Abstract
Macroalgae that inhabit intertidal zones are exposed to the air for several hours during low tide and must endure desiccation and high variations in temperature, light intensity, and salinity. Pyropia yezoensis (Rhodophyta, Bangiales), a typical intertidal red macroalga that is commercially cultivated in the northwestern Pacific Ocean, was investigated under different dehydration stresses of desiccation, high salinity, and high mannitol concentration. Using chlorophyll fluorescence imaging, photosynthetic activities of P. yezoensis thalli were analyzed using six parameters derived from quenching curves and rapid light curves. A distinct discrepancy was revealed in photosynthetic responses to different dehydration stresses. Dehydration caused by exposure to air resulted in rapid decreases in photosynthetic activities, which were always lower than two other stresses at the same water loss (WL) level. High salinity only reduced photosynthesis significantly at its maximum WL of 40% but maintained a relatively stable maximum quantum yield of photosystem II (PSII) (Fv/Fm). High mannitol concentration induced maximum WL of 20% for a longer time (60 min) than the other two treatments and caused no adverse influences on the six parameters at different WL except for a significant decrease in non-photochemical quenching (NPQ) at 20% WL. Illustrated by chlorophyll fluorescence images, severe spatial heterogeneities were induced by desiccation with lower values in the upper parts than the middle or basal parts of the thalli. The NPQ and rETRmax (maximum relative electron transport rate) demonstrated clear distinctions for evaluating photosynthetic responses, indicating their sensitivity and applicability. The findings of this study indicated that the natural dehydration of exposure to air results in stronger and more heterogeneous effects than those of high salinity or high mannitol concentration.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Macroalgae that inhabit intertidal zones are submerged in and emerged from seawater periodically following the tide cycle. During low tides, macroalgae are exposed to the air for several hours and experience a variety of stressful environmental conditions including desiccation, osmotic stress, high light intensity, high or low temperature, and nutrient limitation (Davison and Pearson 1996). Among these, desiccation has received by far the most attention because of its key function in vertical zonation of macroalgae in coastal areas (Contreras-Porcia et al. 2017 and references in; Davison and Pearson 1996; Dring and Brown 1982; Schonbeck and Norton 1978).
The effects of desiccation on macroalgae have been studied from a wide range of aspects, from basic morphology to physiology, such as rates of survival, growth, photosynthesis, respiration, and reproduction (Contreras-Porcia et al. 2017; Flores-Molina et al. 2014; Karsten 2012; Xu et al. 2019). Using physiological, transcriptomic, and proteomic approaches, several studies have determined that the physiological mechanisms of macroalgae are well coordinated in response to desiccation stress, which includes morphological and cell wall changes, photosynthetic activity diminishment, increased expression and synthesis of associated proteins, scavenging of reactive oxygen species (ROS) by antioxidant enzymes and compounds, and osmolyte synthesis (Contreras-Porcia et al. 2013, 2017; Flores-Molina et al. 2014; López-Cristoffanini et al. 2015). As a central physiological process, photosynthesis is significantly inhibited and decreases immediately under desiccation (Blouin et al. 2011; Dring and Brown 1982; Lipkin et al. 1993). During the water loss process in intertidal macroalgae, photosynthetic pigments and related RNAs and proteins, such as phycobiliproteins, ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco), photosystem I and II proteins, and ferredoxin-NADP+, are down-regulated (Contreras-Porcia et al. 2013; Kumar et al. 2011; López-Cristoffanini et al. 2015).
In research studies on dehydration stress, salt and mannitol are commonly used as osmolytes with high solubility that are characteristically transported readily through cell walls and membrane (Chaves et al. 2009; Iwamoto and Shiraiwa 2005). Being able to reduce the cellular water potential, this kind of osmotic dehydration is comparable to desiccation and is commonly utilized to simulate desiccation stress with the benefit of their ease of control and application (Karsten 2012; Munns 2002). However, during natural desiccation by exposure to air, cellular ionic concentrations increase, but the ion ratios remain constant. Furthermore, when using osmolytes, as osmotic stress is prolonged, toxic effects might be induced from excess ions or osmolytes (Bartels and Sunkar 2005; Kirst 1990). On the other hand, most macroalgae could use inorganic ions and small organic osmolytes to create an internal osmotic potential without incurring metabolic damage (Karsten et al. 1997, 1999; Kirst 1990; Yancey 2005). Therefore, it should be considered that in microalgae, different response mechanisms exist to different causes of desiccation.
Pyropia yezoensis is a commercially important macroalga that inhabits the intertidal zone and is widely cultivated in countries of East Asia, such as China, Japan, and South Korea (Blouin et al. 2011; Gao et al. 2019; Kim et al. 2017). Like its congeners and members of sibling genera Porphyra belonging to the same order Bangiales, Pyropia has an extraordinary capacity to withstand the harsh physical and chemical stresses of the upper intertidal zone. Adaptations to these variable environmental conditions include the ability of P. yezoensis thalli to tolerate water losses of up to 85% and to recover metabolic activities upon rehydration within half an hour (Mao et al. 2019; Terada et al., 2020). In addition, due to the simple, plastic morphology of its gametophyte thalli that comprises a single layer cells of 35–45 µm thick, P. yezoensis could be the ideal model species for studying the effects of environmental stress.
Using a chlorophyll fluorescence imaging technique, non-invasive monitoring of photosynthetic activities can be realized synchronously for several samples (Baker 2008; Murchie and Lawson 2013). In the present study, using this technique, we aimed to explore dehydration effects on P. yezoensis photosynthesis under different treatments of desiccation, high salinity, and high mannitol concentration, and compare photosynthetic responses of P. yezoensis to different dehydration stresses. Based on these, further assessment was carried on the feasibility of simulating natural desiccation on macroalgae using osmotic dehydration under high salinity or high mannitol concentration.
Results
Photosynthetic capacity based on quenching curves
The P. yezoensis thalli treated by desiccation presented a rapid decrease in photosynthetic activity along with increased water loss (WL). Except 5% WL, all dehydration levels showed significantly lower Fv/Fm than that of 0% WL (P < 0.05). Under 40% WL, the Fv/Fm dropped to 0.45 ± 0.06, which was 13.2% lower than that of 0% WL (Fig. 1A). Even more rapid decreases were recorded based on other photosynthetic parameters, i.e., QY(II), NPQ, and qP. Compared to 0% WL, under 40% WL, the QY(II) was 48.7% lower at 0.20 ± 0.04 (Fig. 1B), NPQ was 87.1% lower at 0.04 ± 0.01 (Fig. 1C) and qP was 59.8% lower at 0.32 ± 0.04 (Fig. 1D).
The effects of dehydration caused by high salinity differed from those caused by desiccation treatment. Dehydration levels from 5 to 40% WL had no significant effect on Fv/Fm (P > 0.05) (Fig. 1A). Compared to 0% WL, no significant difference in QY(II) or qP (P > 0.05) was observed except at 40% WL (Fig. 1B, D). NPQ decreased but not significantly at 5% WL compared to 0% WL (P > 0.05) (Fig. 1C).
In high mannitol concentration treatments, no distinct dehydration effect on Fv/Fm, QY(II) or qP of P. yezoensis thalli was observed; indeed, there was even a slight increase in activity below 20% WL (P > 0.05, Fig. 1A, B, D). Only NPQ exhibited a decrease and was significantly lower at 20% WL compared to 0% WL (P < 0.05) (Fig. 1C).
The three kinds of treatments caused different effects on P. yezoensis thalli with increased water loss levels (Fig. 1). Comparing at each WL level, the differences were revealed more distinctly. Generally, the thalli treated by desiccation were significantly lower than other two treatments except 5% WL (Fig. 1). Algae exposed to high mannitol concentration always had slightly higher Fv/Fm, QY(II) and qP than those exposed to high salinity treatment from 0 to 20% WL (P > 0.05) (Fig. 1). Generally, the thalli treated by desiccation had a larger interquartile range (IQR) for Fv/Fm, QY(II), and qP than those exposed to the other two treatments. The NPQ of thalli exposed to high salinity showed larger IQR at 10% and 30% WL, and higher median at 10% and 20% WL (P > 0.05), than those exposed to high mannitol concentration (Fig. 1C).
Photosynthetic activity based on rapid light curves (RLCs)
Along with increased dehydration levels, the rapid light curves (RLCs) of Pyropia thalli presented large divergences between different treatments (Fig. 2). The rETR of the thalli treated by desiccation decreased more quickly than other two treatments at each WL level. The thalli treated with high salinity did not present significantly lower rETR at WL levels below 40%. The thalli treated with high mannitol concentration resembled the RLCs from 5 to 30% WL.
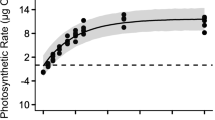
The rETRmax and α derived from RLCs varied among the three dehydration treatments (Fig. 3). The rETRmax of the thalli treated by desiccation descended with increasing WL, differed significantly from 0% WL (P < 0.05), and was always lower than other treatments at the same WL level. Thalli exposed to high salinity had a large variation in rETRmax with a significant increase at 10% WL compared to 5% and 20% WL (P < 0.05). The rETRmax of thalli exposed to high mannitol concentration varied only slightly from 5 to 20% WL (P > 0.05). The α of desiccated thalli exhibited a decreasing trend similar to the rETRmax and also was lower than that of other treatments at each WL level. Thalli exposed to high salinity had a slightly higher α although differences from 5 to 30% WL were not significant (P > 0.05), whereas the decrease at 40% WL compared to 0% WL was significant (P < 0.05). The treatment with high mannitol concentration induced a significant increase in α from 5 to 20% WL compared to 0% WL (P < 0.05).
Spatially heterogeneous effects
Chlorophyll fluorescence images of whole thalli enabled the variations in Fv/Fm, QY(II), NPQ, and qP to be presented visually (Fig. 4). Although showing the same trends as those presented in Fig. 1, the spatial heterogeneity of individual parameters displayed discrepancies among thalli under different treatments at 5%, 10%, and 20% WL. The thalli exposed to high salinity and high mannitol concentration showed relatively homogenous values, with slight differences at the very basal part of the holdfast and at the tip of the thallus. However, thalli exposed to air showed large heterogeneity for all four parameters with much lower values in the upper part, especially at 10% and 20% WL. Furthermore, for any given treatment, differences among individual thalli were larger when treated by desiccation than the other two treatments, resulting in larger standard deviations of parameters, as shown in Fig. 1.
Chlorophyll fluorescence images of Fv/Fm (A), QY(II) (B), NPQ(C), and qP (D) at 5%, 10%, and 20% water loss levels under different dehydration treatments (DE desiccation, HS high salinity, HM high mannitol concentration). Within each panel, P. yezoensis thalli are oriented with the holdfast at the bottom
Discussion
Red macroalgae of the genus Porphyra and Pyropia are considered as marine crops shaped by stress (Blouin et al. 2011). Many species of Porphyra and Pyropia can lose 85–95% of their cellular water during the daytime low tide in intertidal zones when exposed to air for several hours. During the exposure time, desiccation along with other stressors such as high irradiance and salinity inhibits photosynthesis of Porphyra or Pyropia thalli and this effect increases with increased water loss (Blouin et al. 2011; Lipkin et al. 1993; Terada et al. 2020).
In the present study, thalli dehydrated by desiccation showed significant decreases in their maximum photosynthetic capacity (indicated by Fv/Fm). This is consistent with the response of Pyropia haitanensis (previously Porphyra haitanensis), and of other macroalgae, to desiccation (Flores-Molina et al. 2014; Gao et al. 2013; Xu et al. 2016). Moreover, dehydration can inhibit electron transfer and energy conversion in photosynthetic reaction centers, leading to reductions in other measures of photosynthetic activity such as QY(II) and ETR (Jiang and Gao 2009; Yu et al. 2018). Nevertheless, although there is a general decrease in photosynthetic activity with increasing water loss, subtle discrepancies exist in QY(II), ETR, or NPQ among studies on Pyropia species. For instance, Gao et al. (2013) reported that P. haitanensis showed increased ETR in both PSII and PSI reaction centers under moderately desiccated conditions (water loss less than 30%), and its NPQ increased significantly at 20–25% water loss. In contrast, Xu et al. (2016) reported that QY(II) and Fv/Fm continuously decreased with increasing water loss in P. haitanensis. Slight but not significant increases in QY(II) and ETR in PSII were observed when P. yezoensis was exposed to air and directly utilized atmospheric CO2 (Zhou et al. 2014). In the present study, there was a general decrease in six parameters and RLCs in thalli under desiccation, with slight but not significant fluctuations. These discrepancies might due to differences in experimental conditions, the characteristics and growth status of testing organisms, or the response mechanism of photosynthetic physiology itself. Nevertheless, a rapid decrease in photosynthesis invariably occurred in Pyropia thalli as a result of desiccation.
High salinity stress on Pyropia is directly imposed on the whole thallus, unlike in higher plants with well-developed transportation systems connecting their roots, stems and leaves. The water loss under high salinity can occur within a few minutes and is followed by osmotic and ionic stress (Chaves et al. 2009). In the present study, the high salinity treatment, i.e., 100 PSU NaCl-rich seawater, was limited to an exposure time of 10 min which resulted in a maximum individual water loss of 40%. Furthermore, only at 40% WL did the thalli present a significant decrease in most photosynthetic parameters with the except on Fv/Fm. The relative stability of Fv/Fm up to 40% WL indicated that the potential photosynthetic capacity of PSII had not yet been interrupted, although effective photosynthetic efficiency and activity were inhibited at 40% WL as indicated by other parameters. Usually, studies on the effects of high salinity on macroalgae use treatment times of hours or even days (Huan et al. 2014; Nitschke et al. 2014; Yu et al. 2018; Zheng et al. 2019). During such time periods, higher osmotic and ionic stresses such as inhibition on enzyme activities due to excess Na+ ions would likely affect the thalli (Bartels and Sunkar 2005; Kolomeichuk et al. 2020; Sudhir and Murthy 2004). It has also been reported that when exposed to high salinity for 2 h, P. yezoensis can upregulate related enzymes to exploit NAD(P)H as an endogenous electron donor for reducing the plastoquinone pool and stabilizing the ETR (Yu et al. 2018).
In the present study, high mannitol concentration had almost no influence on photosynthesis of P. yezoensis thalli, with the exception of a significantly lower NPQ at 20% WL and a significantly higher α than the control. Mannitol is a universal sugar alcohol compound that is present in many photosynthetic organisms, although its biosynthesis is unusual for red algae, since they usually synthesize heteroside, floridoside, isofloridoside (Bangiales), or digeneaside (Ceramiales) (Iwamoto and Shiraiwa 2005; Kremer and Kirst 1982; Kumar et al. 2011). However, the D-mannitol-1-phosphate dehydrogenase (M1PDH) biosynthesis pathways of mannitol were found in red algae of the genera Caloglossa (Iwamoto et al. 2003; Karsten and West 1993; Karsten et al. 1997), Dixoniella and Rhodella (Karsten et al. 1999) and of the family Bangiophyceae (Tonon et al. 2017). In genome analysis carried out in our laboratory (unpublished data), the D-Mannitol dehydrogenase pathway from D-fructose to D-Mannitol (Richter and Kirst 1987), but not the M1PDH pathways, was found to be possibly responsible for mannitol biosynthesis. With mannitol metabolism, the red algae Caloglossa could cope with dehydration stress caused by high salinity (Karsten and West 1993; Karsten et al. 1997). Therefore, in the present study, endogenous mannitol might have served as an osmotic pressure regulator by balancing exogenous overdose, which resulted in few effects on photosynthesis and even increased the light utilization efficiency (α) of P. yezoensis thalli exposed to high mannitol concentrations.
Comparing the three dehydration treatments revealed the presence of distinct responses of photosynthesis in P. yezoensis thalli. Adverse effects were more severe and faster in thalli exposed to desiccation than those exposed to high salinity or high mannitol concentration. Furthermore, using chlorophyll fluorescence imaging, it was revealed that thalli treated with desiccation had a higher spatial heterogeneity in photosynthesis than other two treatments. In fact, desiccation and osmotic stress due to high salinity or high mannitol concentration reflect two different forms of water deprivation. Under salt or mannitol stress conditions, seaweed cells are still in full contact with liquid water, whereas desiccation due to exposure to air leads to strong cellular dehydration (Karsten 2012). The distinct spatial heterogeneity suggests that more attention should be paid to physiological, biochemical, and gene expression responses to environmental stress. For instance, different parts of the thallus should be considered, especially the tip area which expressed the most adverse effects to desiccation. Although high salinity can be easily applied as substitute for dehydration treatment due to its relatively homogenous effects, it should be kept in mind that the response mechanisms of P. yezoensis thalli to direct desiccation and osmotic dehydration are different. Even at the same levels of osmotic stress, markedly different effects were induced by high salinity and high mannitol concentration. Therefore, for macroalgae such as P. yezoensis, high salinity or high mannitol concentration could not be used to simulate natural dehydration. Detailed comparisons should be done in advance, as in this and other studies (Iwamoto and Shiraiwa 2005; Slama et al. 2007).
The chlorophyll fluorescence techniques used in the present study provide comprehensive information in a non-invasive and instant way for assessing photosynthetic performance in the thalli of red algae. Quenching curves and RLCs can yield information on Fv/Fm, QY(II), NPQ, and ETR in response to various environmental stresses, either on their own or in combination (Adams et al. 1999; Bilger et al. 1995; Maxwell and Johnson 2000). The changes in these parameters are widely used as reliable diagnostic indicators of stress on photosynthesis, especially the Fv/Fm, which is the most important parameter for reflecting the potential photosynthetic capacity of PSII (He et al. 1996; Valladares and Pearcy 1997). In the present study, four parameters derived from quenching curves and two parameters from RLCs were used for assessing the effects of dehydration on photosynthesis in the red alga P. yezoensis. Among these parameters, NPQ and rETRmax exhibited higher variation at different WL levels than the others. This implies that NPQ and rETRmax could be as sensitive indicators of photosynthetic performance for assessing stress on macroalgae such as P. yezoensis.
Materials and methods
Culture of Pyropia thalli
The Pyropia yezoensis strain RZ was maintained by the Laboratory of Algae Genetics and Breeding (Ocean University of China). Pyropia yezoensis thalli were cultured in 2 L bottles with Provasoli’s enriched seawater (PES) medium at 10 °C, under 60 μmol·photons·m−2·s−1 with a 12:12 light:dark (L:D) cycle. The culture was bubbled with filter-sterilized air constantly and the medium was renewed every three days.
Dehydration treatment
When the thalli reached 5–7 cm in length, they were randomly selected for three different dehydration treatments, i.e., desiccation, high salinity, and high mannitol concentration. The temperature and light intensity during the dehydration treatments were the same as the culture conditions. Air humidity remained 60%. Excess water on the thalli surface was removed with tissue paper before treatment.
In the treatment of desiccation, the thalli were spread flat on a Petri dish exposing them to air in a cabinet with stable temperature and moisture. For each sample group, the treatment time was controlled to reach water loss of 5%, 10%, 20%, 30%, and 40%, respectively.
In the salt treatment, the thalli were immersed in one of five NaCl solutions (35, 40, 60, 80, and 100) for 10 min to reach the water loss of 5%, 10%, 20%, 30%, and 40%, respectively. A preliminary experiment showed that salinity more than 100 induced only minimally higher water loss and made P. yezoensis thalli obviously deformed.
In the high mannitol concentration treatment, thalli were incubated in 0.8 mol/L mannitol seawater for 10 min, 20 min, or 60 min to reach water losses of 5%, 10%, and 20%, respectively. No higher water loss could be obtained using the maximum dissolved concentration of mannitol (0.8 mol/L). Six thalli of each treatment level were used for measuring photosynthetic activity.
Measurement of water loss
Dehydration levels of thalli were determined using the formula: \({\text{Water}}\;{\text{loss}}\;\left( {{\text{WL}}} \right) = \;\left( {W_{0} - \;W_{t} } \right)/\left( {W_{0} - \;W_{d} } \right)\; \times \;100\%\), where W0 is the initial weight after the removal of excess water, Wt is the weight of thalli at time t after dehydration, and Wd is the dry weight of thalli after drying to constant weight at 60 °C (Kim et al. 2009).
Determination of photosynthetic activity
Photosynthetic activity of thalli was measured using a chlorophyll fluorescence imaging system (FluorCAM MF800, PSI, Czech). The samples were dark-adapted for 10 min before measurements were taken. For quenching curve analysis, the saturating pulse was set as 45% (1892.65 μmol·photons·m−2·s−1), and actinic light was set as 23% (58.24 μmol·photons·m−2·s−1). Parameters of Fv/Fm (maximum PSII quantum yield), QY(II) (effective PSII quantum yield), NPQ (non-photochemical quenching), and qP (photochemical quenching) were derived from export data of quenching analysis in operating software. The rapid light curves (RLCs) were measured by exposure to six incremental steps of actinic light ranging from 0 to 980 µmol photons m−2 s−1 (PAR, photosynthetically active radiation). For each irradiance level (E), the relative electron transport rates (rETR) were calculated from the product of E and the PSII effective quantum yield (Y(II)), rETR = E × Y(II) (Genty et al. 1989). RLCs were fitted to the model from (Harrison and Platt (1986) and derived corresponding parameters, rETR = rETRmax [1-exp (− α × E/rETRmax)], where rETRmax is the maximum rETR, α is the maximum light utilization coefficient, and E is the light intensity.
Statistical analysis
All the data were expressed as a mean value of six replicates with standard deviation (SD). One-way ANOVA and Tukey tests were used to analyze the differences among treatments using SPSS 17.0 (SPSS Statistics 17.0, IBM). The significance level was set at 0.05.
References
Adams WW, Demmigadams B, Logan BA, Barker DH, Osmond CB (1999) Rapid changes in xanthophyll cycle-dependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australis, growing in the understory of an open Eucalyptus forest. Plant Cell Environ 22:125–136
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Bilger W, Schreiber U, Bock M (1995) Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 102:425–432
Blouin NA, Brodie JA, Grossman AC, Xu P, Brawley SH (2011) Porphyra: a marine crop shaped by stress. Trends Plant Sci 16:29–37
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Contreras-Porcia L, López-Cristoffanini C, Lovazzano C, Flores-Molina MR, Thomas D, Núñez A, Fierro C, Guajardo E, Correa JA, Kube M, Reinhardt R (2013) Differential gene expression in Pyropia columbina (Bangiales, Rhodophyta) under natural hydration and desiccation conditions. Lat Am J Aquat Res 41:933–958
Contreras-Porcia L, López-Cristoffanini C, Andrés M, Kumar M (2017) Tolerance pathways to desiccation stress in seaweeds. In: Kumar M, Ralph P (eds) Systems biology of marine ecosystems. Springer, Cham, pp 13–33
Davison IR, Pearson GA (1996) Stress torlerance in intertidal seaweads. J Phycol 32:197–211
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Flores-Molina MR, Thomas D, Lovazzano C, Nunez A, Zapata J, Kumar M, CorreaJA CL (2014) Desiccation stress in intertidal seaweeds: effects on morphology antioxidant responses and photosynthetic performance. Aquat Bot 113:90–99
Gao S, Niu J, Chen W, Wang G, Xie X (2013) The physiological links of the increased photosystem ii activity in moderately desiccated Porphyra haitanensis (Bangiales, Rhodophyta) to the cyclic electron flow during desiccation and re-hydration. Photosynth Res 116:45–54
Gao G, Gao Q, Bao M, Xu J, Li X (2019) Nitrogen availability modulates the effects of ocean acidification on biomass yield and food quality of a marine crop Pyropia yezoensis. Food Chem 271:623–629
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Harrison WG, Platt T (1986) Photosynthesis-irradiance relationships in polar and temperate phytoplankton populations. Polar Biol 5:153–164
He J, Chee CW, Goh CJ (1996) ‘Photoinhibition’ of Heliconia under natural tropical conditions: the importance of leaf orientation for light interception and leaf temperature. Plant Cell Environ 19:1238–1248
Huan L, Xie X, Zheng Z, Sun F, Wu S, Li M, Gao S, Gu W, Wang G (2014) Positive correlation between PSI response and oxidative pentose phosphate pathway activity during salt stress in an intertidal macroalga. Plant Cell Physiol 55:1395–1403
Iwamoto K, Shiraiwa Y (2005) Salt-regulated mannitol metabolism in algae. Mar Biotechnol 7:407–415
Iwamoto K, Kawanobe H, Ikawa T, Shiraiwa Y (2003) Characterization of salt-regulated mannitol-1-phosphate dehydrogenase in the red alga Caloglossa continua. Plant Physiol 133:893–900
Jiang H, Gao K (2009) Effects of dry-out and UV radiation on photosynthesis of Porphyra latifolia. Prog Nat Sci 19:835–840
Karsten U (2012) Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bischof K (eds) Seaweed biology: ecological studies (analysis and synthesis), vol 219. Springer, Berlin, Heidelberg, pp 87–108
Karsten U, West JA (1993) Ecophysiological studies on six species of the mangrove red algal genus Caloglossa. Aust J Plant Physiol 20:729–739
Karsten U, Barrow KD, Nixdorf O, West JA, King RJ (1997) Characterization of mannitol metabolism in the mangrove red alga Caloglossa leprieurii (Montagne). J Agardh Planta 201:173–178
Karsten U, West JA, Zuccarello GC, Nixdorf O, Barrow KD, King RJ (1999) Low molecular weight carbohydrate patterns in the Bangiophyceae (Rhodophyta). J Phycol 35:967–976
Kim JK, Kraemer GP, Yarish C (2009) Comparison of growth and nitrate uptake by new England Porphyra species from different tidal elevations in relation to desiccation. Phycol Res 57:152–157
Kim CR, Kim YM, Lee MK, Kim IH, Choi YH, Nam TJ (2017) Pyropia yezoensis peptide promotes collagen synthesis by activating the TGF-β/Smad signaling pathway in the human dermal fibroblast cell line Hs27. Int J Mol Med 39:31–38
Kirst GO (1990) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Biol 41:21–53
Kolomeichuk LV, Efimova MV, Zlobin IE, Kreslavski VD, Ol’ga KM, Kovtun IS, Khripach VA, Kuznetsov VV, Allakhverdiev SI (2020) 24-Epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynth Res 146:151–163
Kremer B, Kirst GO (1982) Biosynthesis of photosynthates and taxonomy of algae. Zeitschrift Für Naturforschung C 37:761–771
Kumar M, Gupta V, Trivedi N, Kumari P, Bijo AJ, Reddy CRK, Jha B (2011) Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environ Exp Bot 72:194–201
Lipkin Y, Beer S, Eshel A (1993) The ability of Porphyra linearis (Rhodophyta) to tolerate prolonged periods of desiccation. Bot Mar 36:517–524
López-Cristoffanini C, Zapata J, Gaillard F, Potin P, Correa JA, Contreras-Porcia L (2015) Identification of proteins involved in the tolerance responses to desiccation stress in the red seaweed Pyropia orbicularis (Rhodophyta. Bangiales). Proteomics 15:3954–3968
Mao Y, Chen N, Cao M, Chen R, Guan X, Wang D (2019) Functional characterization and evolutionary analysis of glycine-betaine biosynthesis pathway in red seaweed Pyropia yezoensis. Mar Drugs 17:70
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3893–3898
Nitschke U, Karsten U, Eggert A (2014) Physiological performance of the red alga Stylonema alsidii (Stylonematophyceae) under varying salinities. J Exp Mar Biol Ecol 460:170–176
Richter DF, Kirst GO (1987) D-mannitol dehydrogenase and D-mannitol-1-phosphate dehydrogenase in Platymonas subcordiformis: some characteristics and their role in osmotic adaptation. Planta 170:528–534
Schonbeck M, Norton TA (1978) Factors controlling the upper limits of fucoid algae on the shore. J Exp Mar Biol Ecol 31:303–313
Slama I, Ghnaya T, Messedi D, Hessini K, Labidi N, Savoure A, Abdelly C (2007) Effect of sodium chloride on the response of the halophyte species Sesuvium portulacastrum grown in mannitol-induced water stress. J Plant Res 120:291–299
Sudhir P, Murthy SD (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486
Terada R, Yuge T, Watanabe Y, Mine T, Morikawa T, Nishihara G (2020) Chronic effects of three different stressors, irradiance, temperature, and desiccation, on the PSII photochemical efficiency in the heteromorphic life-history stages of cultivated Pyropia yezoensis f. narawaensis (Bangiales) from Japan. J Appl Phycol 32:3273–3284
Tonon T, Li Y, Mcqueen-Mason S (2017) Mannitol biosynthesis in algae: more widespread and diverse than previously thought. New Phytol 213:1573–1579
Valladares F, Pearcy RW (1997) Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant Cell Environ 20:25–36
Xu K, Xu Y, Ji D, Xie J, Chen C, Xie C (2016) Proteomic analysis of the economic seaweed Pyropia haitanensis in response to desiccation. Algal Res 19:198–206
Xu D, Song X, Li F, Pan X, Zhu W, Zhang X (2019) Effects of desiccation, diurnal temperature changes and irradiance on archeospore production of Pyropia yezoensis. Aquaculture 509:167–170
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Yu B, Niu J, Feng J, Xu M, Xie X, Gu W (2018) Regulation of ferredoxin-NADP+ oxidoreductase to cyclic electron transport in high salinity stressed Pyropia yezoensis. Front Plant Sci 9:1–13
Zheng Z, Gao S, Wang G (2019) High salt stress in the upper part of floating mats of Ulva prolifera, a species that causes green tides, enhances non-photochemical quenching. J Phycol 55:1041–1049
Zhou W, He L, Yang F, Lin A, Zhang B, Niu J, Wang G (2014) Pyropia yezoensis can utilize CO2 in the air during moderate dehydration. Chin J Oceanol Limnol 32:358–364
Acknowledgements
This research was supported by National Key R&D Program of China (2018YFC1406704 and 2020YFD0900702).
Author information
Authors and Affiliations
Contributions
GD and YM conceived the project; GD and XL wrote the manuscript; XL, JW, SC, and XZ performed the experiments, analyzed the data, and prepared the figures. All authors edited and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Animal and human rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Chengchao Chen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, G., Li, X., Wang, J. et al. Discrepancy in photosynthetic responses of the red alga Pyropia yezoensis to dehydration stresses under exposure to desiccation, high salinity, and high mannitol concentration. Mar Life Sci Technol 4, 10–17 (2022). https://doi.org/10.1007/s42995-021-00115-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-021-00115-w