Abstract
Defining species within the Canidae family is challenging due to morphological convergence, behavioral plasticity, traditional taxonomic limitations, and possible hybridisation. This challenge is timely with the recent population and range expansion of the golden jackal (Canis aureus). Exploring their morphological data and sexual dimorphism is essential for identifying factors driving their success in new habitats. The proven hybridization of golden jackals with dogs and wolves may affect species description, population dynamics, and genetic diversity, impacting conservation strategies. This study, for the first time, conducts a morphometric analysis of golden jackals in Somogy County, Hungary, to prove sexual size dimorphism (SSD) in body and skull and sexual shape dimorphism (SShD) in skull across juvenile and adult age groups. 719 golden jackals (362 females and 357 males) were collected between January 2021 and January 2023. Descriptive statistics revealed significant SSD in body and skull measurements among both age groups, with males generally larger than females, particularly in body mass (11.72% in juveniles and 13.37% in adults). Most skull dimensions differed significantly between sexes and age groups, except for foramen magnum height, foramen magnum width, and postorbital breadth among juveniles and foramen magnum height and postorbital breadth among adults. We used principal component analyses (PCA) on raw dimension data and the log shape ratio method to extract shape information. Linear discriminant analysis (LDA) explored skull SShD between sexes. Notably, our study achieved over 71% accuracy in sex classification, illustrating the clear presence of SShD of the skull in golden jackals across both age groups. Our study provides a comprehensive database of golden jackals in the overpopulated Hungarian habitat, which will be helpful for further research on ecology, behavior, and conservation management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, the golden jackal (Canis aureus Linnaeus, 1758) population in Europe has increased significantly in number and geographical range (Arnold et al. 2012; Trouwborst et al. 2015; Jirků et al. 2018). Particularly, in Hungary, the jackal population has increased exponentially since the early 1990s. While the golden jackal’s territory was initially confined to southeastern Eurasia in the nineteenth century (Arnold et al. 2012), significant expansion in its distribution has occurred since the second half of the twentieth century (Trouwborst et al. 2015). Habitat changes, alterations in land use, increased food availability, a flexible diet, and a lack of large carnivores have primarily contributed to the population expansion (Krofel et al. 2017; Macdonald et al. 2019; Spassov and Acosta-Pankov 2019; Lanszki et al. 2022). The species is present in 33 European countries and has an established population in approximately 20 European countries (Trouwborst et al. 2015; Gherman and Mihalca 2017; Hatlauf et al. 2021a, b). Seven subspecies have been described in Eurasia based on differences in craniodental and fur characteristics (Wozencraft 2005; Castelló 2018; Moehlman and Hayssen 2018). Based on genetic, genomic, and morphological evidence, African golden jackal populations are distinguished as a separate species, Canis lupaster (Hemprich and Ehrenberg 1833), also known as the African wolf, which is closer to the gray wolf (Canis lupus Linnaeus, 1758) than the Eurasian golden jackal (Koepfli et al. 2015; Viranta et al. 2017; Krofel et al. 2022). Distinguishing the morphology and genetics of canid species and subspecies is crucial, as recent studies have revealed substantial gene flow between the species, suggesting complex genetic relationships (Gopalakrishnan et al. 2018). Furthermore, recent research has confirmed hybridisation between golden jackals and domestic dogs (Galov et al. 2015; Ninausz et al. 2023), and jackals might also hybridize with wolves (Freedman et al. 2014; Moura et al. 2014) raising conservation concerns. Exploring the causes of this expansion requires cooperation among various stakeholders, including scientists, nature conservationists, and wildlife managers. For effective management, these stakeholders require depth information regarding the species’ ecology, taxonomy, morphology, movement, and reproductive behaviour. Thus, a clear definition of species, which has always been a challenge in canids, is essential.
Morphometric data are often used to assess inter- and intraspecies variation. Variations in skull characteristics are influenced not only by environmental factors and anatomical variations (Forbes-Harper et al. 2017; Tseng and Flynn 2018; Fleming et al. 2020) but also by genetic origin (Basto et al. 2016) and functional implications (Roberts 1979; Brassard et al. 2020a, b, 2021).
The dimensions of the body and skull of mammalian predators have a profound impact on various characteristics, such as their basal metabolism, communication, social hierarchy, reproductive characteristics, and reproductive success (Geffen et al. 1996; Harrington and Asa 2003; Packard 2003; MacNulty et al. 2009).
Carnivores exhibit highly variable traits, including size, diet, social behaviour, movement behaviour, and activity patterns (Gittleman 1985; Meiri et al. 2005). Among these traits, variation in body size is a fundamental characteristic of any animal (Gortázar et al. 2000; Johnson et al. 2017; Law and Mehta 2018; Schwab et al. 2023). However, in carnivores, differences in body dimensions and skull size have been recognized between sexes and across regions (Gittleman and Valkenburgh 1997; Meiri et al. 2005), golden jackals’ cranial and body size traits have only been analyzed from a few locations across their broad geographical range in Europe (Koubek and Červený 2007; Bošković et al. 2015; Porobić et al. 2016; Volokh and Roženko 2016; Raichev et al. 2017; Rezić et al. 2017; Stoyanov 2020; Hatlauf et al. 2021a, b; Hadžiomerović et al. 2022; Rykov et al. 2022). Despite dimorphism being most pronounced in solitary carnivorous species (Law 2019), all canids exhibit some level of SSD (Bidau and Martinez 2016), which strongly correlates with their breeding system. Monogamous pair-bonding species and those that form multi-male, multi-female groups tend to be less dimorphic than uni-male, group-living species (Van Valkenburgh and Sacco 2002). In monogamous species, there tends to be less intense competition between males for mates compared to species with polygynous mating systems. SSD may reflect the effects of group or solitary living since the social organisation may be influenced by body size (Geffen et al. 1996). In monogamous golden jackals, the dimorphism of skull size is generally low and less pronounced (Stoyanov 2012, 2020; Porobić et al. 2016; Markov et al. 2017; Raichev et al. 2017) than in other canid species like grey wolves (Morris and Brandt 2014) and African wild dogs (Lycaon pictus Temminck 1820) (Mcnutt and Gusset 2012).
With the conservation concerns surrounding hybridisation and gene flow, particularly between golden jackals and domestic dogs, a comprehensive understanding of morphological data is indispensable. Morphological analyses of subspecies from different geographical regions can provide an essential basis for comparative population analyses.
In our study regarding the golden jackal, we hypothesize that SSD and SShD exist in body and skull measurements in juvenile and adult age groups, with males larger than females. Our study represents the first morphometric analysis of golden jackals to evaluate these hypotheses by encompassing comprehensive body and skull measurements across a large sample size in southwestern Hungary, using a combination of descriptive statistics.
Methods
Data collection
Golden jackals were collected from hunting management areas in Somogy County in the Pannonian biogeographical region of southwestern Hungary. The study area spans between Lake Balaton and the Drava River, characterized by flat lowlands with dunes and an average elevation of 173 m above sea level. Natural and semi-natural habitats dominate the landscape. The area experiences a continental climate, characterised by moderately humid and mild winters, with an annual mean temperature of 10.3 °C and precipitation ranges between 700 and 750 mm.
The golden jackal is the largest mammalian predator in the area, whose population has increased exponentially in recent decades. Six golden jackals were identified in Hungary in 1995, with the number of identified individuals increasing to 14,831 by 2022 (Csányi et al. 2022). According to the current hunting laws in Hungary, the golden jackal can be legally hunted year-round. Notably, no animals were specifically killed for this study.
A total of 719 golden jackals (362 females and 357 males) were collected from the study area (Fig. 1).
Identifying the golden jackal as a species for this study involved two steps and, in some cases, three steps. (i) According to the Act LV of 1996 on the Protection of Game, Game Management, and Hunting in Hungary, the hunter must make sure that the species is properly identified before shooting (ii) afterward, the carcass is checked based on the zoological parameters (iii) in some cases, there was a genetic check, and the decision was found to be correct for the species (in part unpublished data in part the published article, Ninausz et al. 2023). After biometric characterisation, the body mass and sex of the jackals were recorded. The individuals were classified into two age categories (juveniles and adults) according to the tooth wear of the upper incisor and the first upper molars (Harris 1978; Raichev et al. 1999; Hatlauf et al. 2021a, b) and body measurements. The juvenile category younger than one year, included individuals with permanent teeth. Still, tooth wear had yet to appear, and the body sizes were typical of young individuals (body mass range 4.8–12.25 kg); puppies were excluded from the study. The adult category included individuals one year of age and older, which was determined according to signs of upper-cutting teeth attrition and considering their biological and morphological features (body mass range 7.5–14.9 kg).
The body mass (BM) of each jackal was determined in kg. Six additional body dimensions were measured to the nearest mm using a measuring tape, as follows: body length from the anterior tip of the nose pad to the end of the tail (TBL), tail length (TL), head length (HL), wither height (WH), left back length (LBL), and neck circumference (NC) (Fig. 2a).
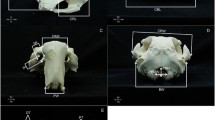
a Diagram of body measurements of the golden jackal (HL head length, LBL left back leg, NC neck circumference, TBL total body length, TL tail length, WH wither height). b Graphical representation of the skull measurements taken on the golden jackal in Hungary. BL basal length, CBL condylobasal length, CL cranial length, CW cranial width, FMH Foramen magnum height, FMW foramen magnum width, HOT height of the occipital triangles, IW interorbital width, LCT length of the lower tooth rows from C to M2, LNP length of the skull from os nasale to prostion, LUT length of the upper tooth row, LUT2 length of the upper tooth row, MH mandible height, ML mandible length, MW mastoid width, NL nasal length, PL palatal length, PB postorbital breadth, TML total mandible length from alveolus I to processus condyloideus, TSL total skull length, VL ventral total length, ZW zygomatic width
Twenty-two dimensions were obtained for each skull using an INSIZE digital caliper with a precision of 0.01. The following morphometric parameters were measured: basal length (BL), condylobasal length (CBL), cranial length (CL), cranial width (CW), foramen magnum height (FMH), foramen magnum width (FMW), height of the occipital triangles (HOT), interorbital width (IW), length of the lower tooth rows from C to M2 (LCT), length of the skull from os nasale to prostion; (LNP), length of the upper tooth row (LUT), length of the upper tooth row (LUT2), mandible height (MH), mandible length (ML), mastoid width (MW), nasal length (NL), palatal length (PL), postorbital breadth (PB), total mandible length from alveolus I to processus condyloideus (TML), total skull length (TSL), ventral total length (VL), zygomatic width (ZW) (Fig. 2b).
Statistical analyses
The body and skull measurements of the golden jackals were analysed to assess the differences and variations between sexes and age groups (juveniles and adults). Descriptive statistics, including the mean, standard deviation (SD), standard error (SE), and minimum and maximum values, were calculated for the body dimensions and skull measurements of all golden jackal samples. Data were first tested to confirm the normality of the distribution. Based on the central limit theorem (Feller 1968) our sample group comprised more than 100 individuals. The homogeneity of variance was tested using Levene’s test (Levene 1961), with most of the data exhibiting a homogenised variance, as indicated by Levene’s test result p > 0.05. When no homogenised variance was observed, we used a robust version of the t-test. The mean values of each dimension were subjected to a comparative analysis between sexes (females and males) and age groups (adults and juveniles) using Student’s t-test (Zar 1999). When homogeneity of variances was not met, the Welch’s t-test was used, considering the absence of equal variances. Our results were also confirmed using the Mann–Whitney U tests, as some variables showed a non-normal distribution according to a Shapiro–Wilk test (Shapiro and Wilk 1965).
We used principal component analysis (PCA) to reduce the dimensionality of the dataset and to identify patterns within the skull measurements of both juveniles and adults. This technique transforms the original variables into a smaller number of uncorrelated variables, known as principal components. The first principal component of the PCA is typically considered a general-size axis, whereas the remaining principal components represent the shape space (Klingenberg 2016). Skulls with missing data (damaged skulls) were excluded from the PCA analysis.
The PCA analysis was performed separately for the two age groups (juveniles and adults). Two types of PCA analysis were performed in both age groups: a raw PCA and a PCA on log-shape ratios. For the raw PCA, the original 22 skull variables were compressed into principal components. MANOVA and t-tests were performed to examine which principal components showed significant differences between sexes in the two age groups.
For a clear separation of shape and size, the PCA was applied to the log-shape ratios of the original measurements (Mosimann 1970; Claude 2013; Baur and Leuenberger 2011), and we calculated the geometric mean of the 22 skull sizes for shape PCA. Then, each skull measurement was divided by the individual’s own geometric mean, and the resulting variables were log-transformed to produce the log-shape ratios; finally, we performed a PCA on these log-shape ratios. We also performed a t-test and MANOVA to examine which main components showed significant differences between the sexes in the two age groups. After the PCA, we tested the predictive power of the principal components using linear discriminant analysis (LDA) to examine how the skull measurements of males and females were separated in both age groups (Baur and Leuenberger 2011). To avoid overtraining, we used the leave-one-out method, whereby we iteratively omitted one individual from the model. Subsequently, we examined the variable combinations that would result in the highest level of accuracy. In the case of raw PCA, the PC1 × PC3 variable combination produced the highest predictive power for distinguishing between the sexes. When we examined the PCA on log-shape ratios, we found that although PC1 and PC2 of the log-shape ratios were significantly different between the sexes, the combination of these variables produced a very low predictive power; this can be explained using the log-shape ratio method, which eliminates most of the size information from the data. Therefore, we decided to include the geometric mean of the data from the 22 skulls as a size component in the LDA classification, as well as in the graphical representation of the PCA results of the log-shape ratios. We obtained the highest LDA predictive power for the sexes using the geometric mean and PC1 of the log-shape ratios as the predictive variables. For the geometric mean, we used the standardised form, which means that we calculated the z-scores of the geometric mean for each individual to obtain a clear graphical representation. Although our PCA on log-shape ratios does not include the geometric mean as a variable, based on the LDA results, we found that the results of PCA on log-shape ratios can be graphically represented by including the standardised geometric mean on the horizontal axis and the PC1 of the PCA on log-shape ratios on the vertical axis (Stoyanov 2020; Ming et al. 2024). IBM SPSS Statistics Version 25 (IBM Corp. 2017) was used for all statistical analyses.
Results
Identification of SSD using descriptive statistics
Juveniles
In our study, SSD in the golden jackal body size among the juvenile age groups was evident, with all except one (tail length) body measurement showing statistically significant results; all measured values were larger in males. Body mass showed the most significant difference at 11.71%. The average difference in body size between the sexes was 4.13%.
We also observed sexual dimorphism in skull size. Regarding the skull measurements, the three dimensions, FMH, FMW, and PB, were not significantly different between the sexes in juveniles (Table 1). The remaining 19 dimensions differed significantly. The average difference in skull measurements between the sexes was 2.79%, with larger values measured in males.
Adults
SSD in the golden jackal body size among the adult age group was also evident, with all body measurements showing statistically significant results; all measured values were larger in males. Body mass showed the most significant difference at 13.37%. The average difference in body size between the sexes was 5.85%.
We also observed sexual dimorphism in skull size. Two dimensions, FMH and PB, were not significantly different between the sexes in adults (Table 2). The average difference in skull measurements between the sexes was 2.81%.
Identification of SSD and SShD using PCA and LDA on raw skull measurement data
Juveniles
The PCA, excluding the missing data of juveniles (N = 68) for the golden jackal craniometrical measurements, explained 67.66% of the variation in the first three principal components (PC1, PC2, and PC3). The PC1 explains the 54.83%, PC2 7.01% and PC3 5.80% of the variation. Most length dimensions (BL, CBL, LNP, ML, PL, TSL, TML, TSL, and VL) contributed almost exclusively to PC1 variation. In contrast, width dimensions (FMH, FMW, IW, and PB) helped explain the variation of PC2 and PC3.
Among the main components of the PCA performed on the raw data for juveniles, based on MANOVA and t-tests, the PC1 and PC3 components showed significant differences between the sexes. In the LDA analysis, we tested which variable combinations produced the most predictive power for the sexes and which combination we could estimate the sex most accurately. Consistent with MANOVA and t-tests (MANOVA: F = 54.96, p < 0.001; t-test PC1: t = − 7.413, p < 0.001, PC3 t-test: t = − 1.798, p = 0.037), the LDA analysis also showed that PC1 and PC3 were the most accurate estimates for the sexes.
Each juvenile individual was represented in the PC1 × PC3 coordinate system (Fig. 3a).
PCA results of the skull measurements of juvenile and adult golden jackals (Canis aureus) on raw data. According to t-tests and MANOVA, the PC1 and PC3 components showed significant sex differences among juvenile and adult golden jackals. a The scatter plot of juvenile golden jackals in the PC1 × PC3 coordinate system shows the distribution of males and females. The variance explained by PC1, PC2, and PC3 were 54.83%, 7.01%, and 5.81%, respectively. b The scatter plot of adult golden jackals in the PC1 × PC3 coordinate system shows the distribution of males and females. The variance explained by PC1, PC2, and PC3 were 54.51%, 7.01%, and 5.68%, respectively
Based on PC1, juvenile females tended to be on the left side, and males were on the right side of the first axis; however, a significant overlap was visible. We used LDA to separate males and females. Wilks’s lambda of the discriminant function was significant, indicating that the discriminant function separates the sexes well (Wilks’s λ = 0.780, χ2 = 52.69, df = 2, p < 0.001). Overall, 74.0% of the juveniles were correctly classified in terms of sex. The ratio was 72.9% for females and 75.6% for males (Supplementary Information: Table A).
Adults
The PCA, excluding the missing data of adults (N = 75) for the golden jackal craniometrical measurements, explained 67.20% of the variation in the first three principal components (PC1, PC2, and PC3). The PC1 explains the 54.51%, PC2 7.01%, and PC3 5.68% of the variation. Most length dimensions (BL, CBL, LNP, ML, PL, TML, TSL, and VL) contributed almost exclusively to PC1 variation. In contrast, width dimensions (CW, FMH, FMW, and PB) helped explain the variation of PC2 and PC3.
Among the main components of the PCA analysis performed on the raw data for adults, based on the MANOVA and t-tests, PC1 and PC3 components showed significant differences between the sexes (MANOVA: F = 124.823, p < 0.001, t-test: t = − 11.172, p < 0.001, PC3: t-test: t = − 2.103, p < 0.018). In the LDA analysis, we checked which variable combinations produced the most predictive power for the sexes and which combination we could estimate the sex most accurately. Each adult was represented in the PC1 × PC3 coordinate system (Fig. 3b).
Based on PC1, adult females tended to be on the left and males on the right. The LDA indicated a significant difference between the sexes (Wilks’s λ = 0.735, χ2 = 113.072, df = 2, p < 0.001). Overall, 75.4% of the adults were correctly classified with regard to sex. This ratio was 77.2% for females and 73.9% for males (Supplementary Information Table A).
Identification of SShD using PCA and LDA on log-shape ratios of the skull measurements
Juveniles
The PCA on log-shape ratios explained 41.2% of the variation in the first three principal components for the golden jackal craniometrical measurements of juveniles. The PC1 of log-shape ratios accounted for 24.7%, the PC2 of log-shape ratios for 9%, and the PC3 of log-shape ratios for 7.5%. The dimensions (BL, CBL, LNP, ML, PB, TML, TSL, ML, and VL) dominated in the first component, whereas IW, LUT, and ZW dominated in the second component, while CW, MW, and NL in the third component.
Based on the MANOVA and t-test results for the PCA on log-shape ratios variables, PC1 and PC2 of log-shape ratios showed a significant difference between sexes in the adult age group. However, based on the results of the LDA test, the combination of standardised geometric mean and PC1 of log-shape ratios variables provided the best estimation accuracy for sexes in the adult age group.
The distribution of data points showed that both males and females were widely distributed along both 1st principal component and standardised geometric mean axes (Fig. 4a) The figure shows that juvenile females are in the lower left corner of the coordinate system, while males are in the upper right corner.
PCA results on log-shape ratios of skull measurements in juvenile and adult golden jackals (Canis aureus). The highest predictive power in LDA was achieved using geometric mean and PC1. The geometric mean was standardized by calculating z-scores for clear representation. a Scatter plot of juvenile golden jackals: males and females, geometric mean on the x-axis, and PC1 on the y-axis. b Scatter plot of adult golden jackals: males and females, geometric mean on the x-axis, and PC1 on the y-axis
The LDA indicated the presence of a significant difference between the sexes (Wilks’s λ = 0.791, χ2 = 49.829, df = 2, p < 0.001). Overall, 71.2% of juveniles were correctly classified by sex. The ratios were 71.3% in females and 70.9% in males (Supplementary Information: Table B).
Adults
For adults’ golden jackal craniometrical measurements, the PCA on log-shape ratios explained 43.4% of the variation in the first three principal components. The PC1 of log-shape ratios accounted for 25.6%, PC2 of log-shape ratios accounted for 9.9%, and the PC3 of log-shape ratios accounted for 7.9%. The dimensions (BL, CBL, LNP, ML, PB, TML, TSL, and VL) dominated in the first component, whereas IW, LUT, and ZW dominated in the second component, while CL, HOT, and TML in the third component.
The data points’ distribution showed that both males and females were widely distributed along the first principal component and standardised geometric mean axes (Fig. 4b). Figure 5 illustrates the distribution of standardised geometric means across juvenile females, juvenile males, adult females, and adult males. Figure 5 highlights the differences in size and shape between the different age and sex groups, which intensify with age.
The LDA indicated the presence of a significant difference between the sexes (Wilks’s λ = 0.749, χ2 = 105.843, df = 2, p < 0.001). Overall, 74.6% of adults were correctly classified according to sex. This ratio was 76.6% for females and 72.9% for males (Supplementary Information: Table B).
Our results confirmed sexual dimorphism at the population level (MANOVA and t-test) and differences in size and shape between both age groups. At the individual level, the LDA test predicted sex with a predictive accuracy higher than 71.2% (highest, 75.4%) in both the juvenile and adult age groups.
Discussion
The results of the present study support our hypothesis that SSD and SShD exist among golden jackals in an overpopulated Hungarian habitat, both in the juvenile and adult age groups.
Morphological studies of the golden jackal’s European distribution area have been ongoing for decades and focus mainly on differences in body and skull sizes and shapes. Although methodological differences and the diversity of measurement techniques make direct comparisons between studies challenging, the previous and present results help us understand the species’ ecological and evolutionary roles.
Our study reveals a significant increase in body mass differences ranging from 11.71% in juveniles to 13.37% in adults, indicating the presence of sexual size dimorphism (SSD) in both age groups. This difference intensifies with age. The substantial differences between the two age groups in our study highlight the role of growth and development in the expression of physical traits that are crucial for survival and reproductive strategies.
Previous studies have also examined body parameters and identified SSD in the golden jackal population. Bošković et al. (2015) investigated differences between golden jackal populations. Their findings indicated that jackals from Eastern Serbia generally exhibited larger total body lengths, ear spans, eye spans, and neck circumferences.
In contrast, the Eastern Croatian population was found to have greater wither heights, ear lengths, and limb measurements. These differences may reflect differences in habitat utilization and resource availability. According to Raichev’s study (2017) body size data, body mass (BM) differences were 6% among males and 4% among females, and individuals were generally heavier in the Hungarian population than in the Central Bulgarian population. Some of these results were comparable with ours. The TBL was 10.27% (in males) and 10.67% (in females), and the TL was 3.25% (in males) and 6.26% (in females) longer in the Central Bulgarian golden jackals population when compared with the Hungarian population. These inter-population morphometric variations agree with previous observations of significant sexual size dimorphism within jackal populations. These results highlight how inter-sexual and geographical differences potentially influence golden jackals’ adaptive traits and ecological strategies.
Previous studies investigating the SSD of the skull demonstrated that males are usually larger than females (Kryštufek and Tvrtković 1990; Demeter and Von Spassov 1993; Porobić et al. 2016; Stoyanov 2020; Hadžiomerović et al. 2022), but the SShD were less pronounced.
Porobić et al.’s (2016) study on Serbian golden jackals found significant shape differences but noted that these differences were not strongly pronounced, potentially due to a strong founder effect. Similarly, Rezić et al.’s (2017) geometric morphometric analysis found significant sexual shape dimorphism, but the differences were not highly expressed. Stoyanov’s (2020) research indicated the presence of differences in skull shape between sexes using LDA, but a high misclassification rate of almost 40% was found.
Our results showed predictive accuracy exceeding 71% for juveniles and adults, highlighting a more pronounced sexual dimorphism in golden jackal skulls. The higher accuracy of our results stresses the importance of using large sample sizes to capture the subtle morphological differences between sexes more effectively.
Our study advances future research by using the large data set of the body and skull size of the golden jackal for the separation and precise definition of the species for biological, nature conservation, and wildlife management purposes.
The sexual dimorphism demonstrated in the study in the two age groups helps better understand how biological differences between males and females manifest across different stages of development, particularly in terms of their ecological roles and dispersal patterns. Ecological factors, such as predation risk or habitat complexity, can cause changes in body shape. For instance, size, coloration, and morphological variations between sexes may correlate with specific ecological niches or mating strategies, thus impacting an individual’s.
movements and expansion (Brönmark and Miner 1992; Frommen et al. 2011; Freudiger et al. 2021). Monogamy is the primary social system of canids, although their social organization, body size, life history characteristics, habitat, and diet are different (Macdonald 1983; Bekoff et al. 1984; Moehlman 1989; Geffen et al. 1996; Sillero-Zubiri et al. 2004; Macdonald et al. 2019). Body size can affect social organization; therefore, SSD may reflect the consequences of living in a group or solitary life (Geffen et al. 1996; Law and Mehta 2018).
Sexual dimorphism in the golden jackals’ juvenile and adult age groups suggests that sexual differences may have significant social or behavioural dynamics. These differences could influence various behavioural functions, such as hierarchy formation, even among juvenile age groups. In species such as golden jackals, which exhibit social behaviour, fights for hierarchy within groups and packs are frequent (Moehlman 1989).
Early sexual dimorphism may promote hierarchy that develops at a young age; juveniles can begin a new pack in a new hierarchical position. The golden jackal’s social flexibility, such as partner exchange outside the mating season (Csányi et al. 2023) and the hierarchies that form at a young age, highlights the golden jackal’s adaptive social structure. This flexible social system may be one of the reasons for the rapid expansion of this species.
Sexual dimorphism can also affect the feeding preferences and diet of each sex, which can affect their place in and the ecosystem and the stability of the ecosystem (Noonan et al. 2016; Kawatsu 2017).
Previous studies have already demonstrated the opportunistic feeding of the golden jackal (Lanszki et al. 2006, 2009; Markov and Lanszki 2012; Bošković et al. 2013; Ćirović et al. 2014; Penezić and Ćirović 2015), and Ćirović et al. (2014) confirmed in Serbia, that the diet of adult jackals was more varied than that of young individuals. Since we verified SSD and SShD in terms of the skull dimensions in the present study, it is likely that such a difference can also exists between the sexes. We will continue the morphological results of our current study with the assignment of stomach contents in the future.
Although our results reported SSD and SShD among golden jackals, it is essential to note that these differences are only sometimes evident in the field or our eyes. Despite this, morphological studies significantly benefit nature conservation and wildlife management. Such studies enable species identification, comparison of populations, and a better understanding of biological diversity. In addition, studying sexual dimorphism can help us understand interspecies roles, social behaviour, and reproductive strategies, which can provide crucial information for population dynamics, conservation, and interspecies relationships. Morphological features provide essential information for classifying organisms, and despite the progress in molecular taxonomy, they are still indispensable for identifying species and constructing accurate taxonomic systems (Hinton and Chamberlain 2014).
Conclusion
Our study presents morphometric data of the Hungarian golden jackal population. In the juvenile and adult age groups, we verified sexual size dimorphism in the body and the skull and sexual shape dimorphism in the skull of the golden jackal, which can be an essential basis for future research. In addition, studying sexual dimorphism can help us understand interspecies roles, social behaviour, and reproductive strategies, providing crucial information for classifying organisms, population dynamics, conservation, and interspecies relationships.
Data availability
The database analysed in this article is available upon reasonable request at the Roth Gyula Doctoral School of Forestry and Wildlife Management Sciences at the University of Sopron; with the authors’ permission, this article (csanyi.erika@phd.uni-sopron; sandor.gyula@uni-sopron.hu).
References
Arnold J, Humer A, Heltai M, Murariu D, Spassov N, Hackländer K (2012) Current status and distribution of golden jackals Canis aureus in Europe. Mamm Rev 42:1–11. https://doi.org/10.1111/j.1365-2907.2011.00185.x
Basto MP, Santos-Reis M, Simões L, Grilo C, Cardoso L, Cortes H, Bruford MW, Fernandes C (2016) Assessing genetic structure in common but ecologically distinct carnivores: The stone marten and red fox. PLoS ONE 11:e0145165. https://doi.org/10.1371/journal.pone.0145165
Baur H, Leuenberger C (2011) Analysis of ratios in multivariate morphometry. Syst Biol 60(6):813–825
Bekoff M, Daniels TJ, Gittleman JL (1984) Life history patterns and the comparative social ecology of carnivores. In: Johnson RF, Frank PW, Michener CO (eds) Annual review of ecology and systematics. Annual Reviews Inc., Palo Alto, pp 191–232
Bidau CJ, Martinez PA (2016) Sexual size dimorphism and Rensch’s rule in Canidae. Biol J Linn Soc 119:816–830. https://doi.org/10.1111/Bij.12848
Bošković I, Šperanda M, Florijančić T, Šprem N, Ozimec S, Degmečić D, Jelkić D (2013) Dietary habits of the golden jackal (Canis aureus L.) in the Eastern Croatia. Agric Conspect Sci 78(3):245–248
Bošković I, Ozimec S, Šperanda M, Šprem N, Degmečić D, Ćurović M, Spalević V, Florijanćić T (2015) Morphometric characteristics of the golden jackal populations in eastern Croatia and eastern Serbia. Agric Forest Podgorica 61:61–68. https://doi.org/10.17707/AgricultForest.61.3.06
Brassard C, Merlin M, Guintard C, Monchâtre-Leroy E, Barrat J, Bausmayer N, Bausmayer S, Bausmayer A, Beyer M, Varlet A, Houssin C, Callou C, Cornette R, Herrel A (2020a) Bite force and its relationship to jaw shape in domestic dogs. J Exp Biol 223:16. https://doi.org/10.1242/jeb.224352
Brassard C, Merlin M, Guintard C, Monchâtre-Leroy E, Barrat J, Callou C, Cornette R, Herrel A (2020b) Interrelations between the cranium, the mandible, and muscle architecture in modern domestic dogs. Evol Biol 47:308–324. https://doi.org/10.1007/s11692-020-09515-9
Brassard C, Merlin M, Monchâtre-Leroy E, Guintard C, Barrat J, Garès H, Larralle A, Triquet R, Houssin C, Callou C, Cornette R, Herrel A (2021) Masticatory system integration in a commensal canid: interrelationships between bones, muscles and bite force in the red fox. J Exp Biol 224:5. https://doi.org/10.1242/jeb.224394
Brönmark C, Miner JG (1992) Predator-induced phenotypical change in body morphology in crucian carp. Science 258:1348–1350. https://doi.org/10.1126/science.258.5086.1348
Castelló JR (2018) Canids of the world: wolves, wild dogs, foxes, jackals, coyotes, and their relatives. Princeton University Press, Princeton
Ćirović D, Penezić A, Milenković M, Paunović M (2014) Winter diet composition of the golden jackal (Canis aureus L., 1758) in Serbia. Mamm Biol 79:132–137
Claude J (2013) Log-shape ratios, procrustes superimposition, elliptic fourier analysis: three worked examples in R. Hystrix Ital J Mammal 24(1):94–102. https://doi.org/10.4404/hystrix-24.1-6316
Csányi E, Lanszki J, Heltai M, Pölös M, Schally G, Gy S (2023) The first evidence of the monogamous golden jackal’s adaptive response to partner loss. Appl Anim Behav Sci 269:106095. https://doi.org/10.1016/j.applanim.2023.106095
Csányi S, Márton M, Bőti Sz, Schally G (2022) Vadgazdálkodási Adattár 2021/2022. vadászati év. Országos Vadgazdálkodási Adattár, Gödöllő, pp 70
Demeter A, Spassov N (1993) Canis aureus Linnaeus, 1758—Schakal, Goldschakal. Handbuch der Säugetiere Europas. AULA-Verlag, Wiesbaden. Europas. Aula-Verlag, Wiesbaden, pp 107–138
Feller W (1968) An introduction to probability theory and its applications, 3rd edn. Wiley, New York
Fleming PA, Crawford HM, Auckland CH, Calver MC (2020) Body size and bite force of stray and feral cats—are bigger or older cats taking the largest or more difficult to handle prey? Animals 10(4):707. https://doi.org/10.3390/ani10040707
Forbes-Harper JL, Crawford HM, Dundas SJ, Warburton NM, Adams PJ, Bateman PW, Calver MC, Fleming PA (2017) Diet and bite force in red foxes: ontogenetic and sex differences in an invasive carnivore. J Zool 303:54–63. https://doi.org/10.1111/jzo.12463
Freedman AH, Gronau I, Schweizer RM, Ortega-Del Vecchyo D, Han E, Silva PM, Galaverni M, Fan Z, Marx P, Lorente-Garos B, Beale H, Ramirez O, Hormozdiari F, Alkan C, Vilà C, Squire K, Geffen E, Kusak J, Boyko AR, Parker HG, Lee C, Tadigotla V, Siepel A, Bustamante CD, Harkins TT, Nelson SF, Ostrander EA, Marques-Bonet T, Wayne RK, Novembre J (2014) Genome sequencing highlights the dynamic early history of dogs. PLoS Genet 10(1):e1004016. https://doi.org/10.1371/journal.pgen.1004016
Freudiger A, Josi D, Thünken T, Herder F, Flury JM, Marques DA, Taborsky M, Frommen JG (2021) Ecological variation drives morphological differentiation in a highly social vertebrate. Funct Ecol 35:2266–2281. https://doi.org/10.1111/1365-2435.13857
Frommen JG, Herder F, Engqvist L, Mehlis M, Bakker TCM, Schwarzer J, Thünken T (2011) Costly plastic morphological responses to predator specific odour cues in three- spined sticklebacks (Gasterosteus aculeatus). Evol Ecol 25:641–656. https://doi.org/10.1007/s10682-010-9454-6
Galov A, Fabbri E, Caniglia R, Arbanasić H, Lapalombella S, Florijančić T, Bošković I, Galaverni M, Randi E (2015) First evidence of hybridization between golden jackal (Canis aureus) and domestic dog (Canis familiaris) as revealed by genetic markers. R Soc Open Sci 2(12):150450. https://doi.org/10.1098/rsos.150450
Geffen E, Gompper ME, Gittleman JL, Luh H-K, Macdonald DW, Wayne RK (1996) Size, life-history traits, and social organization in the Canidae: a reevaluation. Am Nat 147(1):140–160. https://doi.org/10.1086/285844
Gherman CM, Mihalca AD (2017) A synoptic overview of golden jackal parasites reveals high diversity of species. Parasit Vectors 10:419. https://doi.org/10.1186/s13071-017-2329-8
Gittleman JL (1985) Carnivore body size: ecological and taxonomic correlates. Oecologia 67:540–554. https://doi.org/10.1007/BF00790026
Gittleman JL, Valkenburgh BV (1997) Sexual dimorphism in the canines and skulls of carnivores: effects of size, phylogency, and behavioural ecology. J Zool 242:97–117. https://doi.org/10.1111/j.1469-7998.1997.tb02932.x
Gopalakrishnan S, Sinding M-HS, Ramos-Madrigal J, Niemann J, Samaniego Castruita JA, Vieira FG, Carøe C, Montero MDM, Kuderna L, Serres A, González-Basallote VM, Liu Y-H, Wang G-D, Marques-Bonet T, Mirarab S, Fernandes C, Gaubert P, Koepfli K-P, Budd J, Rueness EK, Sillero C, Heide-Jørgensen MP, Petersen B, Sicheritz-Ponten T, Bachmann L, Wiig Ø, Hansen AJ, Gilbert MTP (2018) Interspecific gene flow shaped the evolution of the genus Canis. Curr Biol 28:3441–3449. https://doi.org/10.1016/j.cub.2018.08.041
Gortázar C, Travaini A, Delibes M (2000) Habitat-related microgeographic body size variation in two Mediterranean populations of red fox (Vulpes vulpes). J Zool 250:335–338. https://doi.org/10.1111/j.1469-7998.2000.tb00778.x
Hadžiomerović N, Gündemir O, Kovačević S (2022) Mandible size and shape of the red fox (Vulpes vulpes) and golden jackal (Canis aureus). Adv Anim Vet Sci 10(2):364–368. https://doi.org/10.17582/journal.aavs/2022/10.2.364.368
Harrington FH, Asa CS (2003) Wolf communication. In: Mech LD, Boitani L (eds) Wolves: behavior, ecology, and management. University of Chicago Press, Chicago and London, pp 66–103
Harris S (1978) Age determination in the red fox—an evaluation of technique efficiency as applied to a sample of suburban foxes. J Zool 184:91–117. https://doi.org/10.1111/j.1469-7998.1978.tb03268.x
Hatlauf J, Bayer K, Trouwborst A, Hackländer K (2021a) New rules or old concepts? The golden jackal (Canis aureus) and its legal status in Central Europe. Eur J Wildl Res. https://doi.org/10.1007/s10344-020-01454-2
Hatlauf J, Krendl LM, Tintner J, Griesberger P, Heltai M, Markov G, Viranta S, Hackländer K (2021b) The canine counts! Significance of a craniodental measure to describe sexual dimorphism in canids: golden jackals (Canis aureus) and African wolves (Canis lupaster). Mamm Biol 101:871–879. https://doi.org/10.1007/s42991-021-00133-2
Hemprich W, Ehrenberg CG (1833) Symbolae physicae seu icones et descriptiones animalium evertebratorum. Decas Prima. Officina Academica, Berlin
Hinton JW, Chamberlain MJ (2014) Morphometrics of canis taxa in eastern North Carolina. J Mammal 95(4):855–861. https://doi.org/10.1644/13-MAMM-A-202
Jirků M, Dostál D, Robovský J, Šálek M (2018) Reproduction of the golden jackal (Canis aureus) outside current resident breeding populations in Europe: Evidence from the Czech Republic. Mammalia 82:592–595. https://doi.org/10.1515/mammalia-2017-0141
Johnson PJ, Noonan MJ, Kitchener AC, Harrington LA, Newman C, Macdonald DW (2017) Rensching cats and dogs: feeding ecology and fecundity trends explain variation in the allometry of sexual size dimorphism. R Soc Open Sci 4:170453–170457
Kawatsu K (2017) Ecological effects of sex differ with trophic positions in a simple food web. Ecol Evol 8(2):1239–1246. https://doi.org/10.1002/ece3.3740
Klingenberg CP (2016) Size, shape, and form: concepts of allometry in geometric morphometrics. Dev Genes Evol 226:113–137. https://doi.org/10.1007/s00427-016-0539-2
Koepfli KP, Pollinger J, Godinho R, Robinson J, Lea A, Hendricks S, Schweizer RM, Thalmann O, Silva P, Fan Z, Yurchenko AA, Dobrynin P, Makunin A, Cahill JA, Shapiro B, Álvares F, Brito JC, Geffen E, Leonard JA, Helgen KM, Johnson WE, O’Brien SJ, Van Valkenburgh B, Wayne RK (2015) Genome-wide evidence reveals that African and Eurasian golden jackals are distinct species. Curr Biol 25(16):2158–2165. https://doi.org/10.1016/j.cub.2015.06.060
Koubek P, Červený J (2007) The golden jackal (Canis aureus): a new mammal species in the Czech Republic. Lynx 38:103–106
Krofel M, Giannatos G, Ćirović D, Stoyanov S, Newsome TM (2017) Golden jackal expansion in Europe: a case of mesopredator release triggered by continent-wide wolf persecution? Hystrix Ital J Mammal 28(1):9–15. https://doi.org/10.4404/hystrix-28.1-11819
Krofel M, Hočevar L, Fležar U, Topličanec I, Oliveira T (2022) Golden jackal as a new kleptoparasite for Eurasian lynx in Europe. Glob Ecol Conserv 36:e02116. https://doi.org/10.1016/j.gecco.2022.e02116
Kryštufek B, Tvrtković N (1990) Variability and identity of the jackals (Canis aureus) of Dalmatia. Ann Naturhist Mus Wien 91B:7–25
Lanszki J, Heltai M, Szabó L (2006) Feeding habits and trophic niche overlap between sympatric golden jackal (Canis aureus) and red fox (Vulpes vulpes) in the Pannonian ecoregion (Hungary). Can J Zool 84(11):1647–1656
Lanszki J, Giannatos G, Heltai M, Legakis A (2009) Diet composition of golden jackals during cub-rearing season in Mediterranean marshland in Greece. Mamm Biol Z Säugetierkd 74(1):72–75
Lanszki J, Hayward MW, Ranc N, Zalewski A (2022) Dietary flexibility promotes range expansion: The case of golden jackals in Eurasia. J Biogeogr 49:993–1005. https://doi.org/10.1111/jbi.14372
Law CJ (2019) Solitary meat-eaters: solitary, carnivorous carnivorans exhibit the highest degree of sexual size dimorphism. Sci Rep 9:15344. https://doi.org/10.1038/s41598-019-51943-x
Law CJ, Mehta RS (2018) Carnivory maintains cranial dimorphism between males and females: Evidence for niche divergence in extant Musteloidea. Evolution 72(9):1950–1961. https://doi.org/10.1111/evo.13514
Levene H (1961) Robust tests for equality of variances. In: Olkin I, Ghurye SG, Hoeffding W, Madow WG, Mann HB (eds) Contributions to probability and statistics: Essays in honor of Harold Hotelling. Stanford University Press, Redwood City, pp 279–292
Linné C (1758) Canis aureus Linnaeus, 1758. In: Systema naturae, 10th edition, Holmiæ: Salvius, vol 1, pp 45–46
Macdonald DW (1983) The ecology of carnivore social behaviour. Nature 301:379–384. https://doi.org/10.1038/301379a0
Macdonald DW, Campbell LAD, Kamler JF, Marino J, Werhahn G, Sillero-Zubiri C (2019) Monogamy: cause, consequence, or corollary of success in wild canids? Front Ecol Evol. https://doi.org/10.3389/fevo.2019.00341
MacNulty DR, Smith DW, Mech LD, Eberly LE (2009) Body size and predatory performance in wolves: Is bigger better? J Anim Ecol 78:532–539. https://doi.org/10.1111/j.1365-2656.2008.01517.x
Markov G, Lanszki J (2012) Diet composition of the golden jackal Canis aureus in an agricultural environment. Folia Zool 61(1):44–48. https://doi.org/10.25225/fozo.v61.i1.a7.2012
Markov GG, Kocheva MA, Gospodinova MG (2017) Patterns of sexual dimorphism and phenetic variety among the populations of the golden jackal (Canis aureus) in Bulgaria: insights from craniometric data. Balkan J Wildl Res 4:29–42. https://doi.org/10.15679/bjwr.v4i1.49
Mcnutt JW, Gusset M (2012) Declining body size in an endangered large mammal. Biol J Linn Soc 105(1):8–12. https://doi.org/10.1111/j.1095-8312.2011.01785.x
Meiri S, Dayan T, Simberloff D (2005) Variability and correlations in carnivore crania and dentition. Funct Ecol 19:337–343. https://doi.org/10.1111/j.1365-2435.2005.00964.x
Ming KM, Le Verger K, Geiger M, Schmelzle T, Georgalis GL, Shimbo G, Sasaki M, Ohcachi SD, Sánchez-Villagra MR (2024) Insular dwarfism in horses from the Aegean Sea and the Japanese archipelago. Mamm Biol. https://doi.org/10.1007/s42991-024-00408-4
Moehlman PD (1989) Intraspecific variation in canid social systems. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution. Springer, Boston, pp 143–163. https://doi.org/10.1007/978-1-4757-4716-4_6
Moehlman PD, Hayssen V (2018) Canis aureus (Carnivore: Canidae). Mamm Species 50:14–25. https://doi.org/10.1093/mspecies/sey002
Morris JS, Brandt EK (2014) Specialization for aggression in sexually dimorphic skeletal morphology in grey wolves (Canis lupus). J Anat 225(1):11
Mosimann JE (1970) Size Allometry: Size and shape variables with characterizations of the lognormal and generalized gamma distributions. J Am Stat Assoc 65(330):930–945. https://doi.org/10.1080/01621459.1970.10481136
Moura AE, Tsingarska E, Dąbrowski MJ, Czarnomska SD, Jędrzejewska B, Pilot M (2014) Unregulated hunting and genetic recovery from a severe population decline: the cautionary case of Bulgarian wolves. Conserv Genet 15:405–417. https://doi.org/10.1007/s10592-013-0547-y
Ninausz N, Fehér P, Csányi E, Heltai M, Szabó L, Barta E, Kemenszky P, Gy S, Jánoska F, Horváth M, Sz K, Frank K, Varga L, Stéger V (2023) White and other fur colourations and hybridization in golden jackals (Canis aureus) in the Carpathian basin. Sci Rep 13(1):21969. https://doi.org/10.1038/s41598-023-49265-0
Noonan MJ, Johnson PJ, Kitchener AC, Harrington LA, Newman C, Macdonald DW (2016) Sexual size dimorphism in musteloids: an anomalous allometric pattern is explained by feeding ecology. Ecol Evol 6:8495–8501
Packard JM (2003) Wolf behavior: reproductive, social and intelligent. In: Mech LD, Boitani L (eds) Wolves: behavior, ecology, and management. University of Chicago Press, Chicago and London, pp 35–65
Penezić A, Ćirović D (2015) Seasonal variation in diet of the golden jackal (Canis aureus) in Serbia. Mammal Res 60(4):309–317. https://doi.org/10.1007/s13364-015-0241-1
Porobić J, Ćirović D, Jojić V (2016) Cranial variability of the Serbian golden jackal: geographic variation, sexual dimorphism and allometry. Zool Anz J Comp Zool 261:38–47. https://doi.org/10.1016/j.jcz.2016.03.004
Raichev E, Dimitrov R, Dimova T, Hristov H (1999) How to determine the age of the golden jackal (Canis aureus) by cutting-teeth atrition and ossification of basal cranial synchondroses. Bulg J Agric Sci 5(5):807–810
Raichev E, Peeva S, Masuda R, Kaneko Y, Tsunoda H, Dian G, Georgiev D (2017) Sexual dimorphism in body parameters of the golden jackal Canis aureus L., 1758 (Carnivora, Canidae) in the Sarnena Sredna Gora Mountain and Thracian Plain (Bulgaria). Trakia J Sci 2:135–140. https://doi.org/10.15547/tjs.2017.02.006
Rezić A, Bošković I, Lubinu P, Piria M, Florijančić T, Scandura M, Šprem N (2017) Dimorphism in the skull form of golden jackals (Canis aureus Linnaeus, 1758) in the western Balkans: a geometric morphometric approach. Pak J Zool 49:989–997. https://doi.org/10.17582/journal.pjz/2017.49.3.989.997
Roberts D (1979) Mechanical structure and function of the craniofacial skeleton of the domestic dog. Acta Anat 103(4):422–433. https://doi.org/10.1159/000145044
Rykov AM, Kuznetsova AS, Tirronen KF (2022) The first record of the golden jackal (Canis aureus Linnaeus, 1758) in the Russian subarctic. Polar Biol 45:965–970. https://doi.org/10.1007/s00300-022-03037-0
Schwab JA, Figueirido B, Martín-Serra A, van der Hoek J, Flink T, Kort A, Núñez HME, Jones KE (2023) Evolutionary ecomorphology for the twenty-first century: examples from mammalian carnivores. Proc R Soc B 290:20231400. https://doi.org/10.1098/rspb.2023.1400
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611. https://doi.org/10.2307/2333709
Sillero-Zubiri C, Hoffmann M, Macdonald DW (eds) (2004) Canids: foxes, wolves, jackals and dogs. Status survey and conservation action plan. IUCN/SSC Canid Specialist Group. Gland, Switzerland and Cambridge, UK, pp 430
Spassov N, Acosta-Pankov I (2019) Dispersal history of the golden jackal (Canis aureus moreoticus Geoffroy, 1835) in Europe and possible causes of its recent population explosion. Biodivers Data J 7:34825–34825. https://doi.org/10.3897/BDJ.7.e34825
Stoyanov S (2020) Cranial variability and differentiation among golden jackals (Canis aureus) in Europe, Asia Minor and Africa. ZooKeys 917:141–164. https://doi.org/10.3897/zookeys.917.39449
Stoyanov S (2012) Craniometric differentiation of Golden Jackals (Canis aureus L. 1758) in Bulgaria, pp 48–56. In: Đorđević N (ed) Proceedings. International symposium on hunting “modern aspects of sustainable management of game population”. Zemun-Belgrade, Serbia, pp 189
Temminck CJ (1820) Description de quelques espèces de mammifères nouveaux. Nouv Ann Mus Hist Nat 7:189–194
Trouwborst A, Krofel M, Linnell JDC (2015) Legal implications of range expansions in a terrestrial carnivore: the case of the golden jackal (Canis aureus) in Europe. Biodivers Conserv 24:2593–2610. https://doi.org/10.1007/s10531-015-0948-y
Tseng ZJ, Flynn JJ (2018) Structure-function covariation with nonfeeding ecological variables influences evolution of feeding specialization in Carnivora. Sci Adv 4:2. https://doi.org/10.1126/sciadv.aao5441
Van Valkenburgh B, Sacco T (2002) Sexual dimorphism, social behavior, and intrasexual competition in large Pleistocene carnivorans. J Ver Paleontol 22(1):164–169
Viranta S, Atickem A, Werdelin L, Stenseth NC (2017) Rediscovering a forgotten canid species. BMC Zool 2:6. https://doi.org/10.1186/s40850-017-0015-0
Volokh A, Roženko N (2016) Modern distribution and morphology of the golden jackal (Canis aureus) in Ukraine. Beitr Jagd Wildforsch 41:307–318
Wozencraft WC (2005) Order Carnivora in Mammal species of the world: a taxonomic and geographic reference, 3rd ed. In: Wilson DE, Reeder DM (eds) Johns Hopkins University Press, Baltimore, pp 532–628
Zar JH (1999) Biostatistical analysis. Pearson Education, New Delhi
Acknowledgements
We thank the professional hunters at SEFAG ZRT, specifically Mihály Horváth, who provided the jackals and helped with body measurements. We thank our colleagues at Dél-Dunántúli Fauna Vt. for their assistance in skull cleaning and Roland Szabó, Evelin Buzsáki, Kristóf Kámán, and Kristóf László Tóth for contributing the skull images. Special thanks to the reviewers for their valuable comments and suggestions.
Funding
Open access funding provided by University of Sopron.
Author information
Authors and Affiliations
Contributions
Conceptualization: ECS, GyS; methodology: ECS, GyS; writing—original draft preparation: ECS, GyS; review: GyS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Statement of animal ethics
The body and skull samples used in this study were obtained from individuals who died due to natural causes or as a result of legal hunting. No animal was killed for this study.
Additional information
Handling editor: Kévin Le Verger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Csányi, E., Sándor, G. Sexual dimorphism in the Hungarian golden jackal population: analysing body and skull size and shape. Mamm Biol (2024). https://doi.org/10.1007/s42991-024-00436-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42991-024-00436-0