Abstract
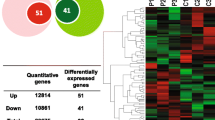
Polycystic ovarian syndrome (PCOS) is often accompanied by overweight/obesity and insulin resistance. The dysfunctions of ovarian granulosa cells (GCs) are closely linked with the pathogenesis of PCOS. Fat mass and obesity-associated gene (FTO), an N6-methyladenosine (m6A) demethylase, has been reported to be implicated in the risks and insulin resistance of PCOS. However, the roles of FTO in the development of GCs along with its m6A-related regulatory mechanisms are poorly defined. Cell proliferative ability was detected by MTT assay. Cell apoptotic rate was measured via flow cytometry. Insulin resistance was assessed by GLUT4 transport potential. The mRNA and protein levels of FTO and flotillin 2 (FLOT2) were determined by RT-qPCR and western blot assays, respectively. FLOT2 was screened out to be a potential FTO target through differential expression analysis for the GSE95728 dataset and target prediction analysis by POSTAR2 and STARBASE databases. The interaction between FTO and FLOT2 was analyzed by RNA immunoprecipitation (RIP) assay. The effect of FTO upregulation on FLOT2 m6A level was measured by methylated RIP (meRIP) assay. FLOT2 mRNA stability was examined by actinomycin D assay. FTO overexpression facilitated cell proliferation, hindered cell apoptosis, and induced insulin resistance in GCs. FTO promoted FLOT2 expression by reducing m6A level on FLOT2 mRNA and increasing FLOT2 mRNA stability. FLOT2 loss weakened the effects of FTO overexpression on cell proliferation/apoptosis and insulin resistance in GCs. FTO induced the dysfunctions of GCs by upregulating FLOT2, suggesting that FTO/FLOT2 might play a role in the pathophysiology of PCOS.





Similar content being viewed by others
Data Availability
The data and material presented in this manuscript is available from the corresponding author on reasonable request.
References
Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132:321–36.
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841–55.
Neven ACH, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med. 2018;36:5–12.
Louwers YV, Laven JSE. Characteristics of polycystic ovary syndrome throughout life. Ther Adv Reprod Health. 2020;14:2633494120911038.
Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet. 2019;12:249–60.
Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34:272–7.
Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–84.
Tu J, Chen Y, Li Z, Yang H, Chen H, Yu Z. Long non-coding RNAs in ovarian granulosa cells. J Ovarian Res. 2020;13:1–12.
Wei D, Xie J, Yin B, Hao H, Song X, Liu Q, et al. Significantly lengthened telomere in granulosa cells from women with polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. 2017;34:861–6.
Baumgarten SC, Armouti M, Ko C, Stocco C. IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology. 2017;158:2309–18.
Yi S, Zheng B, Zhu Y, Cai Y, Sun H, Zhou J. Melatonin ameliorates excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS. Am J Physiol Endocrinol Metab. 2020;319:E91–e101.
Bhardwaj J, Sharma R. Apoptosis and ovarian follicular atresia in mammals. Zoology. 2012:185–206.
Bhardwaj J, Sharma R. Scanning electron microscopic changes in granulosa cells during follicular atresia in Caprine ovary. Scanning. 2011;33:21–4.
Sharma R, Bhardwaj J. Granulosa cell apoptosis in situ in caprine ovary. Cell Tissue Res. 2007;7:1111–4.
Bhardwaj JK, Saraf P. Morphological attributes of granulosa cells perpetuating functional integrity of an ovarian follicle. J Adv Microsc Res. 2017;12:92–6.
Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498:110561–70.
Balen A. The pathophysiology of polycystic ovary syndrome: trying to understand PCOS and its endocrinology. Best Pract Res Clin Obstet Gynaecol. 2004;18:685–706.
Barber TM, Hanson P. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health. 2019;13:1179558119874042–50.
Jeanes YM, Reeves S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: diagnostic and methodological challenges. Nutr Res Rev. 2017;30:97–105.
Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism. 2019;92:108–20.
Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–21.
Chen J, Du B. Novel positioning from obesity to cancer: FTO, an m(6)A RNA demethylase, regulates tumour progression. J Cancer Res Clin Oncol. 2019;145:19–29.
Deng X, Su R, Stanford S, Chen J. Critical enzymatic functions of FTO in obesity and cancer. Front Endocrinol (Lausanne). 2018;9:396–402.
Lan N, Lu Y, Zhang Y, Pu S, Xi H, Nie X, et al. FTO - a common genetic basis for obesity and cancer. Front Genet. 2020;11:559138–48.
Liu Y, Chen Y. Fat mass and obesity associated gene polymorphism and the risk of polycystic ovary syndrome: a meta-analysis. Iran J Public Health. 2017;46:4–11.
Liu AL, Xie HJ, Xie HY, Liu J, Yin J, Hu JS, et al. Association between fat mass and obesity associated (FTO) gene rs9939609 A/T polymorphism and polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med Genet. 2017;18:89–95.
Wang X, Wang K, Yan J, Wu M. A meta-analysis on associations of FTO, MTHFR and TCF7L2 polymorphisms with polycystic ovary syndrome. Genomics. 2020;112:1516–21.
Kowalska I, Malecki MT, Straczkowski M, Skupien J, Karczewska-Kupczewska M, Nikolajuk A, et al. The FTO gene modifies weight, fat mass and insulin sensitivity in women with polycystic ovary syndrome, where its role may be larger than in other phenotypes. Diabetes Metab. 2009;35:328–31.
Tan S, Scherag A, Janssen OE, Hahn S, Lahner H, Dietz T, et al. Large effects on body mass index and insulin resistance of fat mass and obesity associated gene (FTO) variants in patients with polycystic ovary syndrome (PCOS). BMC Med Genet. 2010;11:12–20.
Zhang S, Deng W, Liu Q, Wang P, Yang W, Ni W. Altered m(6) A modification is involved in up-regulated expression of FOXO3 in luteinized granulosa cells of non-obese polycystic ovary syndrome patients. J Cell Mol Med. 2020;24:11874–82.
Fang X, Li M, Yu T, Liu G, Wang J. Reversible N6-methyladenosine of RNA: The regulatory mechanisms on gene expression and implications in physiology and pathology. Genes Dis. 2020;7:585–97.
Dai D, Wang H, Zhu L, Jin H, Wang X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 2018;9:124–36.
Karthiya R, Khandelia P. m6A RNA methylation: ramifications for gene expression and human health. 2020;62:467–84.
Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, et al. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512:479–85.
Ma D, Liu X, Zhang JJ, Zhao JJ, Xiong YJ, Chang Q, et al. Vascular smooth muscle fto promotes aortic dissecting aneurysms via m6A modification of Klf5. Front Cardiovasc Med. 2020;7:592550–9.
Pan W, Liu L, Wei J, Ge Y, Zhang J, Chen H, et al. A functional lncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol Carcinog. 2016;55:90–6.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–8.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3:1101–8.
Tan J, Guo L. Swimming intervention alleviates insulin resistance and chronic inflammation in metabolic syndrome. Exp Ther Med. 2019;17:57–62.
Sullivan WJ, Mullen PJ, Schmid EW, Flores A, Momcilovic M, Sharpley MS, et al. Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell. 2018;175:117–32.
Ma HP, Ming LG, Ge BF, Zhai YK, Song P, Xian CJ, et al. Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J Cell Biochem. 2011;112:916–23.
Huang D, Zhang Y, Qi Y, Chen C, Ji W. Global DNA hypomethylation, rather than reactive oxygen species (ROS), a potential facilitator of cadmium-stimulated K562 cell proliferation. Toxicol Lett. 2008;179:43–7.
Gao X, Zhang X, Hu J, Xu X, Zuo Y, Wang Y, et al. Aconitine induces apoptosis in H9c2 cardiac cells via mitochondria-mediated pathway. Mol Med Rep. 2018;17:284–92.
Liao Y, Wang Z, Wang L, Lin Y, Ye Z, Zeng X, et al. MicroRNA-27a-3p directly targets FosB to regulate cell proliferation, apoptosis, and inflammation responses in immunoglobulin a nephropathy. Biochem Biophys Res Commun. 2020;529:1124–30.
Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–7.
Zhu Y, Xu G, Yang YT, Xu Z, Chen X, Shi B, et al. POSTAR2: deciphering the post-transcriptional regulatory logics. Nucleic Acids Res. 2019;47:D203–11.
Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293–9.
Kang MH, Jeong KJ, Kim WY, Lee HJ, Gong G, Suh N, et al. Musashi RNA-binding protein 2 regulates estrogen receptor 1 function in breast cancer. Oncogene. 2017;36:1745–52.
Wang LJ, Xue Y, Li H, Huo R, Yan Z, Wang J, et al. Wilms’ tumour 1-associating protein inhibits endothelial cell angiogenesis by m6A-dependent epigenetic silencing of desmoplakin in brain arteriovenous malformation. 2020;24:4981–91.
Wang D, Du X, Li Y, Li Q. A polymorphism in the transcriptional regulatory region strongly influences ovine FSHR mRNA decay. 2019;54:83–90.
Liu YD, Li Y, Feng SX, Ye DS, Chen X, Zhou XY, et al. Long noncoding RNAs: potential regulators involved in the pathogenesis of polycystic ovary syndrome. Endocrinology. 2017;158:3890–9.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800.
Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, et al. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20:373–81.
Erion KA, Corkey BE. Hyperinsulinemia: a cause of obesity? Curr Obes Rep. 2017;6:178–86.
Freeman AM, Pennings N. Insulin resistance. In: StatPearls. StatPearls Publishing, Treasure Island (FL). 2020.
Sakumoto T, Tokunaga Y, Tanaka H, Nohara M, Motegi E, Shinkawa T, et al. Insulin resistance/hyperinsulinemia and reproductive disorders in infertile women. Reprod Med Biol. 2010;9:185–90.
Sharma R, Bhardwaj J. In situ evaluation of granulosa cells during apoptosis in caprine ovary. Int J Integr Biol. 2009;5:58–67.
Bhardwaj J, Saraf P. Influence of toxic chemicals on female reproduction: a review. Cell Biol Res Ther. 2014;3:1–10.
Bhardwaj JK, Mittal M, Saraf P, Kumari P. Pesticides induced oxidative stress and female infertility: a review. Toxin Rev. 2018;39:1–13.
Lai Q, Xiang W, Li Q, Zhang H, Li Y, Zhu G, et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front Med. 2018;12:518–24.
Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–7.
Moghetti P. Insulin resistance and polycystic ovary syndrome. Curr Pharm Des. 2016;22:5526–34.
Mohan SS, Perry JJ, Poulose N, Nair BG, Anilkumar G. Homology modeling of GLUT4, an insulin regulated facilitated glucose transporter and docking studies with ATP and its inhibitors. J Biomol Struct Dyn. 2009;26:455–64.
Klip A, McGraw TE, James DE. Thirty sweet years of GLUT4. J Biol Chem. 2019;294:11369–81.
Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic. 2011;12:672–81.
McNay EC, Pearson-Leary J. GluT4: A central player in hippocampal memory and brain insulin resistance. Exp Neurol. 2020;323:113076–106.
Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annu Rev Biochem. 2012;81:507–32.
Stöckli J, Fazakerley DJ, James DE. GLUT4 exocytosis. J Cell Sci. 2011;124:4147–59.
Bryant NJ, Gould GW. Insulin stimulated GLUT4 translocation - size is not everything! Curr Opin Cell Biol. 2020;65:28–34.
Zhang C, Hu J, Wang W, Sun Y, Sun K. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB J. 2020;34:9563–74.
Rice S, Pellatt LJ, Bryan SJ, Whitehead SA, Mason HD. Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J Clin Endocrinol Metab. 2011;96:E427–35.
Chen SH, Liu XN. MicroRNA-351 eases insulin resistance and liver gluconeogenesis via the PI3K/AKT pathway by inhibiting FLOT2 in mice of gestational diabetes mellitus. J Cell Mol Med. 2019;23:5895–906.
Banning A, Tomasovic A, Tikkanen R. Functional aspects of membrane association of reggie/flotillin proteins. Curr Protein Pept Sci. 2011;12:725–35.
Zhao F, Zhang J, Liu YS, Li L, He YL. Research advances on flotillins. Virol J. 2011;8:479–85.
Kwiatkowska K, Matveichuk OV, Fronk J, Ciesielska A. Flotillins: At the intersection of protein S-palmitoylation and lipid-mediated signaling. Int J Mol Sci. 2020;21:2283–305.
Galazis N, Afxentiou T, Xenophontos M, Diamanti-Kandarakis E, Atiomo W. Proteomic biomarkers of type 2 diabetes mellitus risk in women with polycystic ovary syndrome. Eur J Endocrinol. 2013;168:R33–43.
Fecchi K, Volonte D, Hezel MP, Schmeck K, Galbiati F. Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J. 2006;20:705–7.
Acknowledgements
I would like to express my gratitude to all those who have helped me during the writing of the article. I gratefully acknowledge the help of Natural Science Foundation of Science and Technology Department of Jilin Province (grant number 20200201476JC, Jilin, China) that funded our research. Also, I would like to thank Mr. Li Zhou, Xiao Han, Wei Li, Ning Wang, Lan Yao, Yunhe Zhao, and Liqun Zhang, who contributed to the research work.
Code Availability
Not applicable.
Funding
This research was supported by Natural Science Foundation of Science and Technology Department of Jilin Province (grant number 20200201476JC, Jilin, China).
Author information
Authors and Affiliations
Contributions
Li Zhou and Xiao Han designed and performed the experiments, and wrote the manuscript. Wei Li, Ning Wang, and Lan Yao contributed to experimental work and data analysis. Yunhe Zhao conducted the experiments. Liqun Zhang revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., Han, X., Li, W. et al. N6-methyladenosine Demethylase FTO Induces the Dysfunctions of Ovarian Granulosa Cells by Upregulating Flotillin 2. Reprod. Sci. 29, 1305–1315 (2022). https://doi.org/10.1007/s43032-021-00664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00664-6