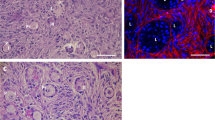
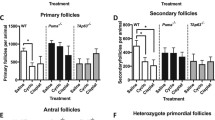
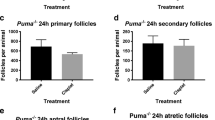
Abstract
Cyclophosphamide (CPM), a part of most cancer treatment regimens, has demonstrated high gonadal toxicity in females. Initially, CPM is believed to damage the ovarian reserve by premature activation of primordial follicles, for the fact that facing CPM damage, primordial oocytes show the activation of PTEN/PI3K/AKT pathways, accompanied by accelerated activation of follicle developmental waves. Meanwhile, primordial follicles are dormant and not considered the target of CPM. However, many researchers have found DNA DSBs and apoptosis within primordial oocytes under CPM-induced ovarian damage instead of premature accelerated activation. A stricter surveillance system of DNA damage is also thought to be in primordial oocytes. So far, the apoptotic death mechanism is considered well-proved, but the premature activation theory is controversial and unacceptable. The connection between the upregulation of PTEN/PI3K/AKT pathways and DNA DSBs and apoptosis within primordial oocytes is also unclear. This review aims to highlight the flaw and/or support of the disputed premature activation theory and the apoptosis mechanism to identify the underlying mechanism of CPM’s injury on ovarian reserve, which is crucial to facilitate the discovery and development of effective ovarian protectants. Ultimately, this review finds no good evidence for follicle activation and strong consistent evidence for apoptosis.



Similar content being viewed by others
Data Availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551.
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–51. https://doi.org/10.3322/caac.21583.
Anderson RA, Brewster DH, Wood R, Nowell S, Fischbacher C, Kelsey TW, Wallace WHB. The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod. 2018;33:1281–90. https://doi.org/10.1093/humrep/dey216.
Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15:587–97. https://doi.org/10.1093/humupd/dmp015.
Anazodo A, Ataman-Millhouse L, Jayasinghe Y, Woodruff TK. Oncofertility-an emerging discipline rather than a special consideration. Pediatr Blood Cancer. 2018;65:e27297. https://doi.org/10.1002/pbc.27297.
Woodruff TK. The emergence of a new interdiscipline: oncofertility. In: Woodruff TK, Snyder KA (eds.) Oncofertility Fertility Preservation for Cancer Survivors. 2007;138:3–11. https://doi.org/10.1007/978-0-387-72293-1_1 .
Xiong J, Xue L, Li Y, Tang W, Chen D, Zhang J, Dai J, Zhou S, Lu Z, Wu M, et al. THERAPY OF ENDOCRINE DISEASE: Novel protection and treatment strategies for chemotherapy-associated ovarian damage. Eur J Endocrinol. 2021;184:R177–92. https://doi.org/10.1530/EJE-20-1178.
Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12:12.
Hanel M, Kroger N, Sonnenberg S, Bornhauser M, Kruger W, Kroschinsky F, Hanel A, Metzner B, Birkmann J, Schmid B, et al. Busulfan, cyclophosphamide, and etoposide as high-dose conditioning regimen in patients with malignant lymphoma. Ann Hematol. 2002;81:96–102. https://doi.org/10.1007/s00277-001-0413-8.
Teske E, Straten G, van Noort R, Rutteman GR. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med. 2002;16:8.
Pennipede D, Mohyuddin GR, Hawkins R, Ganguly S, Shune L, Ahmed N, Mohan MA-O, Cui W, Mahmoudjafari Z, McGuirk J, et al. Carfilzomib, cyclophosphamide, and dexamethasone (KCd) for the treatment of triple-class relapsed/refractory multiple myeloma (RRMM). Eur J Haematol. 2021;107(6):602–8. https://doi.org/10.1111/ejh.13697.
Yimer H, Melear J, Faber E, Bensinger WI, Burke JM, Narang M, Stevens D, Gunawardena S, Lutska Y, Qi K, et al. Daratumumab, bortezomib, cyclophosphamide and dexamethasone in newly diagnosed and relapsed multiple myeloma: LYRA study. Br J Haematol. 2019;185(3):492–502. https://doi.org/10.1111/bjh.15806.
Nakatsukasa K, Koyama H, Oouchi Y, Imanishi S, Mizuta N, Sakaguchi K, Fujita Y, Fujiwara I, Kotani T, Matsuda T, et al. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer. 2017;24:6. https://doi.org/10.1007/s12282-016-0666-7.
Hayashi N, Yagata H, Tsugawa K, Kajiura Y, Yoshida A, Takei J, Yamauchi H, Nakamura S. Response and prognosis of docetaxel and cyclophosphamide as neoadjuvant chemotherapy in ER(+) HER2(-) breast cancer: a prospective phase II study. Clin Breast Cancer. 2020;20:4. https://doi.org/10.1016/j.clbc.2020.09.007.
Madden JA, Keating AF. Ovarian xenobiotic biotransformation enzymes are altered during phosphoramide mustard-induced ovotoxicity. Toxicol Sci. 2014;141:441–52. https://doi.org/10.1093/toxsci/kfu146.
Chemaitilly W, Li Z, Krasin MJ, Brooke RJ, Wilson CL, Green DM, Klosky JL, Barnes N, Clark KL, Farr JB, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab. 2017;102:2242–50. https://doi.org/10.1210/jc.2016-3723.
De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–10. https://doi.org/10.1016/s0140-6736(14)60834-5.
Sonigo C, Beau I, Grynberg M, Binart N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J. 2019;33:1278–87. https://doi.org/10.1096/fj.201801089R.
Zhou L, Xie Y, Li S, Liang Y, Qiu Q, Lin H, Zhang Q. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res. 2017;10:56. https://doi.org/10.1186/s13048-017-0350-3.
Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and “burn-out”, AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra162. https://doi.org/10.1126/scitranslmed.3005402.
Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, Schneider RJ. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci USA. 2017;114:3186–91. https://doi.org/10.1073/pnas.1617233114.
Chen X-Y, Xia H-X, Guan H-Y, Li B, Zhang W. Follicle loss and apoptosis in cyclophosphamide-treated mice: what’s the matter? Int J Mol Sci. 2016;17(6):836. https://doi.org/10.3390/ijms17060836.
Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, Hotte K, Hoffmeister M, Schafer B, De Oliveira T, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018;25:261–9. https://doi.org/10.1038/s41594-018-0035-7.
Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, Mattei M, Candi E, De Felici M, Melino G, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–85. https://doi.org/10.1038/nm.2033.
Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343:533–6. https://doi.org/10.1126/science.1247671.
Luan Y, Edmonds ME, Woodruff TK, Kim S-Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol. 2019;240:243–56. https://doi.org/10.1530/joe-18-0370.
Kano M, Sosulski AE, Zhang L, Saatcioglu HD, Wang D, Nagykery N, Sabatini ME, Gao G, Donahoe PK, Pepin D. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci USA. 2017;114:E1688–97. https://doi.org/10.1073/pnas.1620729114.
Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–8. https://doi.org/10.1038/nature05337.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. https://doi.org/10.1074/jbc.273.10.5858.
Nguyen Q-N, Zerafa N, Liew SH, Morgan FH, Strasser A, Scott CL, Findlay JK, Hickey M, Hutt KJ. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018;9:618. https://doi.org/10.1038/s41419-018-0633-7.
Ganesan S, Keating AF. The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol Appl Pharmacol. 2016;292:65–74. https://doi.org/10.1016/j.taap.2015.12.010.
Nguyen QN, Zerafa N, Liew SH, Findlay JK, Hickey M, Hutt KJ. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol Hum Reprod. 2019;25:433–44. https://doi.org/10.1093/molehr/gaz020.
Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod. 2014;29:107–13. https://doi.org/10.1093/humrep/det391.
Meng Y, Xu Z, Wu F, Chen W, Xie S, Liu J, Huang X, Zhou Y. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil Steril. 2014;102:871–7 e873. https://doi.org/10.1016/j.fertnstert.2014.05.040.
Asadi Azarbaijani B, Sheikhi M, Oskam IC, Nurmio M, Laine T, Tinkanen H, Makinen S, Tanbo TG, Hovatta O, Jahnukainen K. Effect of previous chemotherapy on the quality of cryopreserved human ovarian tissue in vitro. PLoS One. 2015;10:e0133985. https://doi.org/10.1371/journal.pone.0133985.
Lande Y, Fisch B, Tsur A, Farhi J, Prag-Rosenberg R, Ben-Haroush A, Kessler-Icekson G, Zahalka MA, Ludeman SM, Abir R. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod BioMed Online. 2017;34:104–14. https://doi.org/10.1016/j.rbmo.2016.10.005.
Hao X, Anastácio A, Liu K, Rodriguez-Wallberg KA. Ovarian follicle depletion induced by chemotherapy and the investigational stages of potential fertility-protective treatments—a review. Int J Mol Sci. 2019;20:4720–4746. https://doi.org/10.3390/ijms20194720.
Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–3. https://doi.org/10.1126/science.1152257.
Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410. https://doi.org/10.1093/hmg/ddp483.
Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15:765–70. https://doi.org/10.1093/molehr/gap092.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. https://doi.org/10.1038/nrg1879.
Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. https://doi.org/10.1126/science.296.5573.1655.
Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–81. https://doi.org/10.1038/cr.2007.64.
Maidarti M, Anderson RA, Telfer EE. Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the oocyte: implications for primordial follicle activation, oocyte quality and ageing. Cells. 2020;9. https://doi.org/10.3390/cells9010200.
SEIICHI HIROBE, WEI-WU HE, MARY M. LEE, DONAHOE, P.K. Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and sertoli cells coincides with their mitotic activity*. Endocrinology. 1992;131:9.
Titus S, Szymanska KJ, Musul B, Turan V, Taylan E, Garcia-Milian R, Mehta S, Oktay K. Individual-oocyte transcriptomic analysis shows that genotoxic chemotherapy depletes human primordial follicle reserve in vivo by triggering pro-apoptotic pathways without growth activation. Sci Rep. 2021;11:407. https://doi.org/10.1038/s41598-020-79643-x.
Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, Kurita T. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ. 2019;26:502–15. https://doi.org/10.1038/s41418-018-0151-2.
Dupont J, Scaramuzzi RJ. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem J. 2016;473:1483–501. https://doi.org/10.1042/bcj20160124.
Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet. 2014;23:920–8. https://doi.org/10.1093/hmg/ddt486.
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–9. https://doi.org/10.7150/ijms.3635.
Lisio MA, Fu L, Goyeneche A, Gao ZA-O, Telleria CA-O. High-grade serous ovarian cancer: basic sciences, clinical and therapeutic standpoints. Int J Mol Sci. 2019;20:952. https://doi.org/10.3390/ijms20040952.
Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK, et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol Cell. 2012;48:343–52. https://doi.org/10.1016/j.molcel.2012.08.017.
Kerr JB, Hutt KJ, Cook M, Speed TP, Strasser A, Findlay JK, Scott CL. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat Med. 2012;18:1170–2, author reply 1172-1174. https://doi.org/10.1038/nm.2889.
Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra121. https://doi.org/10.1126/scitranslmed.3004925.
Tubbs A, Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 2017;168:644–56. https://doi.org/10.1016/j.cell.2017.01.002.
Liu JC, Xing CH, Xu Y, Pan ZN, Zhang HL, Zhang Y, Sun SC. DEHP exposure to lactating mice affects ovarian hormone production and antral follicle development of offspring. J Hazard Mater. 2021;416:125862. https://doi.org/10.1016/j.jhazmat.2021.125862.
An R, Wang X, Yang L, Zhang J, Wang N, Xu F, Hou Y, Zhang H, Zhang L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. 2021;449:152665. https://doi.org/10.1016/j.tox.2020.152665.
Khedr NF. Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries. Exp Biol Med (Maywood). 2015;240:1682–9. https://doi.org/10.1177/1535370215576304.
Jeelani R, Khan SN, Shaeib F, Kohan-Ghadr HR, Aldhaheri SR, Najafi T, Thakur M, Morris R, Abu-Soud HM. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med. 2017;110:11–8. https://doi.org/10.1016/j.freeradbiomed.2017.05.006.
Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. https://doi.org/10.1016/j.molcel.2012.07.029.
Whitaker AM, Schaich MA, Smith MS, Flynn TS, Freudenthal BD. Base excision repair of oxidative DNA damage: from mechanism to disease. Front Biosci (Landmark Ed). 2017;22:1493–1522. https://doi.org/10.2741/4555.
Reyes GX, Schmidt TT, Kolodner RD, Hombauer H. New insights into the mechanism of DNA mismatch repair. Chromosoma. 2015;124:443–62. https://doi.org/10.1007/s00412-015-0514-0.
Eker AP, Quayle C, Chaves I, van der Horst GT. DNA repair in mammalian cells: direct DNA damage reversal: elegant solutions for nasty problems. Cell Mol Life Sci. 2009;66:968–80. https://doi.org/10.1007/s00018-009-8735-0.
Stringer JM, Winship A, Zerafa N, Wakefield M, Hutt K. Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health. Proc Natl Acad Sci. 2020;117:11513–22. https://doi.org/10.1073/pnas.2001124117.
So S, Davis AJ, Chen DJ. Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. J Cell Biol. 2009;187:977–90. https://doi.org/10.1083/jcb.200906064.
Jungmichel S, Stucki M. MDC1: The art of keeping things in focus. Chromosoma. 2010;119:337–49. https://doi.org/10.1007/s00412-010-0266-9.
Lukas J, Lukas C, Bartek J. More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13:1161–9. https://doi.org/10.1038/ncb2344.
Nazarov IB, Smirnova AN, Krutilina RI, Svetlova MP, Solovjeva LV, Nikiforov AA, Oei S-L, Zalenskaya IA, Yau PM, Bradbury EM, et al. Dephosphorylation of histone γ-H2AX during repair of DNA double-strand breaks in mammalian cells and its inhibition by calyculin A. Radiat Res. 2003;160:309–17. https://doi.org/10.1667/rr3043.
Tomilin NV, Solovjeva LV, Svetlova MP, Pleskach NM, Zalenskaya IA, Yau PM, Bradbury EM. Visualization of focal nuclear sites of DNA repair synthesis induced by bleomycin in human cells. Radiat Res. 2001;156:347–54. https://doi.org/10.1667/0033-7587(2001)156.
Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332:237–48. https://doi.org/10.1016/j.canlet.2012.01.007.
Kujjo LL, Ronningen R, Ross P, Pereira RJ, Rodriguez R, Beyhan Z, Goissis MD, Baumann T, Kagawa W, Camsari C, et al. RAD51 plays a crucial role in halting cell death program induced by ionizing radiation in bovine oocytes. Biol Reprod. 2012;86:76. https://doi.org/10.1095/biolreprod.111.092064.
Kujjo LL, Laine T, Pereira RJ, Kagawa W, Kurumizaka H, Yokoyama S, Perez GI. Enhancing survival of mouse oocytes following chemotherapy or aging by targeting Bax and Rad51. PLoS One. 2010;5:e9204. https://doi.org/10.1371/journal.pone.0009204.
Collins JK, Jones KT. DNA damage responses in mammalian oocytes. Reproduction. 2016;152:R15–22. https://doi.org/10.1530/REP-16-0069.
Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007;67:10159–62. https://doi.org/10.1158/0008-5472.CAN-07-2042.
Pampanini V, Wagner M, Asadi-Azarbaijani B, Oskam IC, Sheikhi M, Sjodin MOD, Lindberg J, Hovatta O, Sahlin L, Bjorvang RD, et al. Impact of first-line cancer treatment on the follicle quality in cryopreserved ovarian samples from girls and young women. Hum Reprod. 2019;34:1674–85. https://doi.org/10.1093/humrep/dez125.
Oktay KH, Bedoschi G, Goldfarb SB, Taylan E, Titus S, Palomaki GE, Cigler T, Robson M, Dickler MN. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency. Fertil Steril. 2020;113:1251–60 e1251. https://doi.org/10.1016/j.fertnstert.2020.01.033.
Bildik G, Akin N, Senbabaoglu F, Sahin GN, Karahuseyinoglu S, Ince U, Taskiran C, Selek U, Yakin K, Guzel Y, et al. GnRH agonist leuprolide acetate does not confer any protection against ovarian damage induced by chemotherapy and radiation in vitro. Hum Reprod. 2015;30(12):2912–25. https://doi.org/10.1093/humrep/dev257.
Amelio I, Grespi F, Annicchiarico-Petruzzelli M, Melino G. p63 the guardian of human reproduction. Cell Cycle. 2012;11:4545–51. https://doi.org/10.4161/cc.22819.
Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G. The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol. 2011;12:259–65. https://doi.org/10.1038/nrm3086.
de la Mata M, Grosshans H. TAp63 as a guardian of female germ line integrity. Nat Struct Mol Biol. 2018;25:195–7. https://doi.org/10.1038/s41594-018-0040-x.
Bursch W, Paffe S, Putz B, Barthel G, Schulte-Hermann R. Determination of the length off the histological stages off apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis. 1990;11:847–53. https://doi.org/10.1093/carcin/11.5.847.
Ji J, Zhang Y, Redon CE, Reinhold WC, Chen AP, Fogli LK, Holbeck SL, Parchment RE, Hollingshead M, Tomaszewski JE, et al. Phosphorylated fraction of H2AX as a measurement for DNA damage in cancer cells and potential applications of a novel assay. PLoS One. 2017;12:e0171582. https://doi.org/10.1371/journal.pone.0171582.
Bellusci G, Mattiello L, Iannizzotto V, Ciccone S, Maiani E, Villani V, Diederich M, Gonfloni S. Kinase-independent inhibition of cyclophosphamide-induced pathways protects the ovarian reserve and prolongs fertility. Cell Death Dis. 2019;10:726. https://doi.org/10.1038/s41419-019-1961-y.
Yang J, Campobasso N, Biju MP, Fisher K, Pan XQ, Cottom J, Galbraith S, Ho T, Zhang H, Hong X, et al. Discovery and characterization of a cell-permeable, small-molecule c-Abl kinase activator that binds to the myristoyl binding site. Chem Biol. 2011;18:177–86. https://doi.org/10.1016/j.chembiol.2010.12.013.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15. https://doi.org/10.1093/humrep/deu353.
McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod. 2014;20:736–44. https://doi.org/10.1093/molehr/gau037.
Maidarti M, Clarkson YL, McLaughlin M, Anderson RA, Telfer EE. Inhibition of PTEN activates bovine non-growing follicles in vitro but increases DNA damage and reduces DNA repair response. Hum Reprod. 2019;34:297–307. https://doi.org/10.1093/humrep/dey354.
Astle MV, Hannan KM, Ng PY, Lee RS, George AJ, Hsu AK, Haupt Y, Hannan RD, Pearson RB. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31:1949–62. https://doi.org/10.1038/onc.2011.394.
Xu N, Hegarat N, Black EJ, Scott MT, Hochegger H, Gillespie DA. Akt/PKB suppresses DNA damage processing and checkpoint activation in late G2. J Cell Biol. 2010;190:297–305. https://doi.org/10.1083/jcb.201003004.
Karimian A, Mir SM, Parsian H, Refieyan S, Mirza-Aghazadeh-Attari M, Yousefi B, Majidinia M. Crosstalk between Phosphoinositide 3-kinase/Akt signaling pathway with DNA damage response and oxidative stress in cancer. J Cell Biochem. 2019;120:10248–72. https://doi.org/10.1002/jcb.28309.
Lai KP, Leong WF, Chau JF, Jia D, Zeng L, Liu H, He L, Hao A, Zhang H, Meek D, et al. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. EMBO J. 2010;29:2994–3006. https://doi.org/10.1038/emboj.2010.166.
Author information
Authors and Affiliations
Contributions
Conceptualization, Qin Xie and Qiuyue Liao, writing—original draft preparation, Qin Xie and Qiuyue Liao, writing—review and editing, Qiuyue Liao, Lingjuan Wang, and Yan Zhang, visualization, Jing Chen and Hualin Bai, supervision, Jihui Ai and Kezhen Li.
Corresponding authors
Ethics declarations
Ethics Approval
This is a comprehensive review. Ethics Committee of the TongJi Hospital has confirmed that no ethical approval is required.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, Q., Liao, Q., Wang, L. et al. The Dominant Mechanism of Cyclophosphamide-Induced Damage to Ovarian Reserve: Premature Activation or Apoptosis of Primordial Follicles?. Reprod. Sci. 31, 30–44 (2024). https://doi.org/10.1007/s43032-023-01294-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01294-w