi.org/10.3758/s13423-015-0942-0
Balota, D. A., & Yap, M. J. (2011). Moving beyond the mean in studies of mental chronometry: The power of response time distributional analyses. Current Directions in Psychological Science, 20(3), 160–166. https://doi.org/10.1177/0963721411408885
Balota, D. A., Yap, M. J., Cortese, M. J., & Watson, J. M. (2008). Beyond mean response latency: Response time distributional analyses of semantic priming. Journal of Memory and Language, 59(4), 495–523. https://doi.org/10.1016/j.jml.2007.10.004
Barrett, L. F., Mesquita, B., & Gendron, M. (2011). Context in emotion perception. Current Directions in Psychological Science, 20(5), 286–290. https://doi.org/10.1177/0963721411422522
Bishop, S. J. (2008). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12(1), 92–98. https://doi.org/10.1038/nn.2242
Bishop, S. J., Jenkins, R., & Lawrence, A. D. (2006). Neural processing of fearful faces: Effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex, 17(7), 1595–1603. https://doi.org/10.1093/cercor/bhl070
Bonanno, G. A., & Burton, C. L. (2013). Regulatory flexibility: An individual differences perspective on coping and emotion regulation. Perspectives on Psychological Science, 8(6), 591–612. https://doi.org/10.1177/1745691613504116
Bonanno, G. A., Maccallum, F., Malgaroli, M., & Hou, W. K. (2020). The Context Sensitivity Index (CSI): Measuring the ability to identify the presence and absence of stressor context cues. Assessment, 27(2), 261–273. https://doi.org/10.1177/1073191118820131
Bylsma, L. M., Morris, B. H., & Rottenberg, J. (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. https://doi.org/10.1016/j.cpr.2007.10.001
Campbell-Sills, L., & Barlow, D. H. (2007). Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In J. J. Gross (Ed.), Handbook of emotion regulation (pp. 542–559). Guilford Press.
Cannito, L., Di Crosta, A., Palumbo, R., Ceccato, I., Anzani, S., La Malva, P., Palumbo, R., & Di Domenico, A. (2020). Health anxiety and attentional bias toward virus-related stimuli during the COVID-19 pandemic. Scientific Reports, 10(1), 16476. https://doi.org/10.1038/s41598-020-73599-8
Clauss, K., Gorday, J. Y., & Bardeen, J. R. (2022). Eye tracking evidence of threat-related attentional bias in anxiety-and fear-related disorders: A systematic review and meta-analysis. Clinical psychology review, 102142. https://doi.org/10.1016/j.cpr.2022.102142
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum.
Derakshan, N., & Eysenck, M. W. (2009). Anxiety, processing efficiency, and cognitive performance: New developments from attentional control theory. European Psychologist, 14(2), 168–176. https://doi.org/10.1027/1016-9040.14.2.168
Dixon-Gordon, K. L., Aldao, A., & De Los Reyes, A. (2015). Repertoires of emotion regulation: A person-centered approach to assessing emotion regulation strategies and links to psychopathology. Cognition and Emotion, 29(7), 1314–1325. https://doi.org/10.1080/02699931.2014.983046
Egloff, B., & Hock, M. (2001). Interactive effects of state anxiety and trait anxiety on emotional Stroop interference. Personality and Individual Differences., 31(6), 875–882. https://doi.org/10.1016/s0191-8869(00)00188-4
Eysenck, M. W., Derakshan, N., Santos, R., & Calvo, M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336. https://doi.org/10.1037/1528-3542.7.2.336
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/bf03193146
Folkman, S., & Moskowitz, J. T. (2000). Positive affect and the other side of coping. American Psychologist, 55(6), 647–654. https://doi.org/10.1037/0003-066X.55.6.647
Fox, E. (2002). Processing emotional facial expressions: The role of anxiety and awareness. Cognitive, Affective & Behavioral Neuroscience, 2(1), 52–63. https://www.doi.org/10.3758/CABN.2.1.52
Fox E., Mathews A., Calder A. J., & Yiend J. (2007). Anxiety and sensitivity to gaze direction in emotionally expressive faces. Emotion, 7(3), 478–486. https://www.doi.org/https://doi.org/10.1037/1528-3542.7.3.478
Fox, E., Derakshan, N., & Shoker, L. (2008). Trait anxiety modulates the electrophysiological indices of rapid spatial orienting towards angry faces. NeuroReport, 19(3), 259–263. https://doi.org/10.1097/wnr.0b013e3282f53d2a
Georgiou, G. A., Bleakley, C., Hayward, J., Russo, R., Dutton, K., Eltiti, S., & Fox, E. (2005). Focusing on fear: Attentional disengagement from emotional faces. Visual Cognition, 12(1), 145–158. https://doi.org/10.1080/13506280444000076
Gruber, J., Oveis, C., Keltner, D., & Johnson, S. L. (2010). A discrete emotions approach to positive emotion disturbance in depression. Cognition & Emotion, 25(1), 40–52. https://doi.org/10.1080/02699931003615984
JASP Team (2023). JASP (Version 0.17.2) [Computer software].
Jiang, Y., Rouder, J. N., & Speckman, P. L. (2004). A note on the sampling properties of the Vincentizing (quantile averaging) procedure. Journal of Mathematical Psychology, 48(3), 186–195. https://doi.org/10.1016/j.jmp.2004.01.002
Johnson, D. R. (2009). Goal-directed attentional deployment to emotional faces and individual differences in emotion regulation. Journal of Research in Personality, 43(1), 8–13. https://doi.org/10.1016/j.jrp.2008.09.006
Jones, E. B., Sharpe, L., Andrews, S., Colagiuri, B., Dudeney, J., Fox, E., Heathcote, L. C., Lau, J. Y. F., Todd, J., Van Damme, S., Van Ryckeghem, D. M. L., & Vervoort, T. (2020). The time course of attentional biases in pain: A meta-analysis of eye-tracking studies. Pain, 162(3), 687–701. https://doi.org/10.1097/j.pain.0000000000002083
Kappenman, E. S., Farrens, J. L., Luck, S. J., & Proudfit, G. H. (2014). Behavioral and ERP measures of attentional bias to threat in the dot-probe task: Poor reliability and lack of correlation with anxiety. Frontiers in psychology, 5. https://doi.org/10.3389/fpsyg.2014.01368
Kleiner, M., Brainard, D., Pelli, D., Ingling, A., Murray, R., & Broussard, C. (2007). What’s new in psychtoolbox-3. Perception, 36(14), 1–16.
Komogortsev, O. V., Gobert, D. V., Jayarathna, S., & Gowda, S. M. (2010). Standardization of automated analyses of oculomotor fixation and saccadic behaviors. IEEE Transactions on Biomedical Engineering, 57(11), 2635–2645. https://doi.org/10.1109/tbme.2010.2057429
Kruijt, A. W., Parsons, S., & Fox, E. (2019). A meta-analysis of bias at baseline in RCTs of attention bias modification: No evidence for dot-probe bias towards threat in clinical anxiety and PTSD. Journal of Abnormal Psychology, 128(6), 563. https://doi.org/10.1037/abn0000406
Langner, O., Dotsch, R., Bijlstra, G., Wigboldus, D. H. J., Hawk, S. T., & van Knippenberg, A. (2010). Presentation and validation of the Radboud Faces Database. Cognition & Emotion, 24(8), 1377–1388. https://doi.org/10.1080/02699930903485076
Larson, C. L., Nitschke, J. B., & Davidson, R. J. (2007). Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion, 7(1), 182–191. https://doi.org/10.1037/1528-3542.7.1.182
Lenzo, V., Quattropani, M. C., Sardella, A., Martino, G., & Bonanno, G. A. (2021). Depression, anxiety, and stress among healthcare workers during the COVID-19 outbreak and relationships with expressive flexibility and context sensitivity. Frontiers in Psychology, 12. https://doi.org/10.3389/fpsyg.2021.623033
MacLeod, C., & Mathews, A. (1988). Anxiety and the allocation of attention to threat. The Quarterly Journal of Experimental Psychology, 40(4), 653–670. https://doi.org/10.1080/14640748808402292
MacLeod, C., Mathews, A., & Tata, P. (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95(1), 15. https://doi.org/10.1037//0021-843x.95.1.15
MacLeod, C., Rutherford, E., Campbell, L., Ebsworthy, G., & Holker, L. (2002). Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology, 111(1), 107–123. https://pubmed.ncbi.nlm.nih.gov/11866165/
Mathews, A., & Macleod, C. (1985). Selective processing of threat cues in anxiety states. Behaviour Research and Therapy, 23(5), 563–569. https://doi.org/10.1016/0005-7967(85)90104-4
Mennin, D. S., Holaway, R. M., Fresco, D. M., Moore, M. T., & Heimberg, R. G. (2007). Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behavior Therapy, 38(3), 284–302. https://doi.org/10.1016/j.beth.2006.09.001
Modi, S., Kumar, M., Kumar, P., & Khushu, S. (2015). Aberrant functional connectivity of resting state networks associated with trait anxiety. Psychiatry Res. Neuroimaging, 234(1), 25–34. https://doi.org/10.1016/j.pscychresns.2015.07.006
Mogg, K., Millar, N., & Bradley, B.P. (2000). Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology, 109(4), 695–704. https://www.doi.org/10.1037/0021-843X.109.4.695
Okon-Singer, H. (2018). The role of attention bias to threat in anxiety: Mechanisms, modulators and open questions. Current Opinion in Behavioral Sciences, 19, 26–30. https://doi.org/10.1016/j.cobeha.2017.09.008
Pacheco-Unguetti, A. P., Acosta, A., Callejas, A., & Lupiáñez, J. (2010). Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychological Science, 21(2), 298–304. https://doi.org/10.1177/0956797609359624
Parsons, S., Kruijt, A. W., & Fox, E. (2016). A cognitive model of psychological resilience. Journal of Experimental Psychopathology, 7(3), 296–310. https://doi.org/10.5127/jep.053415
Parsons, S., Kruijt, A. W., & Fox, E. (2019). Psychological science needs a standard practice of reporting the reliability of cognitive-behavioral measurements. Advances in Methods and Practices in Psychological Science, 2(4), 378–395. https://doi.org/10.1177/2515245919879695
Quigley, L., Nelson, A. L., Carriere, J., Smilek, D., & Purdon, C. (2012). The effects of trait and state anxiety on attention to emotional images: An eye-tracking study. Cognition & Emotion, 26(8), 1390–1411. https://doi.org/10.1080/02699931.2012.662892
Sanchez, A., Everaert, J., & Koster, E. H. (2016). Attention training through gaze-contingent feedback: Effects on reappraisal and negative emotions. Emotion, 16(7), 1074. https://doi.org/10.1037/emo0000198
Schönbrodt, F. D., & Wagenmakers, E. J. (2018). Bayes factor design analysis: Planning for compelling evidence. Psychonomic Bulletin & Review, 25(1), 128–142. https://doi.org/10.3758/s13423-017-1230-y
Sharpe, L., Brookes, M., Jones, E., Gittins, C., Wufong, E., & Nicholas, M. K. (2017). Threat and fear of pain induces attentional bias to pain words: An eye-tracking study. European Journal of Pain, 21(2), 385–396. https://doi.org/10.1002/ejp.936
Skinner, I. W., Hübscher, M., Moseley, G. L., Lee, H., Wand, B. M., Traeger, A. C., Gustin, S. M., & McAuley, J. H. (2018). The reliability of eyetracking to assess attentional bias to threatening words in healthy individuals. Behavior Research Methods, 50(5), 1778–1792. https://doi.org/10.3758/s13428-017-0946-y
Spielberger, C. D. (1983). State-trait anxiety inventory for adults. PsycTESTS Dataset. https://doi.org/10.1037/t06496-000
Taylor, C. T., Bomyea, J., & Amir, N. (2011). Malleability of attentional bias for positive emotional information and anxiety vulnerability. Emotion, 11(1), 127–138. https://doi.org/10.1037/a0021301
Tipura, E., & Fox, E. (2020). Neural mechanisms of eye gaze processing as a function of emotional expression and working memory load. Neuroscience Letters, 742, 135550. https://doi.org/10.1016/j.neulet.2020.135550
Troy, A. S., Shallcross, A. J., & Mauss, I. B. (2013). A person-by-situation approach to emotion regulation: Cognitive reappraisal can either help or hurt, depending on the context. Psychological Science, 24(12), 2505–2514. https://doi.org/10.1177/0956797613496434
Twivy, E., Grol, M., & Fox, E. (2021). Individual differences in affective flexibility predict future anxiety and worry. Cognition and Emotion, 35(2), 425–434. https://doi.org/10.1080/02699931.2020.1843407
Usala, P. D., & Hertzog, C. (1991). Evidence of differential stability of state and trait anxiety in adults. Journal of Personality and Social Psychology, 60(3), 471–479. https://doi.org/10.1037/0022-3514.60.3.471
Van Bockstaele, B., Notebaert, L., MacLeod, C., Salemink, E., Clarke, P. J. F., Verschuere, B., Bögels, S. M., & Wiers, R. W. (2019). The effects of attentional bias modification on emotion regulation. Journal of Behavior Therapy and Experimental Psychiatry, 62, 38–48. https://doi.org/10.1016/j.jbtep.2018.08.010
Veerapa, E., Grandgenevre, P., El Fayoumi, M., Vinnac, B., Haelewyn, O., Szaffarczyk, S., Vaiva, G., & D’Hondt, F. (2020). Attentional bias towards negative stimuli in healthy individuals and the effects of trait anxiety. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-68490-5
Werner, F., Klingelhöfer-Jens, M., Schümann, D., Gamer, M., Kalisch, R., Sommer, T., & Lonsdorf, T. B. (2022, May 6). Limited temporal stability of the Spielberger State-Trait Inventory over 3.5 years. https://doi.org/10.31234/osf.io/mubgv
Willenbockel, V., Sadr, J., Fiset, D., Horne, G. O., Gosselin, F., & Tanaka, J. W. (2010). Controlling low-level image properties: The SHINE toolbox. Behavior Research Methods, 42(3), 671–684. https://doi.org/10.3758/BRM.42.3.671
Funding
Open access funding was provided by the University of Geneva. Both Eda Tipura and Elaine Fox were based at the Department of Experimental Psychology, University of Oxford, when this work was being conducted. During that time, Eda Tipura was supported by the Swiss National Science Foundation (Grant number: P2GEP1_178004/1) and Elaine Fox was supported by a European Research Council Advanced Investigator award (under the European Union’s Seventh Framework Programme [FP7/2007–2013]/ERC grant agreement no. 324176).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tipura, E., Souto, D. & Fox, E. Trait-Anxious People Take Longer to Search for Happy Faces in the Presence of Neutral and Fearful Distractors. Trends in Psychol. (2023). https://doi.org/10.1007/s43076-023-00305-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s43076-023-00305-8