Abstract
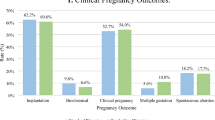
To compare clinical pregnancy rate (CPR) and live birth rate (LBR) after frozen-thawed embryo transfer (FET) of day (D-) 6 blastocysts on D-5 versus D-6. A retrospective cohort study. A university-affiliated single-center tertiary hospital. Women undergoing FET of D-6 blastocysts between August 2015 and March 2019 were included in the study. Exclusion criteria were endometriosis and maternal age ≥ 42. Cycles involving embryo transfer (ET) at D-6 were compared to cycles involving ET on D-5. Primary outcomes assessed were CPR and LBR, and the secondary outcomes were spontaneous abortion and chemical pregnancy rates. Forty-two cycles were assessed, 21 in which ET occurred on D-6 and 21 in which ET occurred on D-5. There were no significant differences between groups regarding age, body mass index (BMI), etiology of infertility, number of oocytes aspirated and blastocysts cryopreserved in the fresh cycle, reason for freezing on D-6, endometrial thickness before ET, and blastocyst grade. A comparison of outcomes of ET on D-5 with those involving ET on D-6 revealed that D-5 transfer produced significantly higher CPR (8, 38% vs. 2, 8.5%; P = 0.030) and LBR (6, 28.6% vs. 1, 4.8%; P = 0.038), respectively. FET of D-6 embryos on D-5 compared with D-6 is associated with increased CPR and LBR values. These findings might be related to the limited time window for optimal rates of implantation and indicate that transferring embryos on D-6 of a FET cycle is likely too late.


Similar content being viewed by others
Data Availability
Data will be made available upon reasonable request to the corresponding author.
Abbreviations
- BMI:
-
Body mass index
- CMA:
-
Chromosomal microarray analysis
- COH:
-
Controlled ovarian hyperstimulation
- CPR:
-
Clinical pregnancy rate
- D:
-
Day
- ET:
-
Embryo transfer
- FET:
-
Frozen-thawed embryo transfer
- GnRH:
-
Gonadotropin-releasing hormone
- HCG:
-
Human chorionic gonadotropin
- HRT:
-
Hormone replacement therapy
- LBR:
-
Live birth rate
- MNC:
-
Modified natural cycle
- PGT-M:
-
Preimplantation genetic testing for monogenic or single-gene defects
References
Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9.
Stanger J, Wong J, Conceicao J, Yovich J. Vitrification of human embryos previously cryostored by either slow freezing or vitrification results in high pregnancy rates. Reprod BioMed Online. 2012;24:314–20.
Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–55.
Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril. 2001;75:1126–30.
Barrenetxea G, López de Larruzea A, Ganzabal T, Jiménez R, Carbonero K, Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. 2005;83:49–53.
Poulsen V, Ingerslev HJ, Kirkegaard K. Elective embryo transfers on day 6 reduce implantation compared with transfers on day 5. Hum Reprod. 2017;32:1238–43.
Elgindy E, Elsedeek MS-E-A. Day 5 expanded blastocysts transferred on same day have comparable outcome to those left for more extended culture and transferred on day 6. J Assist Reprod Genet. 2012;29:1111–5.
Liebermann J, Tucker MJ. Comparison of vitrification and conventional cryopreservation of day 5 and day 6 blastocysts during clinical application. Fertil Steril. 2006;86:20–6.
Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016;106:1370–8.
Haas J, Meriano J, Laskin C, Bentov Y, Barzilay E, Casper RF, et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. 2016;33:1553–7.
Tubbing A, Shaw-Jackson C, Ameye L, Colin J, Rozenberg S, Autin C. Increased live births after day 5 versus day 6 transfers of vitrified-warmed blastocysts. J Assist Reprod Genet. 2018;35:417–24.
Behr B, Gebhardt J, Lyon J, Milki AA. Factors relating to a successful cryopreserved blastocyst transfer program. Fertil Steril. 2002;77:697–9.
Levens ED, Whitcomb BW, Hennessy S, James AN, Yauger BJ, Larsen FW. Blastocyst development rate impacts outcome in cryopreserved blastocyst transfer cycles. Fertil Steril. 2008;90:2138–43.
Wang X, Zhen J, Sun Z, Yu Q, Deng C, Zhou Y, et al. Effects of fifth day (D5) or sixth day (D6) frozen-thawed blastocysts on neonatal outcomes. Zygote. 2016;24:684–91.
Kaye L, Will EA, Bartolucci A, Nulsen J, Benadiva C, Engmann L. Pregnancy rates for single embryo transfer (SET) of day 5 and day 6 blastocysts after cryopreservation by vitrification and slow freeze. J Assist Reprod Genet. 2017;34:913–9.
Boostanfar R, Shapiro B, Levy M, Rosenwaks Z, Witjes H, Stegmann BJ, et al. Large, comparative, randomized double-blind trial confirming noninferiority of pregnancy rates for corifollitropin alfa compared with recombinant follicle-stimulating hormone in a gonadotropin-releasing hormone antagonist controlled ovarian stimulation protocol in older patients undergoing in vitro fertilization. Fertil Steril. 2015;104:94–103.e1.
Racowsky C, Stern JE, Gibbons WE, Behr B, Pomeroy KO, Biggers JD. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011;95:1985–9.
Irani M, Zaninovic N, Rosenwaks Z, Xu K. Does maternal age at retrieval influence the implantation potential of euploid blastocysts? Am J Obstet Gynecol. 2019;220:379.e371–7.
Kroener L, Ambartsumyan G, Briton-Jones C, Dumesic D, Surrey M, Munné S, et al. The effect of timing of embryonic progression on chromosomal abnormality. Fertil Steril. 2012;98:876–80.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81.
Taylor TH, Patrick JL, Gitlin SA, Wilson JM, Crain JL, Griffin DK. Comparison of aneuploidy, pregnancy and live birth rates between day 5 and day 6 blastocysts. Reprod BioMed Online. 2014;29:305–10.
Gasser RF. Atlas of human embryos. 1st ed: Medical Dept; 1975.
O’Rahilly RR, Müller F. Human Embryology and Teratology. 3rd ed: Wiley; 2001.
Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. 1st ed. Philadelphia: Saunders; 2005.
Harper MJ. The implantation window. Baillieres Clin Obstet Gynaecol. 1992;6:351–71.
Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–9.
Horcajadas JA, Pellicer A, Simón C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update. 2007;13:77–86.
Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95:50–60 60.e1.
Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–24.
Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99:508–17.
Neves AR, Devesa M, Martínez F, Garcia-Martinez S, Rodriguez I, Polyzos NP, et al. What is the clinical impact of the endometrial receptivity array in PGT-A and oocyte donation cycles? J Assist Reprod Genet. 2019;36:1901–8.
Bassil R, Casper R, Samara N, Hsieh T-B, Barzilay E, Orvieto R, et al. Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet. 2018;35:1301–5.
Franasiak JM, Forman EJ, Patounakis G, Hong KH, Werner MD, Upham KM, et al. Investigating the impact of the timing of blastulation on implantation: management of embryo-endometrial synchrony improves outcomes. Hum Reprod Open. 2018;2018:hoy022.
Montagut M, Santos-Ribeiro S, De Vos M, Polyzos NP, Drakopoulos P, Mackens S, et al. Frozen-thawed embryo transfers in natural cycles with spontaneous or induced ovulation: the search for the best protocol continues. Hum Reprod. 2016;31:2803–10.
Acknowledgements
The manuscript was professionally proofread by proof-reading-service.com.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Asaf Bilgory, Yael Kalma, and Rotem Kopel. The first draft of the manuscript was written by Asaf Bilgory and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethics Approval
Approval was obtained from the ethics committee of Tel Aviv Sourasky Medical Center. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patients or the public were not involved in the design, conduct, reporting, or dissemination of our research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Blastulation
-
The formation of a blastula from a morula. In mammals, the blastula is called a blastocyst which consists of inner cell mass, trophoblast, and blastocoel.
- Cryopreservation
-
A process that preserves organelles, cells, tissues, or any other biological constructs by cooling the samples to very low temperatures.
- Freeze-all policy
-
Elective cryopreservation of all viable embryos with a later transfer in frozen-thawed embryo transfer cycle.
- Implantation
-
The stage of pregnancy at which the embryo adheres to the wall of the uterus. At this stage of prenatal development, the conceptus is called a blastocyst.
- Vitrification
-
A cryopreservation technique that leads to a glass-like solidification. It is a method in which not only cells but also the whole solution is solidified without the crystallization of ice.
Rights and permissions
About this article
Cite this article
Bilgory, A., Kalma, Y., Kopel, R. et al. Transfer of Day 6 Frozen-Thawed Blastocysts on Day 5 Compared with Day 6: Catching Up with the Window of Implantation—a Retrospective Study. Reprod. Sci. 28, 2208–2215 (2021). https://doi.org/10.1007/s43032-021-00458-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00458-w