Abstract
Kallmann syndrome (KS) is a rare hereditary disease with high phenotypic and genetic heterogeneity. Congenital hypogonadotropic hypogonadism and hyposmia/anosmia are the two major characterized phenotypes of KS. Besides, mirror movements, dental agenesis, digital bone abnormalities, unilateral renal agenesis, midline facial defects, hearing loss, and eye movement abnormalities can also be observed in KS patients. Because of the phenotypic heterogeneity, genetic diagnosis become increasingly valuable to distinguish KS from other disorders including normosmic congenital hypogonadotropic hypogonadism, constitutional delay of growth and puberty, CHARGE syndrome, and functional hypogonadotropic hypogonadism. Application of next-generation sequencing has promoted the discovery of novel pathogenic genes in KS pedigrees. Prenatal diagnosis is an effective method in clinical settings to decrease birth defects and block transmission of genetic disorders. However, pregnant women may suffer from physical and psychological distress when fetuses are diagnosed with congenital defects. Preimplantation genetic testing (PGT) is a prospective approach during the in vitro fertilization process that helps to interrupt transmission of hereditary diseases to offspring at an early stage. Thus, genetic testing and counseling are recommended to KS patients with family histories, prenatal diagnosis and PGT are considered to be useful options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kallmann syndrome (KS) is a hereditary disease characterized by congenital hypogonadotropic hypogonadism (CHH) and hyposmia or anosmia. Isolated gonadotropin-releasing hormone (GnRH) deficiency (IGD) is a common disease with genetic and clinical heterogeneity, and KS is the main component of IGD. The estimated incidence of KS in males (1:30,000) is 4–5 times higher than in females (1:125,000), but the latter is probably underestimated because affected women tend to have milder symptoms than affected men [1]. KS can be sporadic or familial, and approximately 60% of cases present in a sporadic pattern. KS patients exhibit high phenotypic and genotypic heterogeneity, and thus genetic testing provides crucial evidence for diagnosis and guidance of treatment. Approximately 40% of KS patients have one or several rare sequence variants that have been identified [2], while the remaining cases have no identified genetic cause. KS was traditionally considered as a monogenic disorder, but in recent years, the monogenic paradigm was challenged with the digenic and oligogenic modes following the advanced understanding of KS, indicating that KS is not strictly a monogenic Mendelian disease. Therefore, although most pathogenic genes identified in KS follow autosomal dominant, autosomal recessive, or X-linked inheritance patterns, a small number of the genes also inherited in digenic and oligogenic modes [3]. Infertility is one of the problems affecting normal life in male KS patients, and the majority of male patients tend to seek for help for reproductive treatments. Owing to the hereditary nature of KS, genetic counseling, and hereditary risk evaluation are recommended. Prenatal screening/diagnosis is an effective method in clinical settings to decrease birth defects and block transmission of genetic disorders. However, pregnant women suffer from physical and psychological distress when fetuses are diagnosed with KS, and they experience induced abortion. With the deepening of related research, advances have been made in explorations of molecular genetics in KS, especially after the invention of next generation sequencing (NGS). The emergence of preimplantation genetic testing (PGT) has provided families affected by hereditary disorders (including KS) with an optional approach to have healthy offspring. This review summarizes the advances in KS, especially its genetic diagnosis, and genetic interruption of KS in clinical settings.
General Description of KS and Advances in Genetic Diagnosis
Discovery and Causes of KS
CHH and hyposmia or anosmia are the two typical symptoms of KS. The correlation between anosmia and small testes in individuals was first noted by a Spanish pathologist, Aureliano Maestre de San Juan, in 1856 [4]. In 1944, American geneticist Franz Jozef Kallmann first described KS in three families and suggested a hereditary background [5].
GnRH or luteinizing hormone-releasing hormone (LHRH), a decapeptide, is essential for mammalian puberty onset and reproduction. The reproductive function is mainly managed by 1200–1500 GnRH neurons, and these unique neurons have either an olfactory placode/ectodermal or a neural crest cell origin [6,7,8]. GnRH is produced by the arcuate nucleus in the hypothalamus and delivered to the anterior pituitary through pituitary portal blood vessels, where it pulsatively stimulates the synthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) that participate in gonadal maturation and function in both men and women [9, 10].
In 1989, Schwanzel-Fukuda et al. [11] and Wray et al. [12] proved that GnRH neurons were unusual neuroendocrine cells derived from progenitor cells outside the central nervous system in the medial olfactory placode that migrated across the nasal septum, entered the forebrain with the nervus terminalis, and finally arched into the septal-preoptic area and hypothalamus. Several years later, similar spatiotemporal migration and development patterns of GnRH neurons were confirmed in early human embryos [13, 14]. KS, a neuronal migration defect in humans, is caused by disruption of olfactory axon development and GnRH neuron migration. LHRH-expressing cells were found to be absent in the brain of KS fetuses, although dense clusters of LHRH cells and fibers were present in the nose [15]. The developmental relationship between the GnRH system and the olfactory system illustrates some of the pathogenesis and clinical symptoms in KS patients.
The hypothalamic-pituitary-gonadal (HPG) axis is activated from the mid-trimester, restrained at the end of gestation, and reactivated after birth. In early childhood (6 months of age in boys; 3–4 years of age in girls), GnRH pulsatile secretion is actively inhibited and persists until adolescence [16]. Reactivation of GnRH release after this quiescent period marks the initiation of puberty [16], but the mechanisms involved in triggering of puberty remain unclear. During puberty, GnRH regulates the synthesis and release of FSH and LH. In boys, FSH promotes proliferation of immature Sertoli cells and spermatogonia, while LH stimulates Leydig cells to produce testosterone, leading to initiation of spermatogenesis [17]. In girls, FSH and LH are crucial for follicular maturation and ovulation.
Frequent and Rare Clinical Symptoms of KS
CHH and hyposmia or anosmia are the most typical characteristics of KS. Because HPG axis activity and function fluctuate during the lifespan, the axis provides a good time-window for diagnosis of KS. Based on the influence of GnRH participation in different periods of growth and development in humans, the symptoms of KS can be summarized into three stages: (1) neonatal period and childhood, (2) adolescence, and (3) adulthood. Neonatal period and childhood In male infants, cryptorchidism and micropenis (stretched penile length <2.5 cm) [18, 19] are the common signs of GnRH deficiency during the neonatal period and childhood [20]. In GnRH-deficient female neonates, no specific clinical signs are present at this stage. Adolescence Because of their low GnRH levels, KS patients show absent or partial pubertal development in adolescence. In male adolescents, absent and/or minimal virilization, low libido, and lack of sexual function are the common symptoms [20]. Absence of breast development and/or primary amenorrhea are the most common complaints in female adolescents [20]. Adulthood KS diagnosis in adulthood is mainly associated with infertility and sometimes with early appearance of osteoporotic fractures [20, 21]. Some male KS patients suffer from increased breasts or no beard, which cause psychological problems and affect their normal life. For female KS adults, besides the two characteristic symptoms (CHH and hyposmia/anosmia), congenital heart disease (including fatigue, dyspnea, cyanosis, palpitations, syncope) and neurologic manifestation (including hearing defect, epilepsy, paraplegia) may be found in a minority of females with KS, but the symptoms in females are milder than males.
Besides the symptoms mentioned above, small numbers of KS patients show synkinesia (mirror movements), dental agenesis, digital bone abnormalities (polydactyly, syndactyly, campylodactyly), unilateral renal agenesis or urinary tract malformation, midline facial defects (cleft lip/palate), hearing loss, eye movement abnormalities, poor balance through cerebellar ataxia, skeletal anomalies (scoliosis, polydactyly, clinodactyly), and pigmentation defects [22,23,24,25].
The psychological problems of KS patients are non-ignorable, especially among male adolescents, and include depression, anxiety, and decreased quality of life that leads to low self-esteem and other effects [26]. Compared with control patients, male CHH patients were reported to have significantly high scores in a series of test scales, including the Beck Depression Inventory (BDI), Beck Anxiety Inventory, and Arizona Sexual Experiences (ASEX), indicating noteworthy psychological problems [26]. After 6 months of hormone replacement therapy (HRT), the BDI and ASEX scores and psychological symptoms were improved [26]. Because of the self-contempt, KS patients may hesitate to communicate with others, particularly in adolescents who are suffering from emotional fluctuation. Thus, besides medical treatments for painful physical symptoms, psychological care is an indispensable component of the treatment strategy for KS patients.
Diagnosis of KS
In the first several months after birth, transient activation of the HPG axis is defined as “mini-puberty” [27]. During mini-puberty, testosterone is increased in both boys and girls, and girls also have increased estradiol. Such mini-puberty is observed at 1–3 months of age for the diagnosis of IGD. Besides mini-puberty, IGD is mostly diagnosed during adolescence or early adulthood.
The clinical diagnosis of KS includes evaluation of CHH and hyposmia or anosmia. The diagnosis of KS can rely on reproductive symptoms, such as history of cryptorchidism and/or micropenis [28], presence of arrested sexual maturation and/or lack of secondary sexual characteristics, diminished libido, and infertility [25]. Because of the unreliability of self-reporting of a normal sense of smell, formal smell tests are necessary for evaluation of hyposmia or anosmia [29, 30]. Besides the two most common symptoms, some KS patients have other organ complaints such as bimanual synkinesia, dental agenesis, digital bone abnormalities, unilateral renal agenesis or urinary tract malformation, hearing loss, skeletal anomalies, pigmentation defects, and eye movement abnormalities [25, 28].
Hormonal analysis provides useful indicators for KS diagnosis. Male and female KS patients both have very low plasma gonadotrophin, FSH, LH, and inhibin B levels. During mini-puberty in particular, serum levels of testosterone (male infants) and FSH and LH (male and female infants) show transient increases that are extremely low in infants with CHH [31].
Meanwhile, some brain changes have been noted in KS patients. Magnetic resonance imaging examination can show abnormalities in the olfactory fossae, such as unilateral or bilateral olfactory bulb or tract agenesis, reduced depth, reduced curvature, or increased cortical thickness within the olfactory sulcus in KS patients [30, 32, 33]. However, a minority of KS patients with confirmed anosmia show the normal olfactory structure. The anosmia or hyposmia in these patients may be caused by other reasons such as viral infections, trauma, or drug-induced smell disturbance.
Genetic Diagnosis of KS
Although the majority of KS cases are sporadic, many cases are clearly familial, and more than 30 mutated genes in KS patients have been identified to date [20]. These genes either act alone (autosomal dominant [AD], autosomal recessive [AR], and X-linked inheritance patterns) or in combination (digenic or oligogenic modes) [3]. The mutated genes are summarized below.
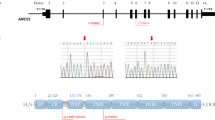
ANOS1 (also known as KAL1), the first and most common pathogenic gene for KS identified in 1991, is inherited through the X-linked recessive mode [34]. ANOS1, located on Xp22.3 and consisting of 14 exons, encodes the protein anosmin-1, a transient and regionally restricted extracellular adhesion protein that participates in GnRH neuron adhesion and axonal migration during organogenesis [34, 35]. Anosmin-1 is expressed from the early stage of pregnancy in the region of the olfactory bulb [36]. Nucleotide insertions and deletions are the main mutation types detected in ANOS1 and cause frameshifts or premature termination codons during protein translation process [37]. A 19-week human male fetus with a deletion in ANOS1 showed disconnection of olfactory, vomeronasal, and terminalis nerve fibers with the brain, and GnRH neurons were unable to migrate to their normal position in the brain [15, 36]. ANOS1 mutations are found in 10%–20% of all KS patients, including 30%–60% of familial cases and 10%–15% of sporadic cases [38,39,40].
FGFR1 was reported to be mutated in KS families by Dodé et al. in 2003, and FGFR1 mutations are the second most common gene mutations found in KS patients. FGFR1 encodes fibroblast growth factor receptor 1 (FGFR1), which belongs to the receptor tyrosine kinase superfamily [41, 42]. The incidence of FGFR1 mutations in KS patients is ~10% [37]. FGFR1 is located on chromosome 8p11.23 and follows the AD/AR/oligogenic inheritance modes. FGFR1 consists of an extracellular domain (three immunoglobulin-like domains that determine the affinity and specificity for its ligands), a transmembrane helix domain, and an intracellular tyrosine kinase domain [43]. FGFR1 mutations include missense, nonsense, splice site, frameshift mutations and intragenic insertions or deletions, and the phenotypes arising from these different mutations are heterogeneous [43,44,45]. FGFR1 mutations are associated with delayed puberty, anosmia, cleft palate, mirror movements, dental agenesis, and bimanual synkinesia, although some familial cases have males and females with FGFR1 mutations who are asymptomatic carriers [42, 46,47,48]. There is no experimental evidence for a potential dominant-negative effect of mutated receptors on wild-type FGFR1, and thus it is speculated that the phenotypes mainly result from haploinsufficiency [43]. Surprisingly, a KS patient with an FGFR1 mutation was reported to show a reversible condition after several years of testosterone treatment, and the same mutation was found in his mother and maternal grandfather [45]. However, the mechanisms behind the FGFR1 mutation dominant and recessive forms in KS remain unclear.
PROKR2 and PROK2 encode the G-protein-coupled receptor prokineticin receptor 2 (PROKR2) and its ligand prokineticin-2 (PROK2), respectively, and the PROKR2/PROK2 pathway plays essential roles during olfactory bulb development and GnRH neuronal progenitor migration and differentiation [49]. PROKR2 and PROK2 mutations can be heterozygous, homozygous, or compound heterozygous, and the prevalence of these mutations in KS patients is about 5%–10% [37, 50]. Prokr2 or Prok2 knockout mice were found to show hypoplasia of the olfactory bulb and reproductive system, without the normal architecture [51, 52]. KS patients who carry loss-of-function mutations in these two genes show different degrees of CHH and hyposmia or anosmia, coincident with the findings in mice [50, 52, 53].
CHD7 located on chromosome 8q12 consists of 38 exons, and encodes chromodomain helicase DNA-binding protein 7 (CHD7). Mutations of CHD7 were identified in patients with CHARGE syndrome, KS, and CHH [54]. Although CHD7 was originally considered to be the causative gene for CHARGE syndrome, subsequent research showed that CHD7 mutations can also be detected in KS patients lacking mutations in KAL1, FGFR1, PROK2, and PROKR2 [55]. The prevalence of CHD7 mutations in all KS/nIHH patients is predicted to be >5% [56, 57].
As well as the major genes described above, the development of NGS has led to the identification of many other pathogenic genes in KS patients during the last several decades. The majority of the mutated genes identified to date participate in GnRH neuron development and migration or secretion processes. FGF8/FGFR1, SOX2, CHD7, FGF17, and IL17RD are involved in the GnRH neuron fate specification process [20], while ANOS1, PROK2/PROKR2, SEMA3A/PLXNA1, SEMA3E, NSMF (NELF), HS6ST1, WDR11, SOX10, FEZF1, IGSF10, DCC/NTN1, TUBB3, and SMCHD1 are associated with olfactory axon guidance, GnRH neuron migration, or axon projection [46] (Table 1).
In addition to the classical Mendelian inheritance pattern, digenic and oligogenic modes have been described in KS patients [58]. First mentioned in 1994, digenic or oligogenic transmission refers to two or more than two synergic deleterious gene mutations in an individual that contribute to a disease phenotype [59]. Digenic and oligogenic inheritance modes were first reported by Dodé et al. in a KS patient with mutations in both PROKR2 (p.L173R) and ANOS1 (p.S396L) [50]. Application of NGS has promoted the discovery of digenic and oligogenic inherited genes in KS patients, which also increases the complexity for genetic counseling.
Differential Diagnosis of KS
Despite the diagnostic criteria mentioned above, the key point for KS diagnosis is to clarify the differential diagnoses. The main differential diagnoses of KS include tumors caused by acquired hypogonadotropic hypogonadism, constitutional delay of growth and puberty (CDGP), CHARGE syndrome, and functional hypogonadotropic hypogonadism [28, 60, 61]. Among these differential diagnoses, CDGP is the most challenging, because KS and CDGP are both associated with delayed puberty. Delayed puberty is defined as delayed puberty onset or progression by >2–2.5SD compared with the population mean, including absence of testicular enlargement (<4 mL) at 14 years of age in boys and absent breast development at 13 years of age in girls [62]. Differences in testicular size, presence of cryptorchidism and/or micropenis, CHH-associated phenotypes, and genetic testing can provide partial diagnostic evidence to distinguish between KS and CDGP [28].
Normosmic congenital hypogonadotropic hypogonadism (nCHH) shares similar phenotypes with KS, including bimanual synkinesia, renal anomalies, facial cleft, dental agenesis, eye movement disorder, and hearing loss, although the prevalences of these abnormalities are more common in KS than in nCHH [63, 64]. Thus, it is difficult to distinguish between nCHH and KS based on clinical phenotypes alone. Genetic analyses have a crucial impact on diagnosis for the two diseases. Some overlapping genes have been identified between nCHH and KS, including FGFR1, FGF8, FGF17, NSMF, SPRY4, IL17RD, DUSP6, GLCE, FLRT3, PROK2/PROKR2, HS6ST1, HESX1, CHD7, KLB, PLXNA1, WDR11, DCC, NTN1, SEMA7A, and SEMA3E [63]. Besides these overlapping genes, OTUD4, RNF216, POLR3A, POLR3B, PNPLA6, PITX2, STUB1, SOX2, SOX3, OTX2, PROP1, PCSK1, LHB, FSHB, LHX4, LHX3, DMXL2, GNRH1, GNRHR, GLI2, GATA2, IGSF10, KISS1/KISSR1, TAC3/TACR3, LEP/LEPR, TBX3, NR0B1, and CCDC141 gene mutations were only reported in nCHH [63]. The combination of clinical symptoms and genetic testing could improve the accuracy of diagnosis to some extent, especially for the patients without overlapping genes. However, overlapping gene mutations may require further explorations for the patients with confusing clinical symptoms. The different clinical manifestations may be related to the variation types of the genes, variations at different gene functional domains, the digenic and oligogenic modes, and even the environmental influence.
Adult-onset hypogonadotropic hypogonadism (AHH) occurs in healthy adult males who have completed normal puberty and have proven fertility [65]. AHH can be caused by anatomic etiologies, infiltrative diseases, space-occupying lesions, other central nervous system tumors, and genetic disorders [66, 67]. The rare DNA variants identified in AHH patients include mutations in GNRHR, FGF8, and PROKR2 [66], which are also found in KS patients.
CHARGE syndrome is a multisystem disorder that includes symptoms of Coloboma, Heart defect, Atresia of choanae, Retardation of growth and/or development, Genital hypoplasia, and Ear anomalies [68]. CHARGE syndrome tends to be sporadic with an incidence of around 1/10,000, but can sometimes show an AD inheritance mode [68]. Mutations in CHD7 are identified in >75% of patients with CHARGE syndrome. KS and nIHH were hypothesized to be milder allelic variants of CHARGE syndrome [69]. KS and CHARGE syndrome share some similar symptoms and common genetic mutations, such as olfactory abnormalities, hearing loss, genital hypoplasia, cleft lip and palate, hand anomalies, and renal anomalies [68, 70]. Therefore, it is important to distinguish KS and CHARGE syndrome using both clinical symptoms and genetic testing.
Treatments for KS
Early diagnosis and treatment of KS are crucial, because they can lead to improved quality of life and prevention of disease-related complications, especially during adolescence as the vital period for secondary sexual maturation and functioning and the critical point for adolescent psychological development. As mentioned before, CHH and hyposmia/anosmia are the two main symptoms of KS, especially CHH causes the most disturbed symptom in KS patients’ life. Surgical operative is the first choice for newborns with cryptorchidism at 6–12 months. HRT promotes virilization, development of muscle mass and strength, pubertal growth spurt, deepening of voice, growth of penis, libido, and sexual function in males [20] and furthers development of breast in females. HRT could also improve skeletal maturation and bone mass density, self-confidence, and well-being in both genders. It should be noted that HRT prescriptions are different depending on the main goal and age of KS patients. Low dose of testosterone is used to induce penis growth of male newborns after surgery. For adolescent males lacking puberty, sex steroid therapy (testosterone in KS male patients) or gonadotropin (including human chorionic gonadotropin (hCG) and follicle-stimulating hormone (FSH)) is used for pubertal induction and development of normal secondary sexual characteristics [20]. To recover the fertility in KS adult males, pulsatile GnRH and gonadotropin are used for treatment. In adolescent girls, 17β-estradiol is administrated to induce puberty, and progesterone is added during the last 14 days of menstrual cycle after breakthrough bleed or full breast development [28, 71]. Estrogen-progesterone therapy is also recommended for the KS females without fertility intention [71]. For the women with fertility desire, pulsatile GnRH/gonadotropin treatment is suggested to mimic physiological condition [71, 72]. After timely and appropriate HRT, KS patients can develop secondary sexual characteristics, maintain normal sex hormone levels, lead a healthy sexual life, and achieve fertility. KS patients generally require lifelong treatment; surprisingly, however, 10–20% patients show spontaneous reversal of reproductive function, although subsequent relapse can occur [73, 74].
Although current treatments markedly improve quality of life in KS patients, the genetic characteristics of KS mean that there is some risk of transmitting the mutated genes to offspring and presenting severe symptoms.
Advances in Genetic Interruption for KS in Clinical Settings
Prenatal Diagnosis of KS
Prenatal screening/diagnosis is an effective method in clinical settings to decrease birth defects and block transmission of genetic disorders. Different methods can be used, including maternal serological screening markers, fetal chromosome karyotype analysis, fetal ultrasound, and non-invasive prenatal testing [75]. KS fetuses can be identified through prenatal diagnosis, such as the case of a male fetus with X-linked recessive chondrodysplasia punctata, steroid sulphatase deficiency, X-linked KS, and chromosome deletion at Xp22.31 [76]. Ultrasound showed nasal hypoplasia with a depressed nasal bridge, short septum, and deep groove between the tip of the nose and the alae nasi, before termination of pregnancy at 19 weeks. Autopsy further showed the absence of bilateral olfactory bulbs and tracts in the brain. Although prenatal diagnosis during pregnancy can help to decrease birth defects, pregnant women suffer from physical and psychological distress when fetuses are diagnosed with congenital defects.
Perspectives for PGT Application in KS Patients
Although the majority of infertile male and female patients with KS have a good reproductive prognosis after appropriate HRT or through assisted reproductive technologies (ART), all KS patients who seek fertility should receive counseling and discuss with their doctors or genetic specialists about the risk of transmitting the disease to their offspring [20, 58, 77]. Considering the genetic characteristics, risks of transmission to their offspring, and possibility of causing defects, KS patients and their families are recommended to evaluate these risks and receive genetic counseling.
In recent years, besides Sanger sequencing and other traditional methods used for PGT, the booming development of high-throughput NGS technology had led to its common use in PGT, providing improved resolution and increased genetic information and reducing the cost and time required for sequencing [78]. Multiple Annealing and Looping-Based Amplification Cycles (MALBAC) is a new whole-genome amplification method which uses quasi-linear preamplification to reduce the bias associated with nonlinear amplification and can achieve 93% genome coverage at ≥1× for a single human cell at 25× mean sequencing [79, 80]. Besides improvements in the MALBAC technology, its combination with linkage analyses has provided a cost-effective method in clinical settings, called Mutated Allele Revealed by Sequencing with Aneuploidy and Linkage Analyses (MARSALA) [81]. MARSALA can achieve simultaneous detection of variant sites and chromosomal abnormalities and linkage analysis of embryos in a single procedure and reduce the amplification bias [80,81,82]. Owing to its advantages of multiple-level genome proofreading, high accuracy, comprehensive analysis, low cost, and easy operation, MARSALA-PGT is commonly used in clinical settings [81]. To date, MARSALA has been successfully used in PGT for AR, AD, and X-linked recessive disorders, including beta-thalassemia [82], spinal muscular atrophy [83], polycystic kidney disease [84], primary open angle glaucoma [85], Marfan syndrome [86], and retinitis pigmentosa [87].
In 2019, Chan et al. reported the first live birth of a term baby girl delivered into a family wherein the husband had KS with anosmia and absence of secondary sexual characteristics [88]. An FGFR1 heterozygous variation (valine to isoleucine) was found in the male patient and his father, but his father was not affected by KS, and the variation was considered most likely to represent a polymorphism. This male KS patient received treatment with human chorionic gonadotropin and human menopausal gonadotropin, ICSI (due to low sperm count), and PGT for aneuploidy (PGT-A) [88]. After two cycles of euploid embryo transfer, his wife eventually achieved pregnancy. Because of the uncertainty regarding the pathogenic gene, PGT for monogenic disease (PGT-M) was not performed.
In this reported case, only 30 KS- or CHH-related genes were tested, and the FGFR1 mutation was found in both the patient and his father. However, the father did not show any symptoms of KS, which may be related to incomplete penetrance and variable expressivity among different individuals or oligogenicity. For this family, whole-exon sequencing would be required to identify other potential pathogenic genes. Thus far, genetic causes can be determined in 50–60% of all KS cases, which is vital for diagnosis and fertility prognosis of KS patients, and PGT has become an optional approach to block transmission among generations.
With the current improvements in its efficiency and security, PGT has become a useful approach for familial inherited diseases to prevent transmission of pathogenic genes to the following generation. However, thus far, KS patients have very rarely chosen to acquire their babies through PGT procedures in clinical settings. This may arise because the majority of male KS patients can produce sperm and acquire offspring after a regular period of HRT, the symptoms are mild in some KS patients, or there is economic pressure. The majority of KS patients choose to acquire their offspring by natural fertilization or IVF after HRT. For patients with family histories, genetic testing and counseling are recommended to establish the mutated genes, associations between pathogenic genes and phenotypes, and inheritance patterns. In families with severe inherited symptoms, such as congenital heart defect, hearing loss, and cleft lip/palate, PGT can be an optional approach to avoid offspring suffering from KS. However, in some cases, there are some limitations and challenges for genetic counseling caused by incomplete penetrance, and oligogenicity for known and unknown genes. Therefore, PGT is a perspective genetic interruption method for KS. Both methods have their advantages and disadvantages, so that it is difficult to evaluate which one is obviously better than the other. For PND, fetal samples are obtained by invasive methods (such as chorionic sampling, amniocentesis, and umbilical vein puncture) for genetic prenatal diagnosis to find whether the fetus carries genetic abnormalities. But the pregnant woman may suffer from psychological stress if the fetus has been diagnosed with KS. While, PGT can avoid such a situation by selecting and transfer an embryo without genetic abnormalities, but OHSS may occur during PGT procedures, and the biopsy procedure is invasive and may be harmful to the embryos.
The current clinical management and genetic interruption perspective of KS are summarized in Fig. 1.
In conclusion, KS is a rare hereditary disease with high phenotypic and genetic heterogeneity. In the last decade, pathogenic genes have gradually been identified in KS patients. Although appropriate HRT or ART can help infertile male and female patients to achieve a good reproductive prognosis, their offspring still have risks of suffering from KS. Although a prenatal diagnosis can detect congenital defects during pregnancy, pregnant women may suffer from physical and psychological distress, especially when the fetus is found to be carrying genetic defects. Meanwhile, with the advances and application of NGS and single-cell amplification technologies, as well as improvements in safety and accuracy, PGT has helped thousands of families with hereditary diseases to obtain a healthy next generation. Although no successful case with genetic interruption of PGT to KS patients was reported until now, genetic counseling is an essential component for KS patients who seek fertility, and it can be an imaginable outlook that PGT becomes an optional approach in the near future to decrease the risk of transmitting the disease of KS to their offspring.
References
Laitinen EM, Vaaralahti K, Tommiska J, Eklund E, Tervaniemi M, Valanne L, et al. Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet J Rare Dis. 2011;6:41. https://doi.org/10.1186/1750-1172-6-41.
Stamou MI, Cox KH, Crowley WF Jr. Discovering genes essential to the hypothalamic regulation of human reproduction using a human disease model: adjusting to life in the "-Omics" era. Endocr Rev. 2016;2016(1):4–22. https://doi.org/10.1210/er.2015-1045.2016.110.1210/er.2015-1045.2016.1.test.
Waldstreicher J, Seminara SB, Jameson JL, Geyer A, Nachtigall LB, Boepple PA, et al. The genetic and clinical heterogeneity of gonadotropin-releasing hormone deficiency in the human. J Clin Endocrinol Metab. 1996;81(12):4388–95. https://doi.org/10.1210/jcem.81.12.8954047.
Cariboni A, Maggi R. Kallmann's syndrome, a neuronal migration defect. Cell Mol Life Sci. 2006;63(21):2512–26. https://doi.org/10.1007/s00018-005-5604-3.
Dode C, Hardelin JP. Kallmann syndrome. Eur J Hum Genet. 2009;17(2):139–46. https://doi.org/10.1038/ejhg.2008.206.
Murdoch B, DelConte C, Garcia-Castro MI. Embryonic Pax7-expressing progenitors contribute multiple cell types to the postnatal olfactory epithelium. J Neurosci. 2010;30(28):9523–32. https://doi.org/10.1523/JNEUROSCI.0867-10.2010.
Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, et al. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2(4):392–403. https://doi.org/10.1016/j.stem.2008.03.005.
Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31(18):6915–27. https://doi.org/10.1523/JNEUROSCI.6087-10.2011.
Naftolin F, Harris GW, Bobrow M. Effect of purified luteinizing hormone releasing factor on normal and hypogonadotrophic anosmic men. Nature. 1971;232(5311):496–7. https://doi.org/10.1038/232496a0.
Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31(4):544–77. https://doi.org/10.1210/er.2009-0023.
Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161–4. https://doi.org/10.1038/338161a0.
Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86(20):8132–6. https://doi.org/10.1073/pnas.86.20.8132.
Schwanzel-Fukuda M, Crossin KL, Pfaff DW, Bouloux PM, Hardelin JP, Petit C. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol. 1996;366(3):547–57. https://doi.org/10.1002/(SICI)1096-9861(19960311)366:3<547::AID-CNE12>3.0.CO;2-M.
Casoni F, Malone SA, Belle M, Luzzati F, Collier F, Allet C, et al. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development. 2016;143(21):3969–81. https://doi.org/10.1242/dev.139444.
Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6(4):311–26. https://doi.org/10.1016/0169-328x(89)90076-4.
Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254–64. https://doi.org/10.1016/S2213-8587(15)00418-0.
Koskenniemi JJ, Virtanen HE, Toppari J. Testicular growth and development in puberty. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):215–24. https://doi.org/10.1097/MED.0000000000000339.
Feldman KW, Smith DW. Fetal phallic growth and penile standards for newborn male infants. J Pediatr. 1975;86(3):395–8. https://doi.org/10.1016/s0022-3476(75)80969-3.
Bin-Abbas B, Conte FA, Grumbach MM, Kaplan SL. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. J Pediatr. 1999;134(5):579–83. https://doi.org/10.1016/s0022-3476(99)70244-1.
Boehm U, Bouloux PM, Dattani MT, de Roux N, Dode C, Dunkel L, et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11(9):547–64. https://doi.org/10.1038/nrendo.2015.112.
Fahy UM, Cahill DJ, Wardle PG, Hull MG. Osteoporotic fractures in young amenorrheic women. Acta Obstet Gynecol Scand. 1994;73(5):417–9. https://doi.org/10.3109/00016349409006255.
Krams M, Quinton R, Ashburner J, Friston KJ, Frackowiak RS, Bouloux PM, et al. Kallmann's syndrome: mirror movements associated with bilateral corticospinal tract hypertrophy. Neurology. 1999;52(4):816–22. https://doi.org/10.1212/wnl.52.4.816.
Bailleul-Forestier I, Gros C, Zenaty D, Bennaceur S, Leger J, de Roux N. Dental agenesis in Kallmann syndrome individuals with FGFR1 mutations. Int J Paediatr Dent. 2010;20(4):305–12. https://doi.org/10.1111/j.1365-263X.2010.01056.x.
Molsted K, Kjaer I, Giwercman A, Vesterhauge S, Skakkebaek NE. Craniofacial morphology in patients with Kallmann's syndrome with and without cleft lip and palate. Cleft Palate Craniofac J. 1997;34(5):417–24. https://doi.org/10.1597/1545-1569_1997_034_0417_cmipwk_2.3.co_2.
Costa-Barbosa FA, Balasubramanian R, Keefe KW, Shaw ND, Al-Tassan N, Plummer L, et al. Prioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypes. J Clin Endocrinol Metab. 2013;98(5):E943–53. https://doi.org/10.1210/jc.2012-4116.
Aydogan U, Aydogdu A, Akbulut H, Sonmez A, Yuksel S, Basaran Y, et al. Increased frequency of anxiety, depression, quality of life and sexual life in young hypogonadotropic hypogonadal males and impacts of testosterone replacement therapy on these conditions. Endocr J. 2012;59(12):1099–105. https://doi.org/10.1507/endocrj.ej12-0134.
Copeland KC, Chernausek S. Mini-puberty and growth. Pediatrics. 2016;138(1). https://doi.org/10.1542/peds.2016-1301.
Young J, Xu C, Papadakis GE, Acierno JS, Maione L, Hietamaki J, et al. Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev. 2019;40(2):669–710. https://doi.org/10.1210/er.2018-00116.
Doty RL. Office procedures for quantitative assessment of olfactory function. Am J Rhinol. 2007;21(4):460–73. https://doi.org/10.2500/ajr.2007.21.3043.
Lewkowitz-Shpuntoff HM, Hughes VA, Plummer L, Au MG, Doty RL, Seminara SB, et al. Olfactory phenotypic spectrum in idiopathic hypogonadotropic hypogonadism: pathophysiological and genetic implications. J Clin Endocrinol Metab. 2012;97(1):E136–44. https://doi.org/10.1210/jc.2011-2041.
Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab. 2005;90(5):3122–7. https://doi.org/10.1210/jc.2004-2465.
Manara R, Salvalaggio A, Favaro A, Palumbo V, Citton V, Elefante A, et al. Brain changes in Kallmann syndrome. AJNR Am J Neuroradiol. 2014;35(9):1700–6. https://doi.org/10.3174/ajnr.A3946.
Hacquart T, Ltaief-Boudrigua A, Jeannerod C, Hannoun S, Raverot G, Pugeat M, et al. Reconsidering olfactory bulb magnetic resonance patterns in Kallmann syndrome. Ann Endocrinol (Paris). 2017;78(5):455–61. https://doi.org/10.1016/j.ando.2016.12.003.
Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353(6344):529–36. https://doi.org/10.1038/353529a0.
Hamada AJ, Esteves SC, Agarwal A. A comprehensive review of genetics and genetic testing in azoospermia. Clinics (Sao Paulo). 2013;68(Suppl 1):39–60. https://doi.org/10.6061/clinics/2013(sup01)06.
Hardelin JP, Julliard AK, Moniot B, Soussi-Yanicostas N, Verney C, Schwanzel-Fukuda M, et al. Anosmin-1 is a regionally restricted component of basement membranes and interstitial matrices during organogenesis: implications for the developmental anomalies of X chromosome-linked Kallmann syndrome. Dev Dyn. 1999;215(1):26–44. https://doi.org/10.1002/(SICI)1097-0177(199905)215:1<26::AID-DVDY4>3.0.CO;2-D.
Bianco SD, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5(10):569–76. https://doi.org/10.1038/nrendo.2009.177.
Georgopoulos NA, Pralong FP, Seidman CE, Seidman JG, Crowley WF Jr, Vallejo M. Genetic heterogeneity evidenced by low incidence of KAL-1 gene mutations in sporadic cases of gonadotropin-releasing hormone deficiency. J Clin Endocrinol Metab. 1997;82(1):213–7. https://doi.org/10.1210/jcem.82.1.3692.
Pedersen-White JR, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of intragenic deletions in patients with idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Mol Hum Reprod. 2008;14(6):367–70. https://doi.org/10.1093/molehr/gan027.
Nie M, Xu H, Chen R, Mao J, Wang X, Xiong S, et al. Analysis of genetic and clinical characteristics of a Chinese Kallmann syndrome cohort with ANOS1 mutations. Eur J Endocrinol. 2017;177(4):389–98. https://doi.org/10.1530/EJE-17-0335.
Simon MA. Receptor tyrosine kinases: specific outcomes from general signals. Cell. 2000;103(1):13–5. https://doi.org/10.1016/s0092-8674(00)00100-8.
Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33(4):463–5. https://doi.org/10.1038/ng1122.
Villanueva C, de Roux N. FGFR1 mutations in Kallmann syndrome. Front Horm Res. 2010;39:51–61. https://doi.org/10.1159/000312693.
Pitteloud N, Acierno JS Jr, Meysing A, Eliseenkova AV, Ma J, Ibrahimi OA, et al. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2006;103(16):6281–6. https://doi.org/10.1073/pnas.0600962103.
Pitteloud N, Acierno JS Jr, Meysing AU, Dwyer AA, Hayes FJ, Crowley WF Jr. Reversible kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab. 2005;90(3):1317–22. https://doi.org/10.1210/jc.2004-1361.
Topaloglu AK. Update on the genetics of idiopathic hypogonadotropic hypogonadism. J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):113–22. https://doi.org/10.4274/jcrpe.2017.S010.
Bachler M, Neubuser A. Expression of members of the Fgf family and their receptors during midfacial development. Mech Dev. 2001;100(2):313–6. https://doi.org/10.1016/s0925-4773(00)00518-9.
Sato N, Katsumata N, Kagami M, Hasegawa T, Hori N, Kawakita S, et al. Clinical assessment and mutation analysis of Kallmann syndrome 1 (KAL1) and fibroblast growth factor receptor 1 (FGFR1, or KAL2) in five families and 18 sporadic patients. J Clin Endocrinol Metab. 2004;89(3):1079–88. https://doi.org/10.1210/jc.2003-030476.
Prosser HM, Bradley A, Caldwell MA. Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci. 2007;26(12):3339–44. https://doi.org/10.1111/j.1460-9568.2007.05958.x.
Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2(10):e175. https://doi.org/10.1371/journal.pgen.0020175.
Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308(5730):1923–7. https://doi.org/10.1126/science.1112103.
Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103(11):4140–5. https://doi.org/10.1073/pnas.0508881103.
Abreu AP, Trarbach EB, de Castro M, Frade Costa EM, Versiani B, Matias Baptista MT, et al. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J Clin Endocrinol Metab. 2008;93(10):4113–8. https://doi.org/10.1210/jc.2008-0958.
Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36(9):955–7. https://doi.org/10.1038/ng1407.
Jongmans MC, van Ravenswaaij-Arts CM, Pitteloud N, Ogata T, Sato N. Claahsen-van der Grinten HL et al. CHD7 mutations in patients initially diagnosed with Kallmann syndrome—the clinical overlap with CHARGE syndrome. Clin Genet. 2009;75(1):65–71. https://doi.org/10.1111/j.1399-0004.2008.01107.x.
Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH, et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2008;83(4):511–9. https://doi.org/10.1016/j.ajhg.2008.09.005.
Marcos S, Sarfati J, Leroy C, Fouveaut C, Parent P, Metz C, et al. The prevalence of CHD7 missense versus truncating mutations is higher in patients with Kallmann syndrome than in typical CHARGE patients. J Clin Endocrinol Metab. 2014;99(10):E2138–43. https://doi.org/10.1210/jc.2014-2110.
Maione L, Dwyer AA, Francou B, Guiochon-Mantel A, Binart N, Bouligand J, et al. GENETICS IN ENDOCRINOLOGY: genetic counseling for congenital hypogonadotropic hypogonadism and Kallmann syndrome: new challenges in the era of oligogenism and next-generation sequencing. Eur J Endocrinol. 2018;178(3):R55–80. https://doi.org/10.1530/EJE-17-0749.
Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264(5165):1604–8. https://doi.org/10.1126/science.8202715.
Xu C, Cassatella D, van der Sloot AM, Quinton R, Hauschild M, De Geyter C, et al. Evaluating CHARGE syndrome in congenital hypogonadotropic hypogonadism patients harboring CHD7 variants. Genet Med. 2018;20(8):872–81. https://doi.org/10.1038/gim.2017.197.
Bozzola M, Bozzola E, Montalbano C, Stamati FA, Ferrara P, Villani A. Delayed puberty versus hypogonadism: a challenge for the pediatrician. Ann Pediatr Endocrinol Metab. 2018;23(2):57–61. https://doi.org/10.6065/apem.2018.23.2.57.
Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366(5):443–53. https://doi.org/10.1056/NEJMcp1109290.
Cangiano B, Swee DS, Quinton R, Bonomi M. Genetics of congenital hypogonadotropic hypogonadism: peculiarities and phenotype of an oligogenic disease. Hum Genet. 2020. https://doi.org/10.1007/s00439-020-02147-1.
Quinton R, Duke VM, Robertson A, Kirk JM, Matfin G, de Zoysa PA, et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin Endocrinol (Oxf). 2001;55(2):163–74. https://doi.org/10.1046/j.1365-2265.2001.01277.x.
Nachtigall LB, Boepple PA, Pralong FP, Crowley WF Jr. Adult-onset idiopathic hypogonadotropic hypogonadism—a treatable form of male infertility. N Engl J Med. 1997;336(6):410–5. https://doi.org/10.1056/NEJM199702063360604.
Dwyer AA, Hayes FJ, Plummer L, Pitteloud N, Crowley WF Jr. The long-term clinical follow-up and natural history of men with adult-onset idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2010;95(9):4235–43. https://doi.org/10.1210/jc.2010-0245.
Stamou MI, Georgopoulos NA. Kallmann syndrome: phenotype and genotype of hypogonadotropic hypogonadism. Metabolism. 2018;86:124–34. https://doi.org/10.1016/j.metabol.2017.10.012.
Blake KD, Prasad C. CHARGE syndrome. Orphanet J Rare Dis. 2006;1:34. https://doi.org/10.1186/1750-1172-1-34.
Topaloglu AK, Kotan LD. Genetics of hypogonadotropic hypogonadism. Endocr Dev. 2016;29:36–49. https://doi.org/10.1159/000438841.
Bergman JE, Janssen N, Hoefsloot LH, Jongmans MC, Hofstra RM, van Ravenswaaij-Arts CM. CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J Med Genet. 2011;48(5):334–42. https://doi.org/10.1136/jmg.2010.087106.
Meczekalski B, Podfigurna-Stopa A, Smolarczyk R, Katulski K, Genazzani AR. Kallmann syndrome in women: from genes to diagnosis and treatment. Gynecol Endocrinol. 2013;29(4):296–300. https://doi.org/10.3109/09513590.2012.752459.
Martin K, Santoro N, Hall J, Filicori M, Wierman M, Crowley WF, Jr. Clinical review 15: Management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J Clin Endocrinol Metab. 1990;71(5):1081A-G. doi:10.1210/jcem-71-5-1081.
Sidhoum VF, Chan YM, Lippincott MF, Balasubramanian R, Quinton R, Plummer L, et al. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99(3):861–70. https://doi.org/10.1210/jc.2013-2809.
Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357(9):863–73. https://doi.org/10.1056/NEJMoa066494.
Carlson LM, Vora NL. Prenatal diagnosis: screening and diagnostic tools. Obstet Gynecol Clin North Am. 2017;44(2):245–56. https://doi.org/10.1016/j.ogc.2017.02.004.
Bick DP, Schorderet DF, Price PA, Campbell L, Huff RW, Shapiro LJ, et al. Prenatal diagnosis and investigation of a fetus with chondrodysplasia punctata, ichthyosis, and Kallmann syndrome due to an Xp deletion. Prenat Diagn. 1992;12(1):19–29. https://doi.org/10.1002/pd.1970120104.
Dwyer AA, Raivio T, Pitteloud N. Gonadotrophin replacement for induction of fertility in hypogonadal men. Best Pract Res Clin Endocrinol Metab. 2015;29(1):91–103. https://doi.org/10.1016/j.beem.2014.10.005.
Liss J, Chromik I, Szczyglinska J, Jagiello M, Lukaszuk A, Lukaszuk K. Current methods for preimplantation genetic diagnosis. Ginekol Pol. 2016;87(7):522–6. https://doi.org/10.5603/GP.2016.0037.
Yan L, Wei Y, Huang J, Zhu X, Shi X, Xia X, et al. Advances in preimplantation genetic diagnosis/screening. Sci China Life Sci. 2014;57(7):665–71. https://doi.org/10.1007/s11427-014-4683-5.
Zong C, Lu S, Chapman AR, Xie XS. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012;338(6114):1622–6. https://doi.org/10.1126/science.1229164.
Yan L, Huang L, Xu L, Huang J, Ma F, Zhu X, et al. Live births after simultaneous avoidance of monogenic diseases and chromosome abnormality by next-generation sequencing with linkage analyses. Proc Natl Acad Sci U S A. 2015;112(52):15964–9. https://doi.org/10.1073/pnas.1523297113.
Liu W, Zhang H, Hu D, Lu S, Sun X. The performance of MALBAC and MDA methods in the identification of concurrent mutations and aneuploidy screening to diagnose beta-thalassaemia disorders at the single- and multiple-cell levels. J Clin Lab Anal. 2018;32(2). https://doi.org/10.1002/jcla.22267.
Ren Y, Zhi X, Zhu X, Huang J, Lian Y, Li R, et al. Clinical applications of MARSALA for preimplantation genetic diagnosis of spinal muscular atrophy. J Genet Genomics. 2016;43(9):541–7. https://doi.org/10.1016/j.jgg.2016.03.011.
Li W, Ma Y, Yu S, Sun N, Wang L, Chen D, et al. The mutation-free embryo for in vitro fertilization selected by MALBAC-PGD resulted in a healthy live birth from a family carrying PKD 1 mutation. J Assist Reprod Genet. 2017;34(12):1653–8. https://doi.org/10.1007/s10815-017-1018-z.
Ji X, Zhang Z, Shi J, He B. Clinical application of NGS-based SNP haplotyping for the preimplantation genetic diagnosis of primary open angle glaucoma. Syst Biol Reprod Med. 2019;65(3):258–63. https://doi.org/10.1080/19396368.2019.1590479.
Qin M, Zhu X, Zhang Z, Li X, Yan Z, Wang Y, et al. Genetic analysis and preimplantation genetic diagnosis of Chinese Marfan syndrome patients. J Genet Genomics. 2019;46(6):319–23. https://doi.org/10.1016/j.jgg.2019.04.003.
Huang X, Liu Y, Yu X, Huang Q, Lin C, Zeng J, et al. The clinical application of preimplantation genetic diagnosis for X-linked retinitis pigmentosa. J Assist Reprod Genet. 2019;36(5):989–94. https://doi.org/10.1007/s10815-019-01434-9.
Chan C, Wang CW, Chen CH, Chen CH. Live birth in male de novo Kallmann syndrome after cross-generational genetic sequencing. J Assist Reprod Genet. 2019;36(12):2481–4. https://doi.org/10.1007/s10815-019-01604-9.
Acknowledgements
Thanks for the support by grants from the National Natural Science Foundation of China (81971440) and Natural Science Foundation of Beijing (7212129).
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Yujun Liu wrote this review, and Xu Zhi guided the writing of this review.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
This paper has not been published before and not under consideration for publication elsewhere. The publication has been approved by all authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Zhi, X. Advances in Genetic Diagnosis of Kallmann Syndrome and Genetic Interruption. Reprod. Sci. 29, 1697–1709 (2022). https://doi.org/10.1007/s43032-021-00638-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00638-8