Abstract
The Egyptian mongoose (Herpestes ichneumon) is considered a generalist and opportunistic predator whose European distribution is restricted to Iberia. However, all the previous studies on the Egyptian mongoose’s diet in the Iberian Peninsula have been performed in Portugal and Doñana National Park (southwestern Spain), covering a small region of the species’ current distribution. To improve the knowledge on the trophic ecology of the species, we estimated the feeding habits of the Egyptian mongoose in central Spain, its north-eastern range edge. Our study was based on the analysis of (1) scats (n = 251) and (2) stomach contents (n = 32); samples were collected between 2018 and 2022. We calculated the relative frequency of occurrence (%RFO) and the percentage consumed biomass (%CB) for 12 food groups across central Spain and we assessed site-seasonal variations in diet composition in three study areas. We also estimated rabbit densities in the three study areas to assess the effect of rabbit abundance on mongoose diets. Overall, our results showed that European rabbits were the group with highest %CB (30.7%) in the diet of the Egyptian mongoose, followed by reptiles (19.9%) and small mammals (19.9%). In the intensively studied areas, the %CB of rabbits was the highest in the area with the highest abundance of this prey, whereas reptiles had the highest %CB in the area with the lowest availability of rabbits. Diet diversity (Shannon index) varied between 1.73 and 2.47 in those areas and was inversely related to the availability of rabbits. Our results confirm the dietary flexibility of this predator that adapts its diet to the relative availability of different prey species in different habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to their diet, predators can be classified in (1) opportunistic, i.e., those which take food depending on its abundance; or (2) selective, i.e., those which take prey that maximizes net energy and nutritional gain (Palomares 1993a). In turn, generalist predators are those which take a wide variety of prey, while specialists prey mostly upon one or several particular prey taxa (Begon and Townsend 2020). The Egyptian mongoose (Herpestes ichneumon) is considered a generalist and opportunistic predator (Bandeira et al. 2018; Palomares 1993a), since its diet includes, among other foods, European rabbits (Oryctolagus cuniculus, rabbits hereafter), small mammals (e.g., Apodemus sylvaticus, Mus musculus, Mus spretus), reptiles, amphibians, birds, insects, and carrion (Palomares 1993a; Rosalino et al. 2009; Bandeira et al. 2018). The European distribution of the Egyptian mongoose is restricted to the southwest of the Iberian Peninsula (Bencatel et al. 2018; Palomares and Román 2020; Descalzo et al. 2021a). It occurs in most of Portugal and, within Spain, in Extremadura, western Castilla-La Mancha and central-western Andalucía regions, with some isolated records in northern and eastern Spanish areas (Descalzo et al. 2021a). This mongoose is a strictly diurnal species (Palomares and Delibes 1993a) and often moves in groups of up to five individuals (Palomares and Delibes 1993b). Egyptian mongooses prefer dense vegetation cover and lineal structures (e.g., vegetation associated to waterways with rushes and blackberries) (Palomares and Delibes 1990a; Pereira and Rodríguez 2010; Curveira-Santos et al. 2017), where they usually defecate in discrete though visible places, forming latrines near breeding and resting places (Palomares 1993b; Descalzo et al. 2021b). All studies about the diet of the Egyptian mongoose agree on a high spatial and seasonal variation of the mongoose trophic habits, although they have been performed in a limited part of the current distribution area of the species within the Iberian Peninsula; in particular, in Portugal (Bandeira et al. 2018; Rosalino et al. 2009) and in Doñana National Park (DNP), southwestern Spain (Delibes et al. 1984; Palomares 1993a; Palomares and Delibes 1991a, b). In contrast, no diet studies have been performed until now in its north-eastern range edge.
Most studies on the diet of the Egyptian mongoose in DNP showed similar results in the frequency of occurrence, with insects being the most frequent prey, followed by rabbits, reptiles, birds, small mammals, and amphibians (Delibes 1976; Delibes et al. 1984; Palomares 1993a; Palomares and Delibes 1991a, b). Regarding the proportion of consumed biomass (CB), the trend is towards higher values for rabbits, followed by reptiles, small mammals, birds, and carrion (Delibes 1976; Delibes et al. 1984; Palomares 1993a; Palomares and Delibes 1991a, b). Studies on the Egyptian mongoose’s diet in DNP show spatial and seasonal variations. Palomares and Delibes (1991a) showed that it changed throughout the year, with a higher consumed biomass of amphibians, reptiles, and insects in autumn. In contrast, rabbits were consumed almost in the same proportion in all seasons. In the south of DNP, the CB varied by season, being higher for small mammals in winter, for reptiles in spring, and for birds in summer (Palomares and Delibes 1991b). Palomares (1993a) compared the diet of the Egyptian mongoose in three areas of DNP with different prey density and found that the diet was most specialized in the area with the highest rabbit density, indicating that the mongoose is a generalist predator that consumes a wide variety of prey. In such areas, rabbits represented most of CB, whereas birds and reptiles predominated in other areas (Palomares 1993a).
The studies of Egyptian mongoose diet in Portugal mostly agree with the results from DNP. Egyptian mongooses in Portugal eat mainly lagomorphs and small mammals (groups with highest CB), followed by reptiles and amphibians, whereas arthropods represent a low CB despite being the most frequent food group (Bandeira et al. 2018; Rosalino et al. 2009).
All these studies agree that the Egyptian mongoose is a generalist and opportunistic predator, although rabbits apparently play an important role in its diet. This lagomorph is a key native prey species in southern Europe (Delibes-Mateos et al. 2007), being included in the diet of more than 40 predators (Delibes-Mateos et al. 2008). Besides, the rabbit is a species with high adaptability, which allows a high colonising ability and potential to reach high densities (Gibb 1990). However, Iberian rabbit populations have declined in most areas in the last decades, mainly because of habitat loss (Delibes-Mateos et al. 2010) and the impact of two viral diseases (rabbit haemorrhagic disease—RHD—and myxomatosis) (Delibes-Mateos et al. 2008; Villafuerte et al. 2017). Rabbit decline has been exacerbated since the early 2000s as result of the outbreak of a new RHD variant (Abrantes et al. 2013). Although this recent rabbit decline could have affected the mongoose's feeding behaviour, possibly focusing on other types of secondary prey, most studies on mongoose diet published until now are previous to the outbreak of the new RHD variant (but see Bandeira et al. 2018). In this context, hunters have recently raised complaints on the potential adverse effects of the Egyptian mongoose on some game species, such as rabbits and red-legged partridges (Alectoris rufa), particularly in areas at the edge of its distribution, where the species has only recently become established (Descalzo et al. 2021a).
Until now, no information on the diet of the Egyptian mongoose was available from the centre of the Iberian Peninsula, namely its north-eastern distribution limit. Therefore, our aim was to study the feeding habits of the Egyptian mongoose in Castilla-La Mancha (central Spain) and their spatial and seasonal variations, based on the analysis of scats (systematically collected) and stomach contents from road-killed individuals (opportunistically collected). According to previous studies (e.g., Palomares and Delibes 1991b), we would expect some spatial and temporal differences in the Egyptian mongoose diet within this region.
Materials and methods
Study area
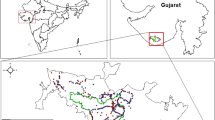
The study was carried out at two spatial scales: at the regional scale (Castilla-La Mancha—CLM—central Spain), and at three areas within CLM (Fig. 1). CLM is dominated by a Mediterranean climate with moderate annual thermal oscillations and notable summer droughts which allows for a great diversity of habitats (e.g., oak woodlands and scrubland, rushes, brambles, brooms, and pastures) (Rivas-Martínez et al. 2004). At the regional scale, we opportunistically collected scats and stomachs from road-killed mongooses (Fig. 1). Locally, we performed systematic, intensive surveys in three areas (Fig. 1) which varied according to habitat configuration and rabbit abundance. One of the areas, in the surroundings of the Finisterre Reservoir (FR, Toledo Province, 39° 37′ 20.8″ N, 3° 40′ 57.9″ W), was dominated by agricultural landscapes and riparian formations of tamarisks (Tamarix canariensis) and rushes (Juncus acutus), with some brambles (Rubus ulmifolius), brooms (Retama sphaerocarpa), giant canes (Arundo donax), and some white poplars (Populus alba). The second area, El Castañar (EC, Toledo province, 39° 41′ 17.5″ N, 4° 06′ 03.7″ W), was dominated by holm oaks (Quercus ilex) and savannah-like pasturelands with scattered holm and cork oak trees (“dehesas”), with some rushes and brambles. The third area, Picón (PI, Ciudad Real Province, 39° 5′ 12.0″ N, 4° 4′ 36.3″ W), was dominated by holm oaks and crimson-spot rockroses (Cistus ladanifer), with some temporary streams fringed by rushes and brambles. As in most of CLM region, the three areas present a Mediterranean pluviseasonal continental bioclimate featured by wet and mild winters and dry and hot summers (Rivas-Martínez et al. 2004).
Data collection
Surveys for scats consisted of transects on foot near to the edge of scrubland vegetation where mongooses usually locate their resting places (Descalzo et al. 2021b). In the three areas of intensive survey, these transects were systematically walked weekly/biweekly during 5–7 months (from February 2020 to August 2020 in PI; from September 2020 to January 2021 in EC), except in FR where scats were collected throughout a full year (from February 2021 to February 2022), to study the diet seasonal variations. At the regional scale, we also collected scats in non-systematic surveys between March and October of 2019 from other favourable zones for the mongoose within CLM (n = 9 municipalities) (Descalzo et al. 2019, 2021a). We also opportunistically collected stomachs from dead Egyptian mongooses throughout the year (monthly sample sizes between 1 and 6), mainly from road kills occurred in different areas across CLM between 2018 and 2022. Overall, our field sampling covered most of the mongoose distribution in CLM (Fig. 1).
Once collected, scats were dried in an oven at 60 °C for 48 h, weighed, and stored until their analysis (Ferreras and Fernandez-de-Simon 2019). Before the analysis, scats were individually soaked in water with some detergent drops for 24 h, and then broken apart under a water stream on a 0.5 mm sieve (Palomares and Delibes 1991b; Reynolds and Aebischer 1991). Food remains were identified under 10 × and 40 × microscopes, with the help of keys of bones, teeth, hairs, feathers, and seeds (Brom 1986; Catalán 1991; Day 1966; Dueñas and Peris 1985; Felix and Montori 1986; Gállego-Castejón 1982; Gállego-Castejón and López 1983; Gállego-Castejón and Alemany 1985; Teerink 2003; Valente et al. 2015). All collected scats were previously identified in the field according to their location, morphology, size, odour, and finally confirmed in the lab as belonging to the Egyptian mongoose by the characteristic hairs of this mongoose found in the scats (Descalzo et al. 2021d).
We estimated the density of rabbits in the three intensive study areas to assess whether rabbit availability was related to the diversity of mongoose diet. Rabbit densities were estimated by distance sampling from counts obtained during transects in vehicles (40.5–75 km total length in each study area) at 10–15 km/h (Fernandez-de-Simon et al. 2011) starting 1 h before sunrise, according to the main activity peaks of rabbits in the study areas (Descalzo et al. 2021c). Transects were repeated in consecutive days until the number of observations allowed precise density estimates in a stacked analysis which has been justified in situations with sparse data (Flockhart et al. 2016). Besides, in FR, we estimated rabbit density for two periods (spring and summer).
Data analysis
Food remains were grouped in 12 food classes: lagomorphs (mainly rabbits, hares Lepus granatensis remains were only detected once), small mammals, reptiles, amphibians, insects, birds, eggs, fruits, carrion, crustaceans, molluscs, and rubbish (e.g., plastic materials). The relative frequency of occurrence (%RFO) was calculated as the percentage of the number of occurrences of one food type from the total number of occurrences of all food types (Mason and Macdonald 1986). %RFO is the most direct method to compare diet composition in different areas (Clavero et al. 2003). We estimated visually the volume proportion of each food group in each scat (Reynolds and Aebischer 1991). Then, we estimated the dry weight for each food class in each scat by multiplying its volume proportion by the scat dry weight (Reynolds and Aebischer 1991). To obtain the percentage of consumed biomass (%CB), we multiplied this value by a correction factor (CF) estimated for each food item (Palomares and Delibes 1990b, 1991b). For rubbish and molluscs (for which no CF was available in the case of the mongoose), we used the CF estimated for carrion and crustaceans, respectively. For fruits, we used the CF value published for the red fox (Vulpes vulpes) (Ferreras and Fernandez-de-Simon 2019).
Since the food items were almost intact in the stomachs (i.e., not yet digested), the %CB for stomach contents were estimated using the weight of each food group over the weight of the full stomach contents, without applying a correction factor. We analysed the diet according to the sex of the mongoose for the stomach samples. We calculated the %CB and %RFO for scats at the regional scale, considering the scats collected not systematically and including ten random samples for each intensive study area to avoid bias due to larger sample sizes in the three intensive study areas, and to have a sample size similar to that of stomach contents for assessing the differences between the two types of samples (scats and stomach contents).
We also analysed diet diversity in the three areas intensively studied, and at the regional scale for scats and stomach contents. As a measure of niche breadth, we calculated the standardized Levins’ index (Bsta) (Feinsinger et al. 1981), which varies between 0 (narrowest niche) and 1 (widest niche)
where pi is the proportion of biomass belonging to food class i and R is the number of food classes (Feinsinger et al. 1981)
We also calculated the Shannon–Wiener index (H′) (Rosalino et al. 2009) as a measure of diet diversity
where pi is the proportion of biomass belonging to food class i, and R is the total number of food classes (Krebs 1989). A diversity curve was calculated by bootstrapping with replacement, in increments of one, and then resampled 10,000 times to obtain a mean for each sample size (Hass 2009). We also calculated the diet diversity from scats including both samples non-systematically collected and the random samples from the three study areas on one hand, and from the stomach contents on the other hand. Adequacy of sampling effort was visually assessed by whether an asymptote was reached in the diversity curve and another curve calculated from the incremental change in H′ (Supplementary Information). On the other hand, we used the Hutcheson t test for two diversity indices to assess the differences in the diet diversity between scats and stomach contents.
First, we compared visually the diet among the three study areas, and among seasons by plotting the major axes of dietary variation identified with correspondence analysis (Ray and Sunquist 2001). Second, we performed a MANOVA to test for statistical differences in the %CB considering the 12 food classes between: (1) scats and stomach contents (regional scale), (2) stomach contents according to sex, (3) the three study areas (local scale), and (4) seasons (spring: April–June; summer: July–September; autumn: October–December; and winter: January–March) in the study area FR with data collected for a full year. If the MANOVA test was significant, we performed an ANOVA for each food class and post hoc Tukey tests to assess differences between pairs of study areas or seasons for which the ANOVA indicated significant effects. All these analyses were performed in R 4.1.2 (R Core Team 2020).
Finally, rabbit densities were estimated through distance sampling analysis from rabbit counts for each study area using the unmarked package in R 4.1.2 (R Core Team 2020). Several detection functions (half-normal, exponential, and hazard-rate) were assessed for each estimate, selecting the function with lowest AIC (Burnham and Anderson 2002).
Results
A total of 251 mongoose scats were collected, including 19 scats collected in non-systematic surveys (for the analysis at regional scale) and 232 scats in intensively study areas (FR: 140, EC: 31, PI: 61). For the analysis at local scale, we only considered the scats collected during the intensive survey to avoid bias between periods (FR: 88, EC: 31, PI: 48). For the analysis of seasonal diet, we considered all scats collected in FR during the full year of intensive survey and non-systematic surveys, totalling 140 scats (46, 30, 15, and 49 for spring, summer, autumn, and winter, respectively, Table S1, Supplementary Information). In all cases, the sample sizes were suitable, since the diversity curves stabilized between 15 and 20 samples (Figs. S1–S4, Supplementary Information).
According to the MANOVA results, the diet composition in terms of %CB at the regional scale was not significantly different between scats and stomachs (p = 0.164; Pillai’s Trace test). However, the ANOVA test showed differences in %CB of amphibians (ANOVA: F1,79 = 6.25, p = 0.014), which were only found in stomachs but not in scats. For this reason, data from these two sources (scats and stomachs) were analysed separately. On the other hand, the MANOVA test showed significant differences in the diet between sexes (18 males and 14 females). The ANOVA test showed differences in the %CB of insects, which was higher in males than in females (ANOVA: F1,30 = 4.39, p = 0.044).
According to the scat analysis (regional scale), rabbits represented overall the highest %CB followed by reptiles and small mammals (Table 1). According to the analysis of %RFO, insects were the most frequent group found in the scats, followed by small mammals and reptiles (Table 1). In the case of stomach contents, rabbits were also the group with the highest %CB, followed by fruits and carrion (Table 1), while small mammals were the most frequent group (%RFO), followed by rabbits and insects (Table 1). The most consumed species of small mammals were Apodemus sylvaticus, Microtus duodecimcostatus, Mus sp.; the most consumed reptiles were Rhinechis scalaris and Psammodromus manuelae, and the most consumed birds belonged to Ralliforme and Passeriforme orders (Table S2, Supplementary Information). The stomach contents had slightly higher diet diversity (H′ = 2.43) than scats (H′ = 2.20), although the trophic niche breadth was very similar (Bsta = 0.41 and 0.415, respectively). Nevertheless, the Hutcheson t test did not show significant differences in the diet diversity between stomach contents and scats (t123 = 0.59, p = 0.558).
At the local scale, rabbits represented the highest %CB (62.0%) in FR (Fig. 2a), whereas insects represented the highest %RFO (Supplementary Information, Table S3). In EC, rabbits represented also the highest %CB (39.2%), (Fig. 2a), and insects also the highest %RFO (Supplementary Information, Table S3). In contrast, reptiles represented the highest %CB (46.3%) in PI, but insects represented again the highest %RFO (Supplementary Information, Table S3). Mongooses in FR had the least diverse diet (H′ = 1.85) and the narrowest trophic niche (Bsta = 0.19), compared to PI (H′ = 2.61, Bsta = 0.31) and EC (H′ = 2.00, Bsta = 0.33) (Figs. S1, S2, S3, S4 Supplementary Information).
Diet composition in terms of %CB significantly differed among the three intensive study areas (MANOVA: p < 0.001; Pillai’s trace test). Subsequent ANOVAs showed differences for the %CB of birds (F2,164 = 3.09, p = 0.048), insects (F2,164 = 3.09, p = 0.048), rabbits (F2,164 = 40.59, p < 0.001), reptiles (F2,164 = 8.83, p < 0.001), and small mammals (F2,164 = 13.10, p < 0.001) (Fig. 3). The correspondence analysis graphically confirmed these differences among study areas (Fig. S6a), showing that the Egyptian mongoose consumed more reptiles in PI, more rabbits in FR and more fruits in EC (Fig. S6a).
The analyses conducted throughout a full year in FR revealed that rabbits represented the highest %CB in all seasons (Fig. 2b, Table S4, Supplementary Information), but the secondary foods varied among seasons: insects and fruits were the secondary foods in spring and summer, respectively, small mammals in winter, and insects and reptiles in autumn (Fig. 2b, Table S4, Supplementary Information). Seasonal variations were characterized by more consumption of reptiles in autumn; rabbits, small mammals, and birds both in winter and spring, and fruits, carrion, and crustaceans in summer (Fig. 2b). Besides, rabbits represented the highest %RFO in winter and spring, and insects in autumn and summer (Table S4, Supplementary Information). The MANOVA test confirmed the significant differences among seasons for the food composition (p < 0.01; Pillai’s Trace test; Fig. S5 Supplementary Information). According to the ANOVAs, the mongoose diet significantly differed among seasons only for the %CB of fruits (F3,136 = 3.30, p = 0.022), insects (F3,136 = 6.01, p < 0.001), rabbits (F3,136 = 3.04, p = 0.031), and reptiles (F3,136 = 2.72, p = 0.047). Post hoc Tukey tests showed that: %CB of fruits was significantly higher in summer than in winter (p = 0.011) (Fig. 4a); %CB of insects was significantly lower in winter and spring than both in autumn and summer (p = 0.020) (Fig. 4b); %CB of rabbits was significantly higher in winter than in summer (p = 0.016) (Fig. 4c); and %CB of reptiles was significantly higher in autumn than in winter (p = 0.041) (Fig. 4d). Seasonal differences are also depicted by the correspondence analysis (Fig. 4b), which shows that the mongoose diet in FR was more associated to rabbits in spring and winter, to fruits in summer, and to reptiles and insects in autumn (Fig. 4b).
The estimated rabbit density (mean ± SE) in FR was 1.680 ± 0.110 rabbits/ha in spring and 2.640 ± 0.509 in summer, 0.135 ± 0.033 in EC and 0.040 ± 0.017 in PI. The highest rabbit density corresponded to the study area with the highest rabbit consumption and the lowest mongoose diet diversity (FR), whereas the lowest rabbit density occurred in the study area with the lowest rabbit consumption and highest diet diversity (PI).
Discussion
The Egyptian mongoose in its north-eastern range edge showed a diverse diet typical of a generalist and opportunistic predator, which agrees with previous studies conducted in other areas of its European range. Although our results show that %CB of scats and stomach contents were not significantly different, the ANOVA test showed significant differences between scats and stomach contents in the %CB of amphibians, which were found in stomach contents but not in scats. This is likely due to the more intense digestion of amphibians compared to other foods, which may reduce the remains of this prey found in scats. In fact, we found three natterjack toads (Epidalea calamita) ingested in full in one stomach. These findings contrast with the lack of differences between scats and stomach contents found in a regional review of red fox diet in the Iberian Peninsula (Díaz-Ruiz et al. 2013). In our study, the highest %CB in stomach contents corresponded to rabbits, followed by fruits and carrion, partially agreeing with another study on mongoose diet in Portugal that analysed stomach contents and found that rabbits were also the group with the highest %CB, but secondary prey were reptiles, arthropods, and other mammals (Rosalino et al. 2009). The diet diversity estimated from the stomach contents (H′ = 2.43) was higher than the mongoose diet diversity estimated in Portugal from stomach contents (H′ = 2.02, as recalculated for our 12 food groups) (Rosalino et al. 2009). Despite the larger sample size (N = 59 vs N = 32 in our study), the study from Portugal was mostly concentrated on the winter and spring seasons, whereas our sample of stomachs was collected throughout the year and, therefore, included a better representation of the seasonal variations in the mongoose diet. On the other hand, we found differences in the %CB of insects among males and females, being higher in males. These results differ from the findings in another study, where males consumed more mammals, whereas females consumed more reptiles and fruits (Rosalino et al. 2009).
According to our results from scats, rabbits were overall the group with highest %CB in the mongoose diet in central Spain, followed by small mammals, reptiles, insects, and fruits. These results agree with most of those published for other Iberian areas, where rabbits were the most important prey (Table S5, Supplementary Information; Delibes 1976; Delibes et al. 1984; Rosalino et al. 2009; Bandeira et al. 2018). Only in one study performed in a specific area of Doñana National Park (SW Spain), rabbits were replaced by reptiles as the prey with the highest %CB (Palomares and Delibes 1991b).
The significant differences in the consumption of some food groups between the three study areas could be partially related to differences in the season when the scats were collected, mostly between EC (September–January) and PI (February–August). Therefore, these differences found between our study areas might be partially explained by the seasonal variations in the mongoose diet (Bandeira et al. 2018), since we collected the scats in different seasons in each study area. The higher consumption of rabbits in FR than in the other areas (Fig. 3c) is likely due to the higher rabbit density found in FR (1.68 rabbits/ha). By contrast, the higher consumption of reptiles in PI than in the other areas (Fig. 3d) might be likely related to the low rabbit density in PI (0.040 rabbits/ha). The Egyptian mongoose is an opportunistic predator, and as consequence of the low rabbit density in PI, this carnivore likely consumed the next more available food. This replacement of rabbits with reptiles was also previously reported in southern Spain (Palomares and Delibes 1991b), and confirms the adaptation of the Egyptian mongoose to hunt on this prey. The higher consumption of small mammals in PI and EC compared to FR (Fig. 3e) is also likely a result of the lower availability of rabbits in the former zones. In addition, we found a less diverse diet (H′ = 1.85), with a narrower trophic niche (Bsta = 0.19) in FR, where the highest rabbit density was estimated, than in PI (H′ = 2.61, Bsta = 0.31) and EC (H′ = 2.00, Bsta = 0.33), where rabbit densities were lower. These findings resemble those obtained by Palomares and Delibes (1991a) and Palomares (1993a) who reported differences in the diet of the Egyptian mongoose between zones nearby, with a specialized diet on rabbits in the area with higher rabbit density.
In addition, our findings revealed that the Egyptian mongoose diet in CLM varied seasonally. In FR, rabbits were the most consumed prey in all seasons, but they were significantly more consumed in winter than in summer (Fig. 4c), which agrees with Palomares and Delibes (1991a). This result is striking as the highest rabbit abundance in the Iberian Peninsula occurs in summer after the reproductive period, while lowest numbers are recorded in winter (Beltrán 1991; Moreno et al. 2004). Nevertheless, this paradox could be due to a potential higher availability in winter of rabbit kittens (which mongooses hunt in the breeding burrows), as result of the start of the breeding season, in addition to a lower availability in this period of the year of alternative prey, such as reptiles, small mammals, insects, or birds (Palomares and Delibes 1991a; Palomares 1993a). These alternative preys are abundant during the summer months, allowing a more diverse diet in that season (Fig. 4). In our study, the consumed biomass of reptiles was higher in autumn than in winter, which agrees with Palomares and Delibes (1991a), but in contrast with the summer peak of reptiles consumption reported in other studies (e.g., Delibes et al. 1984). In our case, the increase of reptiles intake in autumn might be related to the ability of the Egyptian mongoose to find and dig the reptiles in their hideouts, although we cannot confirm this hypothesis, because we did not assess the abundance of other foods; other studies showed that this mongoose eats the resources more abundant in each situation (Delibes et al. 1984; Palomares 1993a; Bandeira et al. 2018). In summary, our results confirm the conclusion raised by Bandeira et al. (2018) that the Egyptian mongoose consumes in each season those most abundant and available prey.
At the regional scale, the least consumed food categories in our study were eggs, amphibians, molluscs, and carrion, likely because of their low availability. The small level of consumption of these food groups prevented the analysis of their spatial and temporal variations. Even so, we found a higher diet diversity (H′ = 2.47) in the study area with lowest rabbit density (PI), due to the contribution of carrion, rubbish, eggs and even amphibians (Fig. 2a). In the case of birds, they represented a low CB both at regional and local scales (Fig. 2a). Most consumed birds were ralliformes, while red-legged partridge remains were found in just two scats (0.79% of samples), suggesting a potential reduced pressure by the Egyptian mongoose on this prey. The contribution of red-legged partridge to the mongoose diet was low, although we did not assess the availability of this prey. Often, the apparent high predation impact of this mongoose on some small game species is presented as a reason to implement predator control actions targeting mongooses, but more studies about its diet and impact on prey should be carried out before reaching any conclusion, which would allow to improve the management and conservation for the species implicated. The potential role of this carnivore on the population dynamics of specific prey would require detailed studies on the predator–prey relationships.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abrantes J, Lopes AM, Dalton KP, Melo P, Correia JJ, Ramada M, Alves PC, Parra F, Esteves PJ (2013) New variant of rabbit hemorrhagic disease virus, Portugal, 2012–2013. Emerg Infect Dis 19:1900
Bandeira V, Virgós E, Carvalho J, Barros T, Cunha MV, Fonseca C (2018) Diet footprint of Egyptian mongoose along ecological gradients: effects of primary productivity and life history traits. Mamm Biol 88:16–25. https://doi.org/10.1016/j.mambio.2017.11.004
Begon M, Townsend CR (2020) Ecology: from individuals to ecosystems. Wiley, Oxford
Beltrán JF (1991) Temporal abundance pattern of the wild rabbit in Doñana, SW Spain. Mammalia 55:591
Bencatel J, Ferreira CC, Barbosa AM, Rosalino LM, Álvares F (2018) Research trends and geographical distribution of mammalian carnivores in Portugal (SW Europe). PLoS One 13:e0207866. https://doi.org/10.1371/journal.pone.0207866
Brom TG (1986) Microscopic identification of feathers and feather fragments of Palearctic birds. Bijdragen Tot De Dierkunde 56:181–204
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York. https://doi.org/10.1007/b97636
Catalán G (1991) Semillas de árboles y arbustos forestales. Ministerio de Agricultura Pesca y Alimentación, Instituto de la Conservación de la Naturaleza (ICONA), Madrid
Clavero M, Prenda J, Delibes M (2003) Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. J Biogeogr 30:761769
Curveira-Santos G, Marques TA, Björklund M, Santos-Reis M (2017) Mediterranean mesocarnivores in spatially structured managed landscapes: community organisation in time and space. Agric Ecosyst Environ 237:280–289
Day MG (1966) Identification of hair and feather remains in the gut and faeces of stoats and weasels. J Zool 148:201–217
Delibes M (1976) Datos sobre la alimentación del meloncillo, Herpestes ichneumon widdringtoni Gray, 1842, en España. Sauget Mitt 24:38–42
Delibes M, Aymerich M, Cuesta L (1984) Feeding habits of the Egyptian mongoose or ichneumon in Spain. Acta Theriol 29:205–218
Delibes-Mateos M, Redpath SM, Angulo E, Ferreras P, Villafuerte R (2007) Rabbits as a keystone species in southern Europe. Biol Conserv 137:149–156. https://doi.org/10.1016/j.biocon.2007.01.024
Delibes-Mateos M, Delibes M, Ferreras P, Villafuerte R (2008) Key role of European rabbits in the conservation of the western Mediterranean Basin hotspot. Conserv Biol 22:1106–1117. https://doi.org/10.1111/j.1523-1739.2008.00993.x
Delibes-Mateos M, Farfán MA, Olivero J, Vargas JM (2010) Land-use changes as a critical factor for long-term wild rabbit conservation in the Iberian Peninsula. Environ Conserv 37:169–176. https://doi.org/10.1017/S0376892910000214
Descalzo E, Díaz-Ruiz F, Jiménez J, Martínez‐Jauregui M, Soliño M, Márquez AL, Farfán MA, Real R, Delibes-Mateos M, Ferreras P (2019) Combining questionnaire survey and biogeographic models to update and forecast the distribution of the only mongoose species in Europe. 8th European Congress of Mammalogy, 23–27 September 2019, Warsaw
Descalzo E, Díaz-Ruiz F, Delibes-Mateos M, Salgado I, Martínez-Jauregui M, Soliño M, Jiménez J, Linares O, Ferreras P (2021a) Update of the Egyptian mongoose (Herpestes ichneumon) distribution in Spain. Galemy 33:29–38
Descalzo E, Jiménez J, Delibes-Mateos M, Díaz-Ruiz F, Ferreras P (2021b) Assessment of methods for detecting an opportunistic and expanding mesocarnivore in southwestern Europe. J Zool 315:138–148. https://doi.org/10.1111/jzo.12912
Descalzo E, Torres JA, Ferreras P, Díaz-Ruiz F (2021c) Methodological improvements for detecting and identifying scats of an expanding mesocarnivore in south-western Europe. Mamm Biol 101:71–81
Descalzo E, Tobajas J, Villafuerte R, Mateo R, Ferreras P (2021d) Plasticity in daily activity patterns of a key prey species in the Iberian Peninsula to reduce predation risk. Wildl Res 48:481–490
Díaz-Ruiz F, Delibes-Mateos M, García-Moreno JL, López-Martín JM, Ferreira C, Ferreras P (2013) Biogeographical patterns in the diet of an opportunistic predator: the red fox Vulpes vulpes in the Iberian Peninsula. Mamm Rev 43:59–70
Dueñas ME, Peris SJ (1985) Clave para los micromamíferos (Insectivora y Rodentia) del Centro y Sur de la Península Ibérica. Claves para la identificación de la fauna española, vol 27. Ediciones Universidad de Salamanca, Salamanca
Feinsinger P, Spears EE, Poole RW (1981) A simple measure of niche breadth. Ecology 62:27–32
Felix J, Montori A (1986) Determinación de las especies de anfibios anuros del nordeste ibérico mediante el hueso ilion. Misc Zool 10:239–246
Fernandez-de-Simon J, Díaz-Ruiz F, Cirilli F, Tortosa FS, Villafuerte R, Delibes-Mateos M, Ferreras P (2011) Towards a standardized index of European rabbit abundance in Iberian Mediterranean habitats. Eur J Wildl Res 57:1091–1100. https://doi.org/10.1007/s10344-011-0524-z
Ferreras P, Fernandez-de-Simon J (2019) Correction factors for estimating food consumption by red fox Vulpes vulpes from scats. Wildl Biol 2019:1–9. https://doi.org/10.2981/wlb.00557
Flockhart DTT, Norris DR, Coe JB (2016) Predicting free-roaming cat population densities in urban areas. Anim Conserv 19:472–483
Gállego-Castejón L (1982) Vertebrados ibéricos (Claves para su determinación). Universidad de Sevilla, Sevilla
Gállego-Castejón L, Alemany A (1985) Vertebrados ibéricos, 6 roedores y lagomorfos. Universitat de les Illes Balears, Palma de Mallorca
Gállego-Castejón L, López S (1983) Vertebrados ibéricos, vol 3: reptiles. Imprenta Sevillana, S. A, Dos Hermanas
Gibb JA (1990) The European rabbit Oryctolagus cuniculus. In: Chapman JA, Flux JE (eds) Rabbits, hares and pikas-status survey and conservation action plan. IUCN, Gland, pp 116–120
Hass CC (2009) Competition and coexistence in sympatric bobcats and pumas. J Zool 278:174–180
Krebs C (1989) Ecological methodology. Harper Collins Publishers, New York
Mason CF, Macdonald SM (1986) Otters: ecology and conservation. Cambridge University Press, Cambridge
Moreno S, Villafuerte R, Cabezas S, Lombardi L (2004) Wild rabbit restocking for predator conservation in Spain. Biol Conserv 118:183–193
Palomares F (1993a) Opportunistic feeding of the Egyptian mongoose, Herpestes ichneumon (L.) in Southwestern Spain. Rev Ecol 48:295–304
Palomares F (1993b) Faecal marking behaviour by free-ranging common genets Genetta genetta and Egyptian mongooses Herpestes ichneumon in southwestern Spain. Z Fur Sauget 58:225–231
Palomares F, Delibes M (1990a) Habitat preference of large grey mongooses Herpestes ichneumon in Spain. Acta Theriol 35:1–6
Palomares F, Delibes M (1990b) Factores de transformación para el cálculo de la biomasa consumida por gineta (Genetta genetta) y meloncillo (Herpestes ichneumon) (Carnivora, Mammalia). Misc Zool 14:233–236
Palomares F, Delibes M (1991a) Dieta del meloncillo (Herpestes ichneumon), en el Coto del Rey (Norte del Parque Nacional de Doñana, SO España). Doñana Acta Vertebr 18:187–194
Palomares F, Delibes M (1991b) Alimentación del meloncillo Herpestes ichneumon y de la gineta Genetta genetta en la Reserva Biológica de Doñana, S.O. de la Península Ibérica. Doñana Acta Vertebr 18:5–20
Palomares F, Delibes M (1993a) Resting ecology and behaviour of Egyptian mongooses (Herpestes ichneumon) in southwestern Spain. J Zool 230:557–566
Palomares F, Delibes M (1993b) Social organization in the Egyptian mongoose: group size, spatial behaviour and inter-individual contacts in adults. Anim Behav 45:917–925
Palomares F, Román J (2020) Nuevos datos sobre la distribución y hábitat usados por el meloncillo en la península ibérica: ¿Es más común y generalista de hábitat de lo que se conocía? Galemys 32:21–30
Pereira M, Rodríguez A (2010) Conservation value of linear woody remnants for two forest carnivores in a Mediterranean agricultural landscape. J Appl Ecol 47:611–620
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ray J, Sunquist M (2001) Trophic relations in a community of African rainforest carnivores. Oecologia 127:395–408. https://doi.org/10.1007/s004420000604
Reynolds JC, Aebischer NJ (1991) Comparison and quantification of carnivore diet by faecal analysis: a critique, with recommendations, based on a study of the fox Vulpes vulpes. Mamm Rev 21:97–122
Rivas-Martínez S, Penas A, Díaz TE (2004) Bioclimatic and Biogeographic maps of Europe. Cartographic Service, University of León, León. http://www.globalbioclimatics.org/form/bi_map/index.htm. Accessed 17 Mar 2022
Rosalino LM, Santos MJ, Pereira I, Santos-Reis M (2009) Sex-driven differences in Egyptian mongoose’s (Herpestes ichneumon) diet in its northwestern European range. Eur J Wildl Res 55:293–299. https://doi.org/10.1007/s10344-008-0248-x
Teerink BJ (2003) Hair of West European mammals: atlas and identification key. Cambridge University Press, Cambridge
Valente AM, Rocha RG, Lozano E, Ferreira JP, Fonseca C (2015) Atlas dos Pelos dos mamíferos terrestres ibéricos. Edições Afrontamento, Porto
Villafuerte R, Castro F, Ramírez E, Cotilla I, Parra F, Delibes-Mateos M, Recuerda P, Rouco C (2017) Large-scale assessment of myxomatosis prevalence in European wild rabbits (Oryctolagus cuniculus) 60 years after first outbreak in Spain. Res Vet Sci 114:281–286. https://doi.org/10.1016/j.rvsc.2017.05.014
Acknowledgements
Special thanks go to Castilla-La Mancha rangers (Agentes Medioambientales) for their collaboration in the selection of survey places in the study area. Thanks to Rafael and Jose María Finat for allowing us to perform the surveys in one of the study areas (El Castañar).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study is part of the research project SBPLY/17/180501/000184, funded by the regional Government of CLM and the European Regional Development Fund (ERDF). Esther Descalzo was supported by a predoctoral research contract funded by the University of Castilla-La Mancha co-supported by the Plus European Social Fund (FSE +). Francisco Díaz-Ruiz was supported by a postdoctoral research contract funded by the University of Málaga through the grant program “Ayudas para la Incorporación de Doctores del I Plan Propio de Investigación de la Universidad de Málaga (Call 2020)”.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collections were performed by all authors. Analysis was performed by ED, PF, and JJ. The manuscript was written by ED, PF, and MD, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in this study were in accordance with the ethical standards of the institution or practice at which the study was conducted.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
All the authors declare their consent to participate in this study. All the authors declare their consent to participate in this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Marcus Clauss.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Descalzo, E., Díaz-Ruiz, F., Delibes-Mateos, M. et al. Seasonal and spatial variations in the diet of the Egyptian mongoose in its north-eastern Iberian range edge. Mamm Biol 103, 467–477 (2023). https://doi.org/10.1007/s42991-023-00364-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-023-00364-5