Abstract
Similar to obstetric outcomes, rates of SARS-CoV-2 (COVID-19) infection are not homogeneously distributed among populations; risk factors accumulate in discrete locations. This study aimed to investigate the geographical correlation between pre-COVID-19 regional preterm birth (PTB) disparities and subsequent COVID-19 disease burden. We performed a retrospective, ecological cohort study of an upstate New York birth certificate database from 2004 to 2018, merged with publicly available community resource data. COVID-19 rates from 2020 were used to allocate ZIP codes to “low-,” “moderate-,” and “high-prevalence” groups, defined by median COVID-19 diagnosis rates. COVID-19 cohorts were associated with poverty and educational attainment data from the US Census Bureau. The dataset was analyzed for the primary outcome of PTB using ANOVA. GIS mapping visualized PTB rates and COVID-19 disease rates by ZIP code. Within 38 ZIP codes, 123,909 births were included. The median COVID-19 infection rate was 616.5 (per 100 K). PTB (all) and COVID-19 were positively correlated, with high- prevalence COVID-19 ZIP codes also being the areas with the highest prevalence of PTB (F = 11.06, P = .0002); significance was also reached for PTB < 28 weeks (F = 15.87, P < .0001) and periviable birth (F = 16.28, P < .0001). Odds of PTB < 28 weeks were significantly higher in the “high-prevalence” COVID-19 cohort compared to the “low-prevalence” COVID 19 cohort (OR 3.27 (95% CI 2.42–4.42)). COVID-19 prevalence was directly associated with number of individuals below poverty level and indirectly associated with median household income and educational attainment. GIS mapping demonstrated ZIP code clustering in the urban center with the highest rates of PTB < 28 weeks overlapping with high COVID-19 disease burden. Historical disparities in social determinants of health, exemplified by PTB outcomes, map community distribution of COVID-19 disease burden. These data should inspire socioeconomic policies supporting economic vibrancy to promote optimal health outcomes across all communities.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The novel coronavirus, SARS-CoV-2 (COVID-19), pandemic has devastated countries worldwide. In the United States (US), as of late June 2021, approximately 48,000,000 cases had been reported, with more than 750,000 cumulative deaths [1]. As data became available and the mechanisms and factors governing community spread were elucidated, it became known that rates of COVID-19 infection were not distributed homogeneously among populations. Similar to obstetrical outcomes, risk factors for COVID-19 accumulate in discrete locations, often due to structural determinants of health, even within geographic confines. Neighborhood-level COVID-19 infection rates [New York City], provided by the New York City Department of Health and Mental Hygiene, unsurprisingly demonstrated higher positivity rates in lower-income areas—an unfortunate trend that persists [2]. Fueled by the presence of structural racism, Black and other minority communities, which have experienced disinvestment for decades, also, are disproportionately impacted by COVID-19 [1, 3,4,5,6].
Racial and ethnic disparities in infant and peripartum mortality in the US clearly pre-date the COVID-19 pandemic [7,8,9,10]. Individuals who identify as Black carry the burden of worse adverse obstetric outcomes, and this disproportion extends across the spectrum of morbidity to include preterm birth (PTB). Non-Hispanic Black individuals experience a preterm birth rate at least 50% higher than non-Hispanic white individuals [11] and rates are comparatively higher in homogeneous geographic communities [12]. Societal factors such as structural racism, influenced, for example, by the legacy of racial discrimination in housing (Home Owner’s Loan Corporation redlining), correlate with modern health inequities, preterm birth being foremost [13, 14]. With propagated structural inequities, PTB continues to remain high in certain ZIP codes.
Assessment of the geographic variation in regional PTB outcomes may provide us with the perspective to better understand communities that are disproportionally at higher risk for COVID-19 infection. Our study, thus, sought to investigate the geographical correlation between pre-COVID-19 regional PTB disparities and subsequent COVID-19 disease burden.
Materials and Methods
This study is a retrospective, ecological cohort study using the Finger Lakes Region Perinatal and Obstetric Data System. The Finger Lakes Region database encompasses a 9-county region and contains birth certificate and supplemental question data that has been collected and processed. Live births from 2004 to 2018 were identified in the city of Rochester, NY, and the eight counties to the south and east of the city, representing a total of 903 ZIP codes. ZIP codes with greater than 100 deliveries during the study period (which, given the noted time frame, excludes ZIP codes with less than 10 births per year) were stratified into cohorts. Publicly available data as described by the US Census Bureau—based on the 2017 American Community Survey [15]—were then merged into the ZIP-stratified dataset to characterize community socioeconomic data including (1) percentage of community members living below the US poverty level ($26,200 for a family of four [16]), (2) median household income for community members, and (3) percentage of community members attaining at least a high school diploma. This fusion facilitated calculation of the frequencies of patient-level obstetric outcomes in each ZIP cohort with retained association between patient-level and community-level socioeconomic information.
We chose to focus our study of COVID-19 within Monroe County, which contains the Rochester city center. The Rochester metropolitan statistical area (MSA) had a population of 1,069,644 as of the 2019 census, the third largest MSA in New York state [15]. Median household income averaged at $62,103, with 41% of household incomes less than $50,000. Approximately 12.7% of individuals have incomes below the poverty line [15]. There are two major healthcare systems encompassing 11 birthing hospitals across Monroe and adjacent counties.
The Monroe County COVID-19 dashboard [16] was utilized to associate encompassed ZIP codes with the cumulative rate of coronavirus diagnosis following the first wave of community spread (March–September 2020). The county-wide distribution of rates was analyzed to generate the median absolute deviation (MAD) of the local infection rates. Distributions of disease concentration in relationship to these local rates were then defined and assigned to “low-” (< 1 MAD), “moderate-” (within 1 MAD), and “high-” (> 1 MAD) prevalence categories of COVID-19 disease burden, normalized to regional rates of infection, rather than infection rates nationally or internationally. ZIP code cohorts were included in the statistical analysis only if they met the obstetric inclusion criteria of greater than 100 births in the study period and had a COVID-19 disease rate available in the dashboard.
The historical ZIP code level cohort of births, categorized by low, medium, and high coronavirus prevalence from March through September 2020 was analyzed for the primary outcome of PTB. Outcomes were analyzed using ANOVA, and odds ratios were calculated to assess the magnitude of disparity. Preterm birth was defined as gestational age of 36 or fewer completed weeks of pregnancy and was further divided into categories of PTB < 28 weeks gestation (i.e., births between 23w0d and 27w6d) and periviable births (i.e., births between 22w0d and 24w6d); groupings were defined by the Statewide Perinatal Data System and were not mutually exclusive. The obstetric data set was collapsed into ZIP code cohorts and was analyzed utilizing Stata 16.0 (StataCorp LLC, College Station, Texas). Graphic representations of the distributions of pandemic and obstetric data were generated in ArcGISPro 2.5.0 (Esri, Redlands, California).
Results
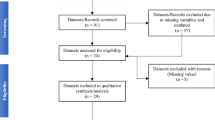
From 2004 until 2018, the birth data system contained detailed information for 210,984 live births in 903 regional ZIP codes. A total of 199,088 pregnancies were eligible for inclusion as being associated with a ZIP code containing greater than 100 deliveries in the study period; this resulted in 120 ZIP code cohorts. Of these births, there were 123,909 within the 38 ZIP codes with publicly available COVID-19 community resource data for Rochester, NY (Monroe County has 42 ZIP codes; 38 ZIP codes were included with both available COVID-19 data and greater than 100 deliveries within the study period, Fig. 1).
Identifying births within COVID-19 cohorts. From 2004 to 2018, the birth data system contained detailed information for 210,984 live births. A total of 199,088 pregnancies were eligible for inclusion as being associated with a ZIP code containing greater than 100 deliveries in the study period; this resulted in 120 ZIP code cohorts. Of these births, there were 123,909 within the 38 ZIP codes with publicly available COVID-19 community resource data for Rochester, New York
The rate of preterm deliveries across all study cohorts was 9.5%. The median COVID-19 infection rate among all Rochester ZIP codes was 616.5 per 100,000 as of September 30, 2020. Preterm birth and COVID-19 were positively correlated, with high-prevalence COVID-19 ZIP codes also being the areas with the highest prevalence of PTB (F = 11.06, P = 0.0002)—8% of all live births in the low-prevalence COVID-19 ZIP codes, 9% of all live births in the moderate-prevalence COVID-19 ZIP codes, and 11% of all live births in the high-prevalence COVID-19 ZIP codes were preterm.
Notably, significance was also demonstrated for PTB < 28 weeks (F = 15.87, P < 0.0001) and periviable birth (F = 16.28, P < 0.0001). Odds of PTB < 28 weeks were significantly higher in the “high-prevalence” COVID-19 cohort compared to the “low-prevalence” COVID 19 cohort (OR 3.27 (95% CI 2.42–4.42)). Percentages of all PTB, PTB < 28 weeks, and periviable birth across “low-,” “moderate-,” and “high-” prevalence COVID-19 cohorts are presented in Fig. 2.
With respect to socioeconomic characteristics—specifically poverty level, median household income, and educational attainment—similar findings were observed in relationship to COVID-19 infection rates. COVID-19 prevalence was positively associated with number of individuals below poverty level and was inversely associated with median household income and educational attainment (Fig. 3).
Notably, geographic information system (GIS) mapping demonstrated ZIP code clustering in the Rochester city center, with highest rates of PTB < 28 weeks overlapping with high COVID-19 disease burden (Fig. 4).
Structured Discussion/Comment
Our data illustrate that community disparities in social determinants of health, as exemplified by preterm birth outcomes, map to geographic areas of COVID-19 disease burden in our region. Historical geographic prevalence of PTB was positively associated with 2020 cohort prevalence of COVID-19, reaching significance not only for all PTB but also for PTB < 28 weeks and periviable births. GIS mapping demonstrated this variance and showed ZIP code clustering in the urban center with highest rates of PTB < 28 weeks overlapping with high COVID-19 disease burden.
This analysis exposes the high rates of both PTB and COVID-19 infection where low-income neighborhoods are concentrated. Assessed within the last 6–7 years, over 50% of Rochester dwellers live in neighborhoods of extreme poverty [17], with individuals identifying as Black and Latinx individuals experiencing poverty at a rate more than three times that of individuals identifying as white [18]. This condensed poverty may be secondary to Rochester’s heritage as a factory town in the mid-twentieth century, which was home to close communities of immigrant blue collar workers. While white immigrants were overall more successful in securing steady employment and income, Black individuals were discriminated against both in terms of employment and housing through redlining and other forms of systemic racism. As higher earning families migrated from the urban center to the suburbs, the urban-suburban schism expanded, and associated racial and economic fissures became more profound [18]. This system of socioeconomic segregation has propagated into the twenty-first century and, likely, has contributed significantly to the specific course of community spread of COVID-19.
Available literature further supports a positive correlation between neighborhood income and physical distancing [19]—those individuals in lower-income neighborhoods were more likely required to work outside the home. State orders for cessation of non-essential work were only associated with small increases in staying home in low-income neighborhoods. COVID-19 infection rates were also found to be higher in neighborhoods that were densely populated, and predominantly Black [6, 20, 21]. Based on his New York City analysis, Whittle et al. suggested that an increase of 10,000 people per km2 was associated with a 2.4% (95% CI 0.6 to 4.2%, P = 0.011) increase in COVID-19 positivity rate [6]. Moreover, in dose–response fashion, a decrease of 10% in the white population was associated with a 1.8% (95% CI 0.8 to 2.8%, P < 0.001) increase in positivity rate, while an increase of 10% in the Black population was associated with a 1.1% (95% CI 0.3 to 1.8%, P < 0.001) increase in positivity rate [6]. These trends may suggest an explanation of why urban clustering may have occurred in the Rochester city center.
Alternatively, outliers to the above discussed trends do exist. Hamlin, for example, is a small, rural, town in the northwest of Monroe County with a widespread ZIP code area and approximately 5000 residents [22]. Rural residence has been consistently linked with elevated preterm birth rates [23], an association that remains significant even after controlling for behavioral risk factors and obstetric characteristics [24, 25]. These considerations are likely applicable to this community and correspond to the increased rates of PTB < 28 weeks observed (Fig. 4). However, a less densely populated ZIP code would, in turn, allow for social distancing in a way that likely prevented widespread COVID-19 infection.
Our analysis of socioeconomic factors demonstrated increased COVID-19 disease burden among those with lower community income levels, higher poverty levels, and lower educational attainment. The persistent association of PTB with increasing COVID-19 burden suggests the disproportionate targeting of a community demographic that is shaped by these, and possibly other, social determinants. As a global indicator of parental and neonatal health, PTB can be utilized as a vital statistic that indicates how well our communities are being served with respect to access to healthcare, among other influences. In suggesting that pre-pandemic regional PTB rates predict COVID-19 distribution, we demonstrate that community disparities unacceptably exist. Ongoing studies seek to explore the geographic correlation of PTB and COVID-19 infection rates over a wide range of communities (i.e., urban, suburban, rural) and assess the influence of other medical and obstetric comorbidities on this correlation. Collectively, these data should inspire socioeconomic policies supporting economic vibrancy to promote optimal health outcomes across all communities.
Our study is unique in that it assesses the geo-spatial association between pre-pandemic PTB rates and COVID-19 infection rates in a mid-sized US city. We included 38 of Monroe County’s 42 ZIP codes, with both available COVID-19 data and greater than 100 deliveries in the study period; inclusion of such a large number of ZIP codes provided for a robust assessment of the city’s geographic disparities. Furthermore, we used publicly available data from the US Census Bureau merged into the ZIP-stratified dataset to characterize community socioeconomic data. This fusion facilitated calculation of the frequencies of patient-level obstetric outcomes in each ZIP cohort with retained association between patient-level and community-level socioeconomic information. In this way, we sought to reduce the influence of ecological fallacy in our study.
Lastly, in choosing to associate ZIP codes with cumulative rates of coronavirus diagnoses (as opposed to current infection rates), we were able to capture a unique snapshot of community disease burden at the close of the first wave of the novel coronavirus pandemic. Analysis of this time frame highlighted in stark contrast the social disparities that emerged during the pandemic’s early months. As these disparities have not been yet fully addressed, our analysis is robust to a changing temporal scale.
Despite application of a novel geo-spatial lens to improve understanding COVID-19 spread, there are limitations to our study. Foremost, our analysis only addressed one mid-sized city. While available literature suggests that these results may be generalizable to other cities with urban centers [6, 19, 20], we are unable to definitively elucidate determinants that may be common across urban, suburban, and rural populations. Additionally, we chose to focus on a particular portion of potential socioeconomic factors that may influence the pandemic and cannot comment on how other parental or obstetric comorbidities may additionally bear on geographic variation of COVID-19 disease burden. Lastly, to clarify, we associated historical preterm birth rates with current COVID-19 disease, not the risk of PTB associated with SARS-CoV-2 infection in a current pregnancy.
In conclusion, merging of COVID-19 community ZIP code datasets into obstetric ZIP code datasets offers a unique opportunity to analyze contemporary, geographic health disparities. We demonstrate that geographic disparities in our regional preterm birth outcomes, ahead of the global pandemic, predicted community distribution of COVID-19 disease burden. Given the distinct impact of the disproportionate burden of both preterm labor and high COVID-19 infection rates, attempts to end these disparities must be multi-faceted and informed by integrated analysis of previously disparate data sets.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Centers for Disease Control and Prevention. COVID-19 data tracker. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed 30 Nov 2021.
New York City Department of Health and Mental Hygiene. Community health profiles. https://www.nyc.gov/site/doh/data/data=publications/profiles.page. Accessed 8 December 2020.
Prevention TCfDCa. Racial/Ethnic disparities in pregnancy-related deaths — United States, 2007–2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/index.html. Accessed 8 December 2020.
Lopez MH, Rainie L, Budiman A. Financial and health impacts of COVID-19 vary widely by race and ethnicity. Pew Research Center. https://www.pewresearch.org/fact-tank/2020/05/05/financial-and-health-impacts-of-covid-19-vary-widely-by-race-and-ethnicity/. Accessed 8 December 2020.
Lynch EE, Malcoe LH, Laurent SE, Richardson J, Mitchell BC, Meier HCS () The legacy of structural racism: Associations between historic redlining, current mortgage lending, and health. SSM Popul Health, 2021;14:100793. https://doi.org/10.1016/j.ssmph.2021.100793
Whittle RS, Diaz-Artiles A. An ecological study of socioeconomic predictors in detection of COVID-19 cases across neighborhoods in New York City. BMC Med. 2020;18:271. https://doi.org/10.1186/s12916-020-01731-6.
Tangel V, White RS, Nachamie AS, Pick JS. Racial and ethnic disparities in maternal outcomes and the disadvantage of peripartum Black women: a multistate analysis, 2007–2014. Am J Perinatol. 2019;36(8):835–48. https://doi.org/10.1055/s-0038-1675207.
Ratnasiri AWG, Parry SS, Arief VN, et al. Temporal trends, patterns, and predictors of preterm birth in California from 2007 to 2016, based on the obstetric estimate of gestational age. Matern Health Neonatol Perinatol. 2018;4:25. https://doi.org/10.1186/s40748-018-0094-0.
Eichelberger KY, Doll K, Ekpo GE, Zerden ML. Black lives matter: claiming a space for evidence-based outrage in obstetrics and gynecology. Am J Public Health. 2016;106(10):1771–2. https://doi.org/10.2105/AJPH.2016.303313.
Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL. Racial and ethnicdisparities in severe maternal morbidity prevalence and trends. Ann Epidemiol. 2019;33:30–6. https://doi.org/10.1016/j.annepidem.2019.02.007.
Grobman WA, Parker CB, Willinger M, et al. Racial disparities in adverse pregnancy outcomes and psychosocial stress. Obstet Gynecol. 2018;131(2):328–35. https://doi.org/10.1097/AOG.0000000000002441.
Jankowska MM, Yang JA, Block J, et al. An online geographic data visualization tool to relate preterm births to environmental factors. Prev Chronic Dis. 2019;16: 180498. https://doi.org/10.5888/pcd16.180498.
Hollenbach SJ, Thornburg LL, Glantz JC, Hill E. Associations between historically redlined districts and current obstetrics outcomes. JAMA Netw Open. 2021;4(9): e2126707. https://doi.org/10.1001/jamanetworkopen.2021.26707.
Chambers BD, Baer RJ, McLemore MR, Jelliffe-Pawlowski LL. Using index of concentration at the extremes as indicators of structural racism to evaluate the association with preterm birth and infant mortality -California, 2011–2012. J Urban Health. 2019;96(2):159–70. https://doi.org/10.1007/s11524-018-0272-4.
National Historical Geographic Information System. download U.S Census data tables & mapping files. https://www.nhgis.org/. Accessed September 1, 2020.
MonroeCounty.gov [online]. https://www.monroecounty.gov/health-COVID-19-archive. Accessed 10 September 2020.
Kneebone E, Holmes N. Brookings Institution: US concentrated poverty in the wake of the great recession. https://www.brookings.edu/research/u-s-concentrated-poverty-in-the-wake-of-the-great-recession/. Accessed 10 September 2020.
Doherty EJ. ACT Rochester and the Rochester area community foundation. Hard facts update: Race and ethnicity in the nine-county greater Rochester area. RACF.org/reports. Accessed 10 December 2020.
Jay J, Bor J, Nsoesie EO, et al. Neighbourhood income and physical distancing during the COVID-19 pandemic in the United States. Nat Hum Behav. 2020. https://doi.org/10.1038/s41562-020-00998-2.
The Annie E. Casey Foundation. Unequal opportunities in education. https://www.aecf.org/m/resourcedoc/aecf-racemattersEDUCATION-2006.pdfpdf iconexternal icon. Accessed 8 December 2020.
Braga JU, Ramos AN, Ferreira AF, Lacerda VM, Freire RMC, Bertoncini BV. Propensity for COVID-19 severe epidemic among the populations of the neighborhoods of Fortaleza, Brazil, in 2020. BMC Public Health. 2020;20:1486. https://doi.org/10.1186/s12889-020-09558-9.
Hamlin. Census.gov. Accessed 19 March 2022.
Amjad S, MacDonald I, Chambers T, Osornio-Vargas A, Chandra S, Voaklander D, Ospina MB. Social determinants of health and adverse maternal and birth outcomes in adolescent pregnancies: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2019;33(1):88–99. https://doi.org/10.1111/ppe.12529.
Leland NL, Petersen DJ, Braddock M, Alexander GR. Variations in pregnancy outcomes by race among 10–14-year-old mothers in the United States. Public Health Rep. 1995;110:53.
Robson S, Cameron CA, Roberts CL. Birth outcomes for teen-age women in New South Wales, 1998–2003. Aust N Z J Obstet Gynaecol. 2006;46:305–10. https://doi.org/10.1111/j.1479-828X.2006.00597.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ponnila Marinescu, Courtney Olson-Chen, J. Christopher Glantz, Elaine Hill, and Stefanie Hollenbach. The first draft of the manuscript was written by Ponnila Marinescu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This retrospective cohort study was approved by the University of Rochester Research Subjects Review Board (RSRB Case Number: RSRB00072789), including a waiver of informed consent consistent with institutional review board approval for use of deidentified data. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marinescu, P.S., Olson-Chen, C., Glantz, J.C. et al. The Geographical Correlation Between Historical Preterm Birth Disparities and COVID-19 Burden. Reprod. Sci. 30, 1343–1349 (2023). https://doi.org/10.1007/s43032-022-01076-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01076-w