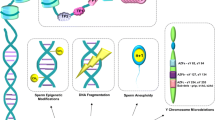
Abstract
Postmortem sperm retrieval for reproductive purposes is an assisted reproduction procedure that offers women an opportunity to have a child using sperm retrieved from their deceased partners. The ethical issues of this procedure have been discussed in previous works. However, an assessment of the procedure using a scientific perspective is still lacking. Here, we aim to ascertain, using a biological standpoint, whether postmortem sperm should be rescued for reproductive purposes. Data suggest that it is premature to use postmortem sperm for reproductive purposes. This procedure should not be clinically applied until appropriate and comprehensive analyses have been completed. Such analyses should be focused not only on fertilization, embryo development, and pregnancy outcomes, but also on potential postmortem alterations of sperm DNA, RNAs, and proteins. In addition, genetic and epigenetic analyses of sperm, pre-implantation embryos, and newborns, as well as mental and physical health follow-up of the resulting offspring during a whole life cycle, using appropriate non-human mammalian models, are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The safety of assisted reproduction techniques (ARTs) is usually assessed after (not before) ARTs are incorporated into a clinical setting. The common end-points typically analyzed in clinical trials and less frequently in randomized controlled trials are fertilization, pre- and post-implantation embryo development, obstetric and perinatal outcomes, and follow-up of the resulting offspring during infancy, childhood, and adolescence. However, before incorporating a new ART into clinical practice, other cellular, molecular, genetic, and epigenetic aspects should be assessed using adequate laboratory and appropriate animal experimentation [1]. Unfortunately, literature shows that this is not the case on most occasions [2,3,4,5,6]. The use of postmortem sperm for reproductive purposes is an example of this praxis.
ART stressors such as gamete/embryo cryopreservation, oocyte in vitro maturation, intracytoplasmic sperm injection (ICSI), in vitro culture system, and embryo biopsy [7], as well as gamete/embryo exposure to suboptimal ART conditions [8], may induce genetic and epigenetic changes in gametes and/or pre-implantation embryos. Likewise, gamete and offspring genetic and epigenetic disturbances may be associated with parental exposure to environmental and occupational xenobiotics [7]. Sperm exposure to the altered body fluids and/or degenerating tissues of dead men may also pose a genetic and/or epigenetic risk for spermatozoa and prospective offspring. Unfortunately, as far as we know, these potential effects have not yet been assessed in men or males of non-human mammalian species.
In the present study, we aim to gather data to support or reject the clinical use of postmortem sperm for reproductive purposes. The study is exclusively focused on biological and clinical data. Readers interested in ethical questions and legal and religious implications of this procedure are referred to several excellent papers dealing with these topics [9,10,11,12,13].
Methods
In order to analyze all the live birth cases reported to date, a literature search was performed to identify all the relevant publications recorded on PubMed from database inception up to October 2021 using the following key words: posthumous/postmortem sperm retrieval, posthumous/postmortem human reproduction, and interval from death to sperm retrieval. References cited in relevant articles were also examined. The literature search was exclusively focused on clinical deaths, not on brain deaths of men on ventilatory support.
Live Births Reported to Date
Table 1 displays the men’s age, cause of death, sperm retrieval schedule, and sperm freezing methodology reported in seven live birth cases of women that underwent standard in vitro fertilization (IVF) and/or ICSI cycles using testicular or epididymal spermatozoa of their deceased husbands. The details of the thirteen oocyte retrieval cycles undergone by these women are shown in Table 2.
The first case described in the literature was a woman that gave birth to a baby girl using the sperm from her deceased husband, who died in 1995 as result of a sudden allergic reaction. This case was reported in the newspaper Los Angeles Times, in March 27, 1999 [14]. The sperm was harvested 30 hours after the husband’s death and frozen for 15 months before the woman’s oocytes were inseminated by ICSI [9, 14]. Likewise, Shefi et al. [15] informed of a case of a woman that gave birth to a healthy female child after undergoing standard IVF and ICSI using epididymal spermatozoa from her deceased husband. As in the live birth disclosed in the Los Angeles Times [14], the interval from death to sperm retrieval was 30 hours and further details of the case were not provided.
Note that the low number of cases and oocyte retrieval cycles undergone by the women analyzed in the present study prevents us from drawing solid conclusions about the potential effect of the interval between the man’s clinical death and sperm retrieval on embryology and pregnancy outcomes. Nevertheless, we have to point out that most of the babies born were girls. In particular, the reported sex ratio is 1:5 (i.e., 16.7% males versus 83.3% females) (in this analysis, we have excluded the full-term baby notified by Check et al. [16] since the sex of the newborn was not specified). Moreover, the four children recorded by Robson et al. [18] displayed an apparently normal development at least up to 1, 4, 5, and 7 years of age, respectively.
The low sex ratio observed, if confirmed after adding future cases, may be a consequence of the psychological disturbance and anxiety likely experienced by women after the death of their husbands, and/or the women’s excitement associated with having made the final decision to fertilize their oocytes using the sperm of their deceased husbands (we should bear in mind that “excitement” is a kind of stress called “eustress” that induces a moderate activation of the sympathetic nervous system, the hypothalamic-pituitary-adrenal axis, and the immune system [19]). Actually, maternal psychological stresses such as depression and anxiety, as well as experiencing stressful life events at the time of conception, are associated with drops in the number of male births in both humans and non-human mammals [20].
An alternative explanation for the preponderance of girls may lie on the fact that human Y spermatozoa under some sperm-storage conditions are more vulnerable to stressful environments than X spermatozoa [21], as may be encountered in the reproductive system of dead men. Nevertheless, we should take into consideration that no significant variation in the sex ratio of 18-day-old mouse fetuses generated after intracytoplasmic injection of cauda epididymis spermatozoa, retrieved from cadavers and maintained at 4°C from 0 to 20 days after death, has been reported [22]. These data suggest that the sex ratio may be unbiased when the number of cases analyzed increases.
Data Against the Current Clinical Use of Postmortem Sperm for Reproductive Purposes
Shefi et al. [15] and Robson et al. [18] proposed to extend the upper 24-h limit of the interval from death to sperm retrieval, previously recommended by Tash et al. [23], to 36 and 48 h, respectively. Their proposal was based on the following facts: (1) Shefi et al. [15] observed that epididymal spermatozoa from a deceased man exhibited 5% motility and 7% viability (determined by vital stain) at 36 h after death; and (2) Robson et al. [18] found that testicular spermatozoa, displaying non-progressive motility at 48 h after death, were able to fertilize 5 out of 10 microinjected oocytes and contributed to the generation of a healthy baby girl. Their proposal, however, did not take into account other studies carried out in non-human mammalian species showing that the interval from death to sperm retrieval as well as the storage conditions, mainly temperature, for both the cadaver and sperm may affect sperm motility [24,25,26,27,28,29], sperm viability [22, 25, 27], fertilization [22], pre- and/or post-implantation embryo development [22, 24], and live birth incidence [27]. The source of spermatozoa (i.e., testes, epididymides, or vasa deferens) is another important factor we should bear in mind. For instance, at an ambient temperature of ≈ 22°C, degenerative changes in mouse testes are observed within 6 h after death. The alterations include disruption of intercellular bridges and sloughing of the germ cells into the lumen of the seminiferous tubules, and increased incidence of pyknotic nuclei in germ cells [24]. In contrast, postmortem degenerative changes in epididymides at the same ambient temperature are not clearly evidenced up to 12 h postmortem. On this occasion, the specific modifications observed in the epididymides are pyknosis of epithelial cells and release of their intracellular contents into the lumen [24].
Although animal models are essential to biological research, it may be argued that not all the results obtained on non-human mammalian species can be directly translated to humans [30]. However, even if we disregard the studies performed in animal models and assume a safe upper limit interval for human sperm retrieval between 24 [23] and 48 h [18], we could not be confident enough yet about the safety of this procedure. In fact, sperm motility and viability, fertilization, pre- and post-implantation embryo development, live birth incidence, and postnatal development from 1 to 7 years of age are not the only parameters that we should rely on to recommend the clinical use of postmortem sperm for reproductive purposes. Other lines of biological evidence demonstrating this procedure is harmless are needed, for instance, the assessment of the potential genetic and epigenetic anomalies in spermatozoa, pre-implantation embryos, and newborns, as well as short-, medium-, and long-term mental and physical health follow-up of the resulting offspring using appropriate animal models. In fact, although there may be differences among various tissues, organs, and genetic markers [31], postmortem degradation of DNA [32, 33] and RNA [31, 34] begins immediately after death because of endogenous nuclease activity and hydrolytic attack. Protein degradation also takes place after death although is slower and more reproducible than degradation of RNAs [35]. Moreover, we should remember that spermatids and spermatozoa may be transcriptionally and translationally active [36, 37]. They likely translate both nuclear and mitochondrial transcripts using mitochondrial ribosomes [36] and a stored heterogeneous population of sperm protein-coding messenger RNAs that may contribute to fertilization and early embryo development [36, 37]. Mature spermatozoa also store long non-coding RNAs (lncRNAs) and small non-coding regulatory RNAs (sncRNAs) including, among others, microRNAs, PIWI interacting RNAs, and transfer RNA-derived small RNAs [37, 38]. Notably, spermatozoa acquire fertilization and normal embryonic development capabilities after the sperm profiles of these lncRNAs and sncRNAs classes are remodeled during epididymal transit [37, 38]. That is to say, in order for spermatozoa to be functionally mature, they must interact with extracellular components secreted by epithelial cells lining the lumen of the epididymis, including not only proteins, DNA, lipids, ions, and nutrients but also lncRNAs and sncRNAs [37, 39].
Importantly, although microRNAs are less susceptible to degradation than other types of RNAs [40, 41], the sperm lncRNAs and sncRNAs profiles exhibit substantial plasticity and may be modified by paternal and sperm exposure to a variety of environmental conditions and stressful environments [37, 39]. Such epigenetic modifications may affect both early embryo development and the subsequent health of the offspring, including altered behavioral and metabolic phenotypes [37, 38]. Likewise, the stressful environment to which spermatozoa are exposed in the reproductive system of dead men may induce adverse long-term effects on offspring.
Conclusion
Several lines of biological evidence suggest that postmortem sperm should not be rescued for reproductive purposes until appropriate and comprehensive analyses have been completed. Such analyses should be focused not only on fertilization, embryo development, and pregnancy outcomes, but also on potential postmortem alterations of sperm DNA, RNAs, and proteins. In addition, genetic and epigenetic analyses of sperm, pre-implantation embryos, and newborns, as well as mental and physical health follow-up of the resulting offspring during a whole life cycle, using appropriate non-human mammalian models, are warranted. These analyses should be performed even taking into account that the clinical demand for this ART procedure is relatively scarce.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. https://doi.org/10.1001/jama.2013.281053.
Evers JLHH. Do we need an RCT for everything? Hum Reprod. 2017;32(3):483–4. https://doi.org/10.1093/humrep/dex003.
Harper J, Jackson E, Sermon K, et al. Adjuncts in the IVF laboratory: where is the evidence for ‘add-on’ interventions? Hum Reprod. 2017;32(3):485–91. https://doi.org/10.1093/humrep/dex004.
Mulder CL, Serrano JB, Catsburg LAE, Roseboom TJ, Repping S, van Pelt AMM. A practical blueprint to systematically study life-long health consequences of novel medically assisted reproductive treatments. Hum Reprod. 2018;33(5):784–92. https://doi.org/10.1093/humrep/dey070.
Trias E, Nijs M, Rugescu IA, et al. Evaluating risk, safety and efficacy of novel reproductive techniques and therapies through the EuroGTP II risk assessment tool. Hum Reprod. 2020;35(8):1821–38. https://doi.org/10.1093/humrep/deaa146.
Chimote BN, Chimote NM. Fertility interventions ‘add-ons’ in clinical ART practice: ethical, moral and commercial considerations. J Assist Reprod Genet. 2021;38(10):2579–80. https://doi.org/10.1007/s10815-021-02288-w.
Tarín JJ, García-Pérez MA, Cano A. Assisted reproductive technology results: why are live-birth percentages so low? Mol Reprod Dev. 2014;81(7):568–83. https://doi.org/10.1002/mrd.22340.
Ramos-Ibeas P, Heras S, Gómez-Redondo I, et al. Embryo responses to stress induced by assisted reproductive technologies. Mol Reprod Dev. 2019;86(10):1292–306. https://doi.org/10.1002/mrd.23119.
Batzer FR, Hurwitz JM, Caplan A. Postmortem parenthood and the need for a protocol with posthumous sperm procurement. Fertil Steril. 2003;79(6):1263–9. https://doi.org/10.1016/s0015-0282(03)00384-4.
Tremellen K, Savulescu J. A discussion supporting presumed consent for posthumous sperm procurement and conception. Reprod BioMed Online. 2015;30(1):6–13. https://doi.org/10.1016/j.rbmo.2014.10.001.
Ethics Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org; Ethics Committee of the American Society for Reproductive Medicine. Posthumous retrieval and use of gametes or embryos: an Ethics Committee opinion. Fertil Steril. 2018;110(1):45–9. https://doi.org/10.1016/j.fertnstert.2018.04.002.
Maddox N. Children of the dead: posthumous conception, critical interests and consent. J Law Med. 2020;27(3):645–62.
Negro F, Beck R, Cotoia A, Varone MC. Posthumous sperm retrieval: a procreative revolution. Med Glas (Zenica). 2021;18(1):114–21. https://doi.org/10.17392/1256-21.
Planchon S. The application of the dead man’s statutes in family law. J Am Acad Matrim Lawyers Lawyers. 2000;16:561–78.
Shefi S, Raviv G, Eisenberg ML, et al. Posthumous sperm retrieval: analysis of time interval to harvest sperm. Hum Reprod. 2006;21(11):2890–3. https://doi.org/10.1093/humrep/del232.
Check ML, Check JH, Summers-Chase D, Choe JK, Check DJ, Nazari A. Live birth after posthumous testicular sperm aspiration and intracytoplasmic sperm injection with cryopreserved sperm: case report. Clin Exp Obstet Gynecol. 2002;29(2):95–6.
Check M, Summers-Chase D, Check JH, Choe J, Nazari A. Sperm extracted and cryopreserved from testes several hours after death results in pregnancy following frozen embryo transfer: case report. Arch Androl. 1999;43(3):235–7. https://doi.org/10.1080/014850199262553.
Robson SJ, Campbell S, McDonald J, Tremellen K, Carlin E, Maybury G. Pregnancy and childhood health and developmental outcomes with the use of posthumous human sperm. Hum Reprod. 2015;30(10):2259–62. https://doi.org/10.1093/humrep/dev191.
Xiao R, Ali S, Caligiuri MA, Cao L. Enhancing effects of environmental enrichment on the functions of natural killer cells in mice. Front Immunol. 2021;12:695859. https://doi.org/10.3389/fimmu.2021.695859.
Navara KJ. Programming of offspring sex ratios by maternal stress in humans: assessment of physiological mechanisms using a comparative approach. J Comp Physiol B. 2010;180(6):785–96. https://doi.org/10.1007/s00360-010-0483-9.
You YA, Kwon WS, Saidur Rahman M, Park YJ, Kim YJ, Pang MG. Sex chromosome-dependent differential viability of human spermatozoa during prolonged incubation. Hum Reprod. 2017;32(6):1183–91. https://doi.org/10.1093/humrep/dex080.
Kishikawa H, Tateno H, Yanagimachi R. Fertility of mouse spermatozoa retrieved from cadavers and maintained at 4 degrees C. J Reprod Fertil. 1999;116(2):217–22. https://doi.org/10.1530/jrf.0.1160217.
Tash JA, Applegarth LD, Kerr SM, Fins JJ, Rosenwaks Z, Schlegel PN. Postmortem sperm retrieval: the effect of instituting guidelines. J Urol. 2003;170(5):1922–5. https://doi.org/10.1097/01.ju.0000092832.37190.94.
Songsasen N, Tong J, Leibo SP. Birth of live mice derived by in vitro fertilization with spermatozoa retrieved up to twenty-four hours after death. J Exp Zool. 1998;280(2):189–96. https://doi.org/10.1002/(sici)1097-010x(19980201)280:2<189::aid-jez10>3.0.co;2-h.
Hishinuma M, Suzuki K, Sekine J. Recovery and cryopreservation of sika deer (Cervus nippon) spermatozoa from epididymides stored at 4 degrees C. Theriogenology. 2003;59(3–4):813–20. https://doi.org/10.1016/s0093-691x(02)01154-8.
Martinez-Pastor F, Guerra C, Kaabi M, et al. Decay of sperm obtained from epididymes of wild ruminants depending on postmortem time. Theriogenology. 2005;63(1):24–40. https://doi.org/10.1016/j.theriogenology.2004.03.003.
Santiago-Moreno J, Toledano-Díaz A, Pulido-Pastor A, Gómez-Brunet A, López-Sebastián A. Birth of live Spanish ibex (Capra pyrenaica hispanica) derived from artificial insemination with epididymal spermatozoa retrieved after death. Theriogenology. 2006;66(2):283–91. https://doi.org/10.1016/j.theriogenology.2005.11.012.
Chatdarong K, Thuwanut P, Suksamai P, Patanatiradaj S, Sangwornrachasup A. Survival of frozen-thawed cat spermatozoa pre-cooled in the epididymides. Reprod Domest Anim. 2009;44(Suppl 2):377–80. https://doi.org/10.1111/j.1439-0531.2009.01412.x.
Roth TL, Stoops MA, Robeck TR, O'Brien JK. Factors impacting the success of post-mortem sperm rescue in the rhinoceros. Anim Reprod Sci. 2016;167:22–30. https://doi.org/10.1016/j.anireprosci.2016.01.019.
Barré-Sinoussi F, Montagutelli X. Animal models are essential to biological research: issues and perspectives. Future Sci OA. 2015;1(4):FSO63. https://doi.org/10.4155/fso.15.63.
Peng D, Lv M, Li Z, et al. Postmortem interval determination using mRNA markers and DNA normalization. Int J Legal Med. 2020;134(1):149–57. https://doi.org/10.1007/s00414-019-02199-7.
Liu L, Shu X, Ren L, et al. Determination of the early time of death by computerized image analysis of DNA degradation: which is the best quantitative indicator of DNA degradation? J Huazhong Univ Sci Technolog Med Sci. 2007;27(4):362–6. https://doi.org/10.1007/s11596-007-0404-7.
Tozzo P, Scrivano S, Sanavio M, Caenazzo L. The role of DNA degradation in the estimation of post-mortem interval: a systematic review of the current literature. Int J Mol Sci. 2020;21(10):3540. https://doi.org/10.3390/ijms21103540.
Scrivano S, Sanavio M, Tozzo P, Caenazzo L. Analysis of RNA in the estimation of post-mortem interval: a review of current evidence. Int J Legal Med. 2019;133(6):1629–40. https://doi.org/10.1007/s00414-019-02125-x.
Lee DG, Yang KE, Hwang JW, et al. Degradation of kidney and psoas muscle proteins as indicators of post-mortem interval in a rat model, with use of lateral flow technology. PLoS One. 2016;11(8):e0160557. https://doi.org/10.1371/journal.pone.0160557.
Ellis PJ, Yu Y, Zhang S. Transcriptional dynamics of the sex chromosomes and the search for offspring sex-specific antigens in sperm. Reproduction. 2011;142(5):609–19. https://doi.org/10.1530/REP-11-0228.
Santiago J, Silva JV, Howl J, Santos MAS, Fardilha M. All you need to know about sperm RNAs. Hum Reprod Update. 2021;28(1):67–91. https://doi.org/10.1093/humupd/dmab034.
Trigg NA, Eamens AL, Nixon B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction. 2019;157(6):R209–23. https://doi.org/10.1530/REP-18-0480.
Chen H, Alves MBR, Belleannée C. Contribution of epididymal epithelial cell functions to sperm epigenetic changes and the health of progeny. Hum Reprod Update. 2021;28(1):51–66. https://doi.org/10.1093/humupd/dmab029.
Tu C, Du T, Shao C, Liu Z, Li L, Shen Y. Evaluating the potential of housekeeping genes, rRNAs, snRNAs, microRNAs and circRNAs as reference genes for the estimation of PMI. Forensic Sci Med Pathol. 2018;14(2):194–201. https://doi.org/10.1007/s12024-018-9973-y.
Maiese A, Scatena A, Costantino A, et al. MicroRNAs as useful tools to estimate time since death. A systematic review of current literature. Diagnostics (Basel). 2021;11(1):64. https://doi.org/10.3390/diagnostics11010064.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Juan J. Tarín conceived and designed the study, and wrote the first draft of the manuscript. All authors contributed to acquisition of data, analysis, and interpretation of data, revised the article critically for important intellectual content, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This article is a review. No animal or human experiment was carried out in this study.
Consent for Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarín, J.J., García-Pérez, M.A. & Cano, A. It Is Premature to Use Postmortem Sperm for Reproductive Purposes: a Data-Driven Opinion. Reprod. Sci. 29, 3387–3393 (2022). https://doi.org/10.1007/s43032-022-00874-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00874-6