Abstract
Due to the influence of economic, social and many other factors, there are more and more reproductive problems. Originally introduced for managing male factor infertility, intracytoplasmic sperm injection had become the most commonly used fertilization treatment in the world, with broadened indications including low oocyte yield, prior fertilization failure with conventional in vitro fertilization etc. However, academic evidence for better live-birth outcomes of intracytoplasmic sperm injection over conventional in vitro fertilization is limited. Thus, we aimed to compare the reproductive outcomes of conventional in vitro fertilization and intracytoplasmic sperm injection in patients with non-severe male factor infertility across poor and different sub-optimal ovarian response categories. The fertility rate, implantation rate, clinical pregnancy rate, live birth rate and other obstetric outcomes were mainly compared. Our results showed that independent of the number of oocytes retrieved, intracytoplasmic sperm injection significantly increased the fertilization rate, while conventional in vitro fertilization cycles showed a higher implantation rate, clinical pregnancy rate, and live birth rate. No differences were observed in most obstetric outcomes. Our study indicates that poor ovarian response is not an indication for intracytoplasmic sperm injection in couples with non-severe male infertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Backgrounds
Due to the influence of economic, social, and many other factors, the postponement of childbearing age, poor living habits, mental stress, and other factors lead to an increasing number of reproductive problems [1]. According to the 2022 assisted reproductive technology (ART) fact sheet from the European Society of Human Reproduction and Embryology (ESHRE) (Monitoring 2022), one in six couples suffer from infertility problems more than once during their reproductive lifetime across the world. A few years after the first application of conventional in vitro fertilization (IVF), the emergence of intracytoplasmic sperm injection (ICSI) has broadened the application of ART [2]. Although ICSI was initially introduced for managing male factor infertility, it has become the most commonly used fertilization treatment worldwide. According to data from the ESHRE in 2018 and the U.S. Centers for Disease Control and Prevention (CDC), ICSI accounted for approximately 39.7% of all cycles in 39 registered European countries and 68.3%–79.3% across all age groups in America [3, 4]. Considering the wider use of ICSI, the American Society of Reproductive Medicine (ASRM) analyzed the outcomes of ICSI for couples with non-male factor infertility in the latest practice committee opinion [5]. It was concluded that academic evidence for better live-birth outcomes of ICSI over conventional IVF is limited [5]. And according to its procedure, ICSI bypasses the natural barriers of the oocyte through directly injecting a single sperm into an oocyte. This invasive operation may increase the risk of the transmission of genetic defects [6, 7]. Collectively, extra time, costs, and possible risks put an additional note of caution in the broader use of ICSI in fertilization treatments [8].
The primary outcome of ART depends on many factors, among which, ovarian response is one of the most important factors [9]. In conventional IVF, fewer retrieved oocytes were related to fertilization failure and a higher cycle cancellation rate [10]. Although the definition of low ovarian response and the criteria for the number of oocytes retrieved after ovarian stimulation have been the subject of debate in ART, the most commonly used criteria were the Bologna criteria by the ESHRE in 2011 and the POSEIDON criteria in recent years [11]. The commonly used definition of poor ovarian response is the retrieval of < 4 oocytes, and suboptimal ovarian response is the retrieval of 4–9 oocytes after ovarian stimulation [11]. Impaired ovarian reserve and poor ovarian response are common infertility factors, in which the reproductive outcome is closely related to the number of oocytes retrieved [12]. Considering this close link, poor ovarian response can indicate ICSI, in theory, to increase the fertilization rate. However, to the best of the authors’ knowledge, there is a paucity of comparisons of the clinical effects of conventional IVF and ICSI across poor (1–3 oocytes retrieved) and suboptimal (4–9 oocytes retrieved) ovarian response categories [13, 14]. There were some limitations in the previous studies, such as limited sample size [15], less detailed study outcomes [16], and failure to conform to standards [17].
Therefore, our study aimed to compare the reproductive outcomes of conventional IVF and ICSI in couples with non-severe male factor infertility across poor (1–3 oocytes retrieved) and different suboptimal (4–6; 7–9 oocytes retrieved) ovarian response categories. Furthermore, we compared the effect of ICSI over conventional IVF on clinical pregnancy rate (CPR), live birth rate (LBR), abortion rate (AR), and preterm delivery rate after adjusting for potential confounding factors, which may provide information for clinical decision-making.
Materials and Methods
Subjects and Data
This retrospective study was conducted at the Reproductive Medicine Center of the Peking University Third Hospital, Beijing, China. Based on the inclusion criteria, patients who underwent conventional IVF/ICSI procedures at the Medical Center for Reproductive Medicine of Peking University Third Hospital between 2009 and 2019 were included in this study. Data were collected from the medical records kept by the center. Cycles were included based on fresh embryo transfer cycles. Cycles were excluded from this study based on the following: (1) cycles with missing or incorrect data (n = 6,541; 4.4%); (2) cycles that had > 9 retrieved oocytes or no oocytes (n = 83,316; 55.9%); (3) cycles with severe male infertility including oligospermia, severe oligospermia, azoospermia or other male factors including ejaculatory disorder, male genetic abnormality, necrospermia, or cycles performed TESA, PESA or MESA, or any cycle using donor gametes (n = 26,824; 18.0%); (4) cycles received preimplantation genetic testing (PGT, n = 1,002; 0.7%); (5) cycles performed half-ICSI, rescue ICSI, IVM, OP-IVM and oocyte cryopreservation (n = 1,019; 0.7%). Severe oligospermia and astheno-spermia were defined as: sperm concentration < 10 × 106/ml, and sperm with progressive motility rate (a + b) < 10% in our clinical diagnostic criteria. A total of 30,352 cycles (22,472 conventional IVF cycles and 7,880 ICSI cycles) were included. Based on the number of oocytes retrieved, the included cycles were divided into three groups: 1–3 (n = 8,680), 4–6 (n = 1,071), and 7–9 (n = 11,101).
Outcome Measures
The primary outcomes included fertilization rate, clinical pregnancy rate (CPR; clinical pregnancy cycles/transplanted cycles), and live birth rate (LBR; live birth cycles/transplanted cycles). Secondary outcomes were embryonic condition (mean number of 2PN embryos, cleavage stage embryos, transferable embryos, and high-quality embryos), implantation rate (IR, the number of gestational sacs/the number of embryos transferred), and abortion rate (AR, abortion cycles/clinical pregnancy cycles). High quality embryos were defined as ≥ 5 G2 on day 3 of culture. Embryos were graded based on their blastomere number, size and symmetry as well as the percentage of fragmentation that was present: G1: cell with even sized blastomeres and without fragmentation; G2: cell with ≤ 10% fragmentation; G3: even sized cell with 10–30% fragmentation; G4: cell with ≥ 30% fragmentation [18].
Statistical Analysis
All analyses were performed using IBM SPSS Statistics 24. Visual inspection of histograms and normality Q-Q plots were used to check whether all quantitative variables were normally distributed within each type of explanatory variable. The mean values were compared to assess the association between the quantitative outcomes. Independent sample t-tests and non-parametric tests were conducted to assess the statistical significance between the study groups. Categorical outcomes between the conventional IVF and ICSI groups were compared using the chi-square test. A binary logistic regression model was used to compare the effect of ICSI over conventional IVF on CPR, LBR, AR, and preterm delivery rate after adjustment for potential confounding factors, which were defined as non-equally distributed between ICSI and conventional IVF in each retrieved oocyte group. Statistical significance was set at P < 0.05.
Ethical Approval
This study was approved by the Ethics Committee of Peking University Third Hospital for data handling process (No. IRB00006761-M2020007). All the participants and procedures followed the required guidelines and provided informed consent.
Results
Characteristics of Patients
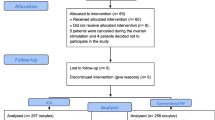
A total of 149,054 fresh embryo transfer cycles were performed at the Medical Center for Reproductive Medicine of Peking University Third Hospital from to 2009–2019. Finally, we reviewed 30,352 cycles (22,472 conventional IVF cycles and 7,880 ICSI cycles) after screening. The data selection process is illustrated in Fig. 1.
The baseline characteristics of female patients and infertility workup are presented in Table 1. Baseline data were compared between conventional IVF and ICSI cycles in three groups across different ovarian response categories: 1–3, 4–6, and 7–9 oocytes retrieved. In all three groups, patients in the ICSI group were significantly older and had longer durations of infertility than those in the conventional IVF group, whereas body mass index (BMI), AMH, and bFSH levels were similar. ICSI cycles had a significantly higher level of bLH, whereas conventional IVF cycles had higher bE2 levels in groups 1–3 and 4–6 oocytes, respectively. Patients with ≤ 3 oocytes were more likely to suffer from secondary infertility in the conventional IVF group whereas the ICSI group showed the opposite trend in groups with ≥ 4 oocytes. Also, the main infertility factor for patients with ≤ 3 oocytes was diminished ovarian reserve while patients were more likely to suffer from other factors(hyperprolactinemia, unexplained infertility etc.) if ≥ 4 oocytes were retrieved. The ovulation induction protocols varied between conventional IVF and ICSI cycles (p < 0.05).
Comparison of Laboratory Outcomes
Laboratory outcomes in groups with 1–3, 4–6, and 7–9 oocytes retrieved were compared between conventional IVF and ICSI in Table 2. The 2PN fertility rate was higher in ICSI cycles, independent of the number of oocytes retrieved (Group 1:58.1% versus 67.2%; Group 2:60.1% versus 65.1%; Group 3:60.0% versus 66.7%, all P < 0.001). There were more transferable D3 embryos observed in conventional IVF cycles in all three groups (1.5 ± 0.9 versus 1.4 ± 0.8; 3.8 ± 1.4 versus 3.0 ± 1.4; 6.0 ± 1.9 versus 4.7 ± 1.9, all p < 0.001). When ≥ 4 oocytes were retrieved, conventional IVF cycles obtained more 2PN embryos and high-quality embryos (3.0 ± 1.4 versus 2.6 ± 1.4; 4.8 ± 1.9 versus 4.0 ± 1.9; 2.0 ± 1.5 versus 1.7 ± 1.4; 3.2 ± 2.1 versus 2.8 ± 2.0, all p < 0.001) and higher transplantation rate (82.3% versus 79.2%; 88.5% versus 84.8%). Most cases received Day 3 embryos transfer. In groups of ≤ 6 oocytes were retrieved, no differences were observed in the proportion of Day 3/Day 5 embryo transfer between IVF and ICSI groups. When ≥ 7 oocytes were retrieved, ICSI cycles had higher proportion of Day 3 embryos transfer.
Comparison of Clinical Outcomes
The clinical outcomes were also compared between the conventional IVF and ICSI groups (Table 3). Conventional IVF cycles showed a higher implantation rate (IR, 17.6% versus 13.2%; 22.2% versus 20.2%; 26.3% versus 23.9%, p < 0.05) and clinical pregnancy rate (CPR, 23.2% versus 17.2%; 34.3% versus 30.9%; 41.3% versus 38.6%, p < 0.05) than ICSI cycles independent of the number of oocytes retrieved. The live birth rate was significantly higher in the conventional IVF cycles than in the ICSI cycles (LBR, 17.1% versus 12.6%; 26.6% versus 24.2%; 32.7% versus 30.8%, p < 0.05). No differences were observed in most obstetric outcomes, including abortion rate, early abortion rate, twin births rate, cesarean delivery rate, gestational age, and congenital malformations rate, between conventional IVF and ICSI in all three groups (p ≥ 0.05). However, conventional IVF cycles showed higher preterm delivery rates and lower birth weights in groups with 4–6 and 7–9 oocytes retrieved. If twin births were excluded, this result was only significant in the group with 7–9 oocytes retrieved (8.5% versus 5.5%, p = 0.022; 3297.2 ± 540.3 versus 3359.4 ± 523.5, p = 0.016). Interestingly, more female infants born in ICSI cycles with 4–6 oocytes were retrieved (male:53.4% vs. 48.7%; female:46.6%vs. 51.3%, p = 0.038).
Binary Logistic Regression for Effect Comparison
We compared the effect of ICSI over conventional IVF on CPR, LBR, AR, and preterm delivery rate after adjusting for potential confounding factors (Table 4). The results demonstrated that only in cycles with 1–3 oocytes retrieved, ICSI was associated with a significant decrease in CPR (OR = 0.763, 95% CI, 0.636, 0.916, p = 0.004) and LBR (OR = 0.795, 95% CI, 0.646, 0.979, p = 0.030). There was no evidence that fertilization methods were associated with AR (OR = 1.033, 95% CI, 0.709, 1.505; OR = 0.869, 95% CI, 0.684, 1.104; OR = 1.048, 95% CI, 0.859, 1.278, all p > 0.05). The results also indicated that ICSI was associated with a significant decrease in the preterm delivery rate, independent of the inclusion of multiple births when ≥ 4 oocytes were retrieved.
Discussion
The results of our study demonstrated that while ICSI cycles reached higher fertilization rates, conventional IVF cycles still resulted in higher clinical pregnancy rates and live birth rates in patients with non-severe male factor infertility across poor and different suboptimal ovarian response categories, which were more significant when ≤ 3 oocytes were retrieved. No significant differences were found in most obstetric outcomes across the different ovarian response categories.
The number of ART cycles performed has grown by 5–10% each year in many developed countries over the last few years. ICSI plays a significant role in broadening the application of ART by treating male factor infertility. It is a micromanipulation technique that helps the abnormal spermatozoon bypass natural barriers around the oocyte by inserting the spermatozoon into the cytoplasm [19]. ICSI has been considered to decrease the occurrence of fertilization failure [5]. A retrospective study based on 62,641 stimulated fresh cycles of the POR cohort suggested that the fertilization rate was lower in conventional IVF cycles [16]. However, another retrospective study revealed that in patients with ≤ 4 oocytes retrieved, the fertilization rate was better via conventional IVF, whereas implantation rates, live birth rates, and miscarriage rates were similar [15]. Despite its debatable efficacy and safety, ICSI has become the most commonly used ART technique worldwide, with broadened indications including low oocyte yield, prior fertilization failure with conventional IVF, and advanced maternal age [20]. ICSI has undergone relatively strict indications in China and is not as commonly used in European countries and America [21]. Low oocyte yield is a suitable case because fewer oocytes retrieved are related to fertilization failure [10, 22]. Our results showed that ICSI significantly increased the fertilization rate among poor and suboptimal ovarian response cycles, which is in line with most previous studies.
Several studies have compared conventional IVF and ICSI in terms of embryonic outcomes [23,24,25]. In most studies, there was no significant difference in the proportion of good-quality embryos and 2PN embryos [24, 25]. However, in another study, conventional IVF cycles showed a higher cleavage rate, which is consistent with our results. Our results demonstrated that more transferable D3 embryos were observed in conventional IVF cycles in all the groups. When ≥ 4 oocytes were retrieved, the conventional IVF cycles resulted in more 2PN and high-quality embryos. This may help explain the better reproductive outcomes in conventional IVF cycles, as the internal developmental potential of oocytes is a critical factor in the process of embryonic development.
In terms of post-fertilization reproductive outcomes, our result was consistent with some previous studies, that is conventional IVF showed significant advantages over ICSI in terms of CPR and LBR [16, 26, 27]. Possible reasons why ICSI improved the fertilization rate but was inferior to conventional IVF in IR, CPR, and LBR have been discussed [28,29,30,31,32]. Unpredictable oocyte damage brought by invasiveness of ICSI has always been a key point. First, the injection needle may damage the cytoplasm of oocytes and cause clusters of smooth endoplasmic reticulum or vacuoles, which may influence the oocyte survival [28, 29]. Furthermore, the inaccurate positioning of the injection needle may do damage to the metaphase II (MII) spindle, leading to an increased risk of producing aneuploid embryos [30]. The reproductive outcome is closely related to the number and quality of oocytes retrieved, and oocytes quality is more likely to be impaired by ICSI procedure in patients with poor ovarian response. Apart from the better clinical outcomes, conventional IVF also shows advantages in lower costs and less time consuming [33]. Thus, the choice of the treatment should be considered comprehensively, both in the efficacy and efficiency side, in the absence of the male factor.
Regarding the majority of obstetric outcomes, another study based on subgroup analysis found no significant differences in multiple birth rate, cesarean delivery rate, and gestational age [34]. Significant decreases were noted in the preterm delivery rate and low birth weight in infants born after ICSI treatment [35, 36]. Similar to these studies, our results demonstrated that multiple fetal, abortion, early abortion, congenital malformations, and cesarean delivery rates, and gestational weeks were similar in each subgroup, but lower preterm delivery rates and higher birth weights in ICSI cycles were observed when ≥ 4 oocytes were retrieved. Wennerholm et al. [37] also concluded that ICSI had a lower incidence of preterm delivery and low birth weight, suggesting that ICSI had a better obstetric outcome. A possible reason for this is that ICSI is usually related to poor semen quality in men, while the reproductive situation in women is better. Therefore, the difference is more significant especially in women with a better ovarian response (≥ 4 oocytes retrieved).
Another finding is the impact of the fertilization method on the gender balance of infants born after ART. According to our results, more male newborns were born after conventional IVF cycles than after ICSI cycles, and the opposite was true for female infants. A similar study from the UK demonstrated that more female infants were born after ICSI, while conventional IVF increased the number of male births, which is consistent with our study [38]. Several hypotheses regarding the underlying mechanism have been proposed in previous studies, including bias towards females when performing sperm selection in ICSI cycles and reduced normally functioning sperm with the Y chromosome caused by the invasive operation during ICSI [39, 40]. Given the widespread use of ART, its long-term effect on sex imbalance should be a concern; thus, further research is needed.
Our study had several strengths and limitations. First, it was based on a large cohort of 30,352 fresh cycles from 2009–2019. Furthermore, our study focused on couples with non-severe male infertility and poor ovarian response, for whom the indication of ICSI has always been the subject of debate. The information used in this study was sourced from hospital records to ensure the detail and accuracy of the data. However, there are some limitations. For example, cycles and not patients have been analyzed due to the condition of hospital records. And our study was limited by the lack of randomization due to the research type; hence, prospective randomized controlled trials are required to provide a higher level of evidence.
Conclusion
In conclusion, in the presence of a poor ovarian response, our results showed that even if ICSI increased the fertilization rate, conventional IVF exhibited significant advantages over ICSI in IR, CPR, and LBR for patients with non-severe male infertility. Furthermore, the advantages of conventional IVF are independent of the number of eggs retrieved. There were no significant differences in most of the obstetric outcomes. Therefore, considering that the goal of treatment is live birth, poor ovarian response is not an indication of ICSI in couples with non-severe male infertility.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICSI:
-
Intracytoplasmic sperm injection
- conventional IVF:
-
Conventional in vitro fertilization
- ART:
-
Assisted Reproductive Technology
- IR:
-
Implantation rate
- CPR:
-
Clinical pregnancy rate
- LBR:
-
Live birth rate
- AR:
-
Abortion rate
References
Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2–10.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet (London, England). 1992;340(8810):17–8.
Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, et al. ART in Europe, 2018: Results generated from European registries by ESHRE. Hum Reprod Open. 2022;2022(3):hoac022.
Centers for Disease Control and Prevention. 2019 assisted reproductive technology fertility clinic and national summary report. US Dept of Health and Human Services, 2021. Available from: https://archive.cdc.gov/www_cdc_gov/art/reports/2019/pdf/2019-Report-ART-Fertility-Clinic-National-Summary-h.pdf.
Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non-male factor indications: a committee opinion. Fertil Steril. 2020;114(2):239–45.
Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19(4):330–53.
Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015;313(3):255–63.
Giacobbe M, Conatti M, Gomes A, Bonetti TC, Monteleone PA. Effectivity of conventional in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) when male factor is absent: a perspective point of view. JBRA Assist Reprod. 2022;26(1):123–8.
Haahr T, Roque M, Esteves SC, Humaidan P. GnRH agonist trigger and LH activity luteal phase support versus hCG trigger and conventional luteal phase support in fresh embryo transfer IVF/ICSI cycles-a systematic PRISMA review and meta-analysis. Front Endocrinol. 2017;8:116.
Baker VL, Brown MB, Luke B, Conrad KP. Association of number of retrieved oocytes with live birth rate and birth weight: An analysis of 231,815 cycles of in vitro fertilization. Fertil Steril. 2015;103(4):931–938.e2.
Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, Alviggi C. Defining low prognosis patients undergoing assisted reproductive technology: POSEIDON criteria-the why. Front Endocrinol. 2018;9:461.
Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod (Oxford, England). 2016;31(2):370–6.
Haahr T, Esteves SC, Humaidan P. Poor definition of poor-ovarian response results in misleading clinical recommendations. Human Reproduction (Oxford, England). 2018;33(5):979–80.
Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod (Oxford, England). 2015;30(9):2005–8.
Isikoglu M, Ceviren AK, Cetin T, Avci A, Aydinuraz B, Akgul OK, et al. Comparison of ICSI and conventional IVF in non-male factor patients with less than four oocytes. Arch Gynecol Obstet. 2022;306(2):493–9.
Supramaniam PR, Granne I, Ohuma EO, Lim LN, McVeigh E, Venkatakrishnan R, Becker CM, Mittal M. ICSI does not improve reproductive outcomes in autologous ovarian response cycles with non-male factor subfertility. Hum Reprod. 2020;35(3):583–594. https://doi.org/10.1093/humrep/dez301. Erratum in: Hum Reprod. 2021;36(6):1732–1735.
Guo N, Hua X, Li YF, Jin L. Role of ICSI in non-male factor cycles as the number of oocytes retrieved decreases from four to one. Curr Med Sci. 2018;38(1):131–6.
Liu P QJ. Reproductive medicine laboratory technology. Beijing: Peking University Medical Press; 2013. 106–8 p.
Palermo GD, Cohen J, Alikani M, Adler A, Rosenwaks Z. Development and implementation of intracytoplasmic sperm injection (ICSI). Reprod Fertil Dev. 1995;7(2):211–7; discussion 217–8.
Bai F, Wang DY, Fan YJ, Qiu J, Wang L, Dai Y, Song L. Assisted reproductive technology service availability, efficacy and safety in mainland China: 2016. Hum Reprod. 2020;35(2):446–452. https://doi.org/10.1093/humrep/dez245. Erratum in: Hum Reprod. 2020;35(6):1477.
Haddad M, Stewart J, Xie P, Cheung S, Trout A, Keating D, et al. Thoughts on the popularity of ICSI. J Assist Reprod Genet. 2021;38(1):101–23.
Papathanasiou A, Mawal N. The risk of poor ovarian response during repeat IVF. Reprod Biomed Online. 2021;42(4):742–7.
Biliangady R, Kinila P, Pandit R, Tudu NK, Sundhararaj UM, Gopal IST, et al. Are we justified doing routine intracytoplasmic sperm injection in nonmale factor infertility? A retrospective study comparing reproductive outcomes between in vitro fertilization and intracytoplasmic sperm injection in nonmale factor infertility. J Hum Reprod Sci. 2019;12(3):210–5.
Liu H, Zhao H, Yu G, Li M, Ma S, Zhang H, et al. Conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI): Which is preferred for advanced age patients with five or fewer oocytes retrieved? Arch Gynecol Obstet. 2018;297(5):1301–6.
Sfontouris IA, Kolibianakis EM, Lainas GT, Navaratnarajah R, Tarlatzis BC, Lainas TG. Live birth rates using conventional in vitro fertilization compared to intracytoplasmic sperm injection in Bologna poor responders with a single oocyte retrieved. J Assist Reprod Genet. 2015;32(5):691–7.
Drakopoulos P, Garcia-Velasco J, Bosch E, Blockeel C, de Vos M, Santos-Ribeiro S, et al. ICSI does not offer any benefit over conventional IVF across different ovarian response categories in non-male factor infertility: A European multicenter analysis. J Assist Reprod Genet. 2019;36(10):2067–76.
Sustar K, Rozen G, Agresta F, Polyakov A. Use of intracytoplasmic sperm injection (ICSI) in normospermic men may result in lower clinical pregnancy and live birth rates. Aust N Z J Obstet Gynaecol. 2019;59(5):706–11.
Nagy ZP, Liu J, Joris H, Bocken G, Desmet B, Van Ranst H, et al. The influence of the site of sperm deposition and mode of oolemma breakage at intracytoplasmic sperm injection on fertilization and embryo development rates. Hum Reprod (Oxford, England). 1995;10(12):3171–7.
Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, et al. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005;83(6):1635–40.
Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: Influence of the fertilization procedure. Fertil Steril. 2005;83(5):1397–403.
Watanabe H. Risk of chromosomal aberration in spermatozoa during intracytoplasmic sperm injection. J Reprod Dev. 2018;64(5):371–6.
Kato Y, Nagao Y. Changes in sperm motility and capacitation induce chromosomal aberration of the bovine embryo following intracytoplasmic sperm injection. PLoS One. 2015;10(6):e0129285.
Giacobbe M, Conatti M, Gomes A, Bonetti TCS, Monteleone PAA. Effectivity of conventional in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) when male factor is absent: a perspective point of view. JBRA Assist Reprod. 2022;26(1):123–8.
Liu L, Wang H, Li Z, Niu J, Tang R. Obstetric and perinatal outcomes of intracytoplasmic sperm injection versus conventional in vitro fertilization in couples with nonsevere male infertility. Fertil Steril. 2020;114(4):792–800.
Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: A retrospective cohort study. Sci Rep. 2016;6:35141.
Schwarze JE, Jeria R, Crosby J, Villa S, Ortega C, Pommer R. Is there a reason to perform ICSI in the absence of male factor? Lessons from the Latin American registry of ART. Hum Reprod Open. 2017;2017(2):hox013.
Wennerholm UB, Bergh C. Perinatal outcome in children born after assisted reproductive technologies. Upsala J Med Sci. 2020;125(2):158–66.
Supramaniam PR, Mittal M, Ohuma EO, Lim LN, McVeigh E, Granne I, et al. Secondary sex ratio in assisted reproduction: an analysis of 1 376 454 treatment cycles performed in the UK. Hum Reprod Open. 2019;2019(4):hoz020.
Tarín JJ, García-Pérez MA, Hermenegildo C, Cano A. Changes in sex ratio from fertilization to birth in assisted-reproductive-treatment cycles. Reprod Biol Endocrinol. 2014;12:56.
Dean JH, Chapman MG, Sullivan EA. The effect on human sex ratio at birth by assisted reproductive technology (ART) procedures--an assessment of babies born following single embryo transfers, Australia and New Zealand, 2002–2006. BJOG. 2010;117(13):1628–34.
Acknowledgements
We wish to thank all study participants.
Funding
This study was funded by Beijing Science and Technology Planning Project (Z191100006619085); National Key Research and Development Program 2021YFC2700605; National Natural Science Foundation of China 82171632.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Rui Yang, Tian Tian and Dina Jiesisibieke. The first draft of the manuscript was written by Dina Jiesisibieke and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and Consent to participate
This study was approved by the Ethics Committee of the Peking University Third Hospital for data handling (No. IRB00006761-M2020007). All methods were performed in accordance with the relevant guidelines and regulations stipulated in the Declaration of Helsinki.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiesisibieke, D., Tian, T., Zhu, X. et al. Reproductive Outcomes of Conventional In Vitro Fertilization and Intracytoplasmic Sperm Injection in Patients with Non-Severe Male Infertility Across Poor and Different Sub-Optimal Ovarian Response Categories: A Cohort Study Based on 30,352 Fresh Cycles from 2009–2019. Reprod. Sci. 31, 1353–1362 (2024). https://doi.org/10.1007/s43032-023-01444-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01444-0