Abstract
In conservation biology, flagship species are defined as species that can raise support for biodiversity conservation in a given place or social context. In the newly established Gishwati–Mukura National Park in Rwanda, the Eastern chimpanzee was considered such an ambassador. To test whether the flagship species concept was effective in the park, we studied species richness, relative abundance, and species distribution (occupancy) of medium- to large-sized, ground-dwelling mammals using camera trapping technology. The impact of three environmental and three anthropogenic variables on species distribution was investigated and the ecological diversity of the fauna in the national park assessed. Over a period of 9 months, two 4 × 4 camera trapping grids were deployed in Gishwati and Mukura Forest. Sampling effort in each forest equated to 32 and 29 camera trapping locations, yielding 258 and 242 independent photographic events of eight and six species, respectively. In both forests, the Emin's giant poached rat was the most frequently encountered species, while all other species showed high relative abundance only in Gishwati Forest. The relative abundance of the endangered Eastern chimpanzee in Gishwati Forest was 0.54, the estimated occupancy was 0.31. Single-species, single-season occupancy models revealed that forest cover, altitude and distance to forest edge influenced the detectability of L'Hoest's monkey and squirrel species, while no effect was found on their occupancy. Notably, no larger herbivore or carnivore species were observed in the park, while the flagship species, i.e., the Eastern chimpanzee, was relatively abundant. Moreover, in both forests, all detected carnivores were small- to medium-sized, suggesting a meso-predator release phenomenon, i.e., populations of medium-sized predators increased after the removal of larger, top carnivores, due to relaxed competition. It appears that the prioritization of the flagship species resulted in the neglect of other mammalian species, leading eventually into the demise of entire functional guilds. Based on these results, the Gishwati–Mukura NP was categorized as a ‘depleted forest’. We, therefore, strongly object chimpanzees as a suitable flagship species—at least in the Gishwati–Mukura NP—and recommend collating more knowledge on the release of meso-predators and the loss of forest ungulates to improve their future conservation in Afro-montane forest habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared to primates, African ungulate diversity has attracted relatively little attention and conservation funding although ungulates account for 8.2% (90 species) of Africa’s mammal fauna, while primates account for only 4.1% (45 species; Kingdon et al. 2013; Dickson et al. 2020). The decline of most African forest ungulates in recent decades (East 1999; O'Brien et al. 2020) implies that many vital ecological (e.g., functional guilds) and socio-economic services (e.g., seed dispersal) will be lost and thus irrevocably change the ecosystem. The loss of these functional traits is framed by other conservation aspects, such as the management of overexploitation, habitat fragmentation, disease transmission, climate change, competition with livestock, and the international, illegal bushmeat trade (Bro-Jørgensen and Mallon 2016).
The Albertine Rift Valley in East Africa—stretching from the northern end of Lake Albert to the southern end of Lake Tanganyika—has been identified as one of the world’s biodiversity hotspots and is internationally recognized as an area of global conservation significance (Plumptre et al. 2003, 2007; Kanyamibwa 2013). Gishwati–Mukura National Park in western Rwanda—a tiny remnant of the once continuous forest complex fringing both sites of the Albertine Rift Valley—is the youngest National Parks to preserve this outstanding biodiversity. The National Park was mainly gazetted to protect an isolated population of Eastern chimpanzee (Pan troglodytes schweinfurthii) but also the populations of golden monkey (Cercopithecus mitis kandti) and L'Hoest’s monkey (Cercopithecus l’hoesti). Great efforts were made by the Gishwati Area Conservation Program, the ‘Great Ape Trust of Iowa’ and ‘Earthpark’ to make the National Park a forest of hope, especially for the endangered chimpanzee. Chimpanzees were hereby considered a flagship species (i.e., a species selected to act as an ambassador for the newly established National Park and that may incidentally facilitate the persistence of other species; Caro et al. 2004; McGowan et al. 2020), or an umbrella species (i.e., a species that has large habitat needs and whose conservation facilitates the conservation of many other species at the ecosystem level; Hassan et al. 2005), jointly acting as a ‘surrogate for biodiversity’ for the newly established conservation area.
Primate conservationists argue that targeting flagship species such as apes (or other primates) will also benefit other species through the continued protection of their habitats (Mittermeier 1986, 1988; Mallinson 1991; Wrangham et al. 2008). Collins et al. (2011), for example, proposed that some carnivore species will automatically benefit if their habitat is conserved due to the presence of rare or endangered primates. Given similar threats faced by primates, ungulates and larger felids, it was argued that primates are good candidates to be flagship species for larger carnivores and possibly some ungulates (Macdonald et al. 2012). Other studies, however, urged for caution, warning that the prioritization of flagship species for conservation may result in the neglect of other wildlife species (Caro et al. 2004; Ozaki et al. 2006). However, such flagship (or umbrella) species approaches are rarely validated or screened for the proposed success.
In Gishwati–Mukura NP, it was argued that conservation actions targeted at primates, will indirectly benefit ungulates and other mammalian wildlife through the continued protection of charismatic species, such as chimpanzee and golden monkey. Numerous studies have reported on the population development and conservation status of these primates (e.g., Barakabuye et al. 2007; Chancellor et al. 2012a, b; Mc Guinness and Taylor 2014; Chancellor et al. 2016), but none of them considered ungulates or other mammalian wildlife. Currently, the status of carnivores, ungulates and other ground-dwelling mammals in the newly established National Park remains nebulous, scanty and often based on anecdotal reports (RoR 2014; RDB 2015; Kisioh 2015). Ground-dwelling mammals, others than primates, alleged to occur in the park include bush pig (Potamochoerus larvatus), black-fronted duiker (Cephalophus nigrifrons), southern tree hyrax (Dendrohyrax arboreus), servaline genet (Genetta servalina), African civet (Civettictis civetta), side-striped jackal (Canis adustus) and serval cat (Felis serval; Musabyimana 2014; RoR 2014; RDB 2015; Kisioh 2015). Based on species inventories from neighbouring Nyungwe NP in Rwanda and Magahinga NP in Uganda, some additional species might be expected in Gishwati–Mukura NP (Erickson Wilson 1995; Chao 2008). Moreover, recent reports from neighbouring Nyungwe NP confirmed the presence of the rare Lestrade’s duiker (Cephalophus weynsi lestradei; Moore et al. 2018). Since this duiker species was also reported from Bwindi NP in Uganda (both Nyungwe and Bwindi comprise the same habitat as Gishwati–Mukura NP, i.e., afro-montane rainforest) there is some hope that this rare ungulate also persists in Gishwati–Mukura NP.
Our study, therefore, aimed to close this gap and attempted to compile data on the richness, relative abundance, and occupancy of medium- to large-sized, ground-dwelling mammals. We used camera trap data to survey ground-dwelling mammals in two pristine parts of the National Park (i.e., the Gishwati and Mukura Forest fragments) and to explore whether the flagship species concept applied in the park was effective, i.e., whether its application benefitted other ground-dwelling mammals than primates. In a first step, we compiled information on the persistence (species richness) of mammalian species and compared our inventory to that of previous reports by Kisioh (2015), RDB (2015) and REMA (2015) as well as to inventories from neighboring Nyungwe and Volcanoes NP (Erickson Wilson 1995; Chao 2008). Given that only Gishwati Forest harbors Eastern chimpanzees and golden monkeys, we tested for differences between the Gishwati and the Mukura Forest fragment, assuming the presence of primates to have a positive effect on the richness, relative abundance, and occupancy of other ground-dwelling mammals. To exclude the possibility that environmental or anthropogenic variables in and around the National Park account for the differences between fragments, we predicted that altitude, forest cover, human encounter rate, distance to the nearest forest edge, distance to nearest settlement, or distance to nearest tourist trail had no substantial impact on the detectability and occupancy of ground-dwelling mammals. Subsequently, we used the results to indirectly assess the intactness, i.e., the ecological integrity (Theobald 2013), of the mammal fauna as well as the status of functional guilds within the Gishwati–Mukura ecosystem, and to discuss the success and efficacy of applying the flagship species concept in the park.
Methods
Study area
The Gishwati–Mukura National Park in western Rwanda is part of the Congo-Nile Divide forest complex at the eastern side of the Albertine Rift Valley, characterized by steep slopes (mean 35%), low mean annual temperatures (15–17 °C) and high mean annual rainfall (1200–1500 mm; Safari 2012). Within the Gishwati–Mukura National Park, our study focused on two isolated forest fragments of primary, afro-montane rainforest (Gishwati Forest: 1°49′S, 29°22′E; Mukura Forest: 1°59′S, 29°31′E) at an altitude of 2300–2700 m (Kindt et al. 2014; REMA 2015; Fig. 1). Both forests have a history of deforestation extending over the past 50 years: protected as a forest reserve in 1933 (700 km2), Gishwati Forest measured 280 km2 in the 1970’s and had shrunk to only 6 km2 of pristine forest by 2002 (Kisioh 2015; Ordway 2015). In 2008 the Rwandan government launched a campaign to restore Gishwati Forest and its size was increased to 14.8 km2 by 2016 (Kisioh 2015). In Mukura Forest, about half of the forest area was lost to deforestation since 1951, and from 1990 to 2006 the pristine forest area declined from 21 to 16 km2 (REMA 2015). Despite the high degree of deforestation, the two forest fragments still hold a significant biodiversity, including 250 plant species, more than 200 birds (including 20 that are endemic to the Albertine Rift) and three species of primates (RDB 2015; Kisioh 2015). Since 2008, impressive conservation outcomes were achieved, such as the reforestation of 6 km2 naturally regenerating forest that was added to the cores of primary forest (Kisioh 2015). In early 2016, both forests were eventually protected as Gishwati–Mukura National Park and managed by the Forest of Hope Association in collaboration with the Rwanda Development Board (RDB) and the Rwanda Natural Resources Authority (Kisioh 2015). The newly established National Park has a core area of 34.3 km2 (Gishwati: 14.4 km2, Mukura: 19.9 km2) and is surrounded by a buffer zone of 9.9 km2 consisting of farm and rangeland as well as reforested areas (RDB 2015; UNEP-WCMC 2020).
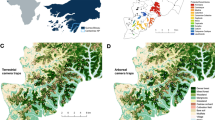
Location of Rwanda in Africa (A), location of Gishwati–Mukura NP in western Rwanda (B), and locations of four camera trapping grids in Gishwati (C) and Mukura Forest (D). Grey shades in C and D represent forest cover (tree density in %) in the year 2000 (Hansen et al. 2013)
Camera trapping
Our survey design consisted of 16 camera trapping points located at 300 m intervals on a regular 4 × 4 square grid, oriented to fit the remaining fragments of natural habitat (pristine, primary afro-montane rainforest, i.e., the core zone of the National Park; Kisioh 2015). Beside these core areas, the National Park’s total surface area also included sections of secondary forest, forest plantations as well as pastures and farmland (REMA 2015). However, such areas were excluded from our considerations as these land use differences would have likely influenced the composition of faunal communities. Each study site (i.e., Gishwati and Mukura Forest; Fig. 1) contained two camera trapping grids, extending over an area of 1.2 × 1.2 km (i.e., 2.88 km2 per forest fragment), resulting in a total of 64 camera trap sites and a trapping density of one trap per 0.05 km2. Apart from serval cat (home range size: 10–32 km2; Hunter and Bowland, 2013) and Eastern chimpanzee (home range size: 6–32 km2; Emery Thompson and Wrangham, 2013), home ranges of potential target species could be traversed at least every 1–4 days, with an average daily ranging distance of about 3 km (0.48–5.84 km; home range sizes were obtained from Kingdon et al., 2013). Therefore, selected camera trap locations and intervals represented a random sample of the entire population of most target species (Gotelli and Colwell 2011; Rovero et al. 2013; van Berkel 2014).
BaseCamp software vs. 4.6.2 (Garmin Ltd., USA) and a handheld GPS receiver were used to locate camera trapping points in the centre of each grid cell. Camera traps were placed within 50 m of each centroid at heights of 30–50 cm. We applied Ltl Acorn 6310 (Ltl Acorn, Shenzhen, China) camera traps with a passive infra-red sensor, set to take only one picture per trigger. Ltl Acorn 6310 camera traps have a trigger time of 0.1 s with a detection range of approximately 25 m. All other settings were used as suggested by the default (Ltl Acorn, 2019). During daylight, images were in natural colour (RGB), while at night (or at low light levels in the daytime) pictures were taken using the infra-red flash. Due to financial and logistical constraints, camera trap grids were sampled consecutively rather than simultaneously. The overall camera trapping period extended from 7 May 2017 to 11 May 2018, covering one dry and one wet season in each forest (Gishwati I, dry season: 10 May to 18 August 2017; Gishwati II, wet season: 16 August to 17 November 2017; Mukura I, dry season: 4 December 2017 to 4 February 2018; Mukura II, wet season: 5 February to 11 May 2018; Supplementary Information Table S1).
Data collation and processing
The Exiv2 software tool (Huggel 2012) was used to extract image metadata into Excel spreadsheets (Microsoft Office, USA). Camera trap label, date and time records were compiled for each image. If possible, pictures containing ground-dwelling mammals were identified to species level and grouped into independent photographic events. In our study, an ‘event’ was defined as a sequence of pictures of a certain species occurring after an interval of more than 60 min from the previous image of that species (Tobler et al. 2008; Amin et al. 2014). Time elapse between two images was calculated using ‘lubridate’ package (Spinu et al. 2020) in R (version 4.0.3, Development Core Team 2018). Species identification was based on Dorst and Dandelot (1970), Halthenorth and Diller (1977) and Kingdon et al. (2013). Identification of squirrel species was difficult and potential squirrel species occurring in the study area (fire-footed tree squirrel, Funisciurus pyrrhopus; Carruther's mountain squirrel, Funisciurus carruthersi and Rwenzori sun squirrel, Heliosciurus ruwenzorii) were, therefore, lumped at family level (Sciuridae).
Environmental and anthropogenic variables
Six environmental and anthropogenic variables were identified and collected at each camera trapping locations, including the percentage of forest cover, the human encounter rate, the distance to the nearest forest edge, the distance to the nearest settlement, the distance to the nearest tourist trail (RDB 2015), and the altitude (obtained from Google Earth Pro version 7.3.2). Distance to forest edge, distance to next settlement and tourist trail were measured in QGIS (version 3.2.3), using Google Satellites (WGS 84, UTM zone 35S, EPSG code: 32,735).
Data on percentage forest cover at a 30 × 30 m resolution were obtained from Global Forest Change (https://earthenginepartners.appspot.com/science-2013-global-forest; Hansen et al. 2013), and further processed in QGIS. The human encounter rate was calculated using the number of human independent photographic events divided by the number of camera trapping days at each camera trapping location, resulting in the mean number of human events per day per camera trap.
Data analyses
Occupancy models were used to determine whether camera survey periods were adequate to confidently assign site-specific absences of ground-dwelling mammals in the study area. Based on species-by-sampling-unit incidence matrices, species accumulation curves were constructed using the ‘specaccum’ function in the ‘vegan’ package in R (Development Core Team 2018; Oksanen et al. 2019). The Mao Tau estimator, also known as the ‘exact’ method, was chosen to create the expected species accumulation curves. Smoothed, rarefied species accumulation curves were finally obtained as the cumulative number of detected species (mean and 95% CIs) plotted against the respective sampling effort in each forest fragment.
Naïve occupancy (ψ0) was calculated as the ratio of camera trapping locations at which a target species was detected to the total number of operational locations. For each species, the relative abundance index was established at each trapping location by dividing the number of independent photographic events by the total number of camera trapping days, multiplied by 100 (i.e., the number of events per 100 trapping days). To test the species-specific differences in the relative abundance index between Gishwati and Mukura Forests, a Brunner–Munzel test (Brunner and Munzel 2000) was applied using 'brunner.munzel.test' function in the R package 'lawstat' (Gastwirth et al. 2020).
To test whether the relevant environmental and anthropogenic variables affected the detectability (p) and occupancy (ψ) of frequently detected species, as well as to estimate the relevant p and ψ, a single-species, single-season occupancy model was applied for the detected species (> 10 independent photographic events) with maximum likelihood estimation using the logit link function of the ‘unmarked’ package in R (Development Core Team 2018, Fiske and Chandler 2019).
Some environmental and anthropogenic variables included in our modelling approach were significantly correlated (i.e., distance to nearest forest edge and distance to nearest settlement in Gishwati Forest: r = 0.93, P < 0.001; distance to nearest forest edge and percentage forest cover in Mukura Forest: r = 0.44, P = 0.02; distance to nearest forest edge and altitude in Mukura Forest: r = − 0.43, P = 0.02; and distance to nearest tourist trail and human encounter rate in Mukura Forest: r = − 0.41, P = 0.03; Spearman rank correlation). To ensure independence of site-specific covariates and to simplify the occupancy model structure, the six environmental and anthropogenic variables were, therefore, z-transformed (mean = 0 and SD = 1) and subjected to a factor reduction procedure (Principal Components Analysis, PCA). PCAs were conducted using the ‘psych’ and ‘factoextra’ packages in R (Revelle and Revelle 2015; Kassambara and Mundt 2017). The resulting principal components (PCs) were used as the site-specific covariates in our occupancy models. Prior to model construction, camera trapping efforts were aggregated into 10-day sampling survey periods (i.e., Gishwati Forest: 20 ten-day survey periods, Mukura Forest: 13 ten-day survey periods), to enhance the detection probability and to aid occupancy estimates by stabilising the detectability of each species, particularly if they were only sporadically detected. This approach implies that our reported detection probabilities represent the likelihood a species was detected within a 10-day sampling period.
Subsequently, a common set of models were applied for each detected species. In the basic null model p(۔)ψ(۔)—which was used as the reference model—detectability and occupancy were fixed, meaning that they were not altered by any covariate. A series of occupancy models were built to estimate the detectability (p) with all possible combinations of site-specific PCs and compared with the Akaike Information Criterion (AIC; Akaike 1974). Any model with a ∆AIC < 2 was considered to have support. If strong similarities among the top-ranked models were detected, a model averaging process was applied using the ‘MuMIn’ package in R (Barton and Barton 2020). We used Nagelkerke’s R-squared index to quantify the explanatory power of each model (Nagelkerke, 1991). The relative importance of the averaged model parameters was computed using the ‘AICmodavg’ package in R (Mazerolle 2019). After estimating p, another set of occupancy models were conducted to determine the significant occupancy-related covariates and to estimate the parameters for ψ. Within these models the best supported detection probability model was incorporated, and a final model selection and averaging procedure was performed among all models. The estimated ψ and p, their standard errors (SE) and 95% CIs were back transformed and expressed as a continuous value (ranging from 0 to 1) using the logit link functions. For model validation, we applied the MacKenzie and Bailey goodness-of-fit test to the most complex global model, using 1000 bootstrap simulations (MacKenzie and Bailey 2004).
Results
Camera trapping effort and species richness
Of the 64 camera traps, three were stolen from the Mukura Forest trapping grid. The 61 remaining camera traps amounted to a total sampling effort of 2704 days (mean per location: 85 days, range: 31–98 days) for Gishwati Forest and 2050 days (mean per location: 71 days, range: 62–133 days) for Mukura Forest. The sampling effort in Gishwati Forest yielded a total of 254 independent photographic events of eight medium- to large-sized, ground-dwelling mammals, while that of Mukura Forest yielded a total of 93 independent photographic events of six species (Table 1, Fig. 2). Two species detected in Gishwati Forest, i.e., chimpanzee and the African palm civet (Nandinia binotata) were not detected in Mukura Forest. The list of species detected during this study did not match the expected number and taxa (Musabyimana 2014; RoR 2014; RDB 2015; Kisioh 2015), with less species detected and no new species discovered. Moreover, humans were recorded on 29 independent photographic events obtained from 14 camera trapping locations in Gishwati Forest (average human encounter rate: 0.01, i.e., one human encounter in 100 days), and on 177 independent photographic events from 11 locations in Mukura Forest (average human encounter rate: 10.7). Numerous small-sized, unidentified rodents (superfamily: Muroidea) as well as 11 bird species were also recorded in both forests but not included in the analyses (Table S1). Rarefied species accumulation curves for each forest were plotted against the number of camera trapping locations (Fig. 3a) and against the number of camera trapping days (Fig. 3b), indicating that the camera trapping effort was sufficient to capture most, if not all, medium- to large-sized, ground-dwelling mammals present in Gishwati–Mukura NP.
The rarefied species accumulation curves plotting the cumulative number of detected medium- to large-sized, ground-dwelling mammals against A camera trapping locations and B camera trapping days in Gishwati Forest (black) and Mukura Forest (grey). The Mao Tau estimate (‘exact’ method) was applied to find the expected mean species richness (dotted lines). Error bars indicate the lower and upper bounds of the 95% confidence intervals
Relative abundance
In general, most of the detected species had a higher relative abundance index (i.e., were more frequently encountered) in Gishwati Forest than in Mukura Forest (Fig. 4). Emin's giant pouched rat was the most frequently recorded species in both forests and no significant difference in the relative abundance index was observed between the two forests (Brunner–Munzel test: BM = − 0.13, df = 55.12, P = 0.89; Fig. 4). L'Hoest's monkey was the second-most frequently encountered species in Gishwati Forest, while in Mukura Forest it was the least commonly recorded mammal (Fig. 4) and the relative abundance index was higher in Gishwati than in Mukura Forest (Brunner–Munzel test: BM = − 7.80, df = 31.97, P < 0.001). The relative abundance index of servaline genet in Gishwati Forest was three times higher than that of Mukura Forest (Brunner–Munzel test: BM = − 2.83, df = 52.24, P = 0.01; Fig. 4), while that of squirrel species was seven times higher (Brunner–Munzel test: BM = − 3.04, df = 54.00, P < 0.01; Fig. 4). No difference in the relative abundance index was found between the two forests for side-stripped jackal (Brunner–Munzel test: BM = − 0.25, df = 58.90, P = 0.81) and serval cat (Brunner–Munzel test = − 0.07, df = 55.25, P = 0.94).
Site-specific covariates (principal components)
The PCA analysis condensed six environmental and anthropogenic variables from Gishwati Forest into two PCs with an eigenvalue > 1.0, cumulating 61% of the total variance. PC1 received high factor loadings from forest cover, distance to forest edge and distance to settlement (Table 2), indicating that dense forest canopy cover was located far from settlements and the forest edge. PC2 received high factor loadings from distance to trail, human encounter rate and altitude (Table 2), suggesting areas far from tourist trails at high elevations that encountered a high degree of human activities. For Mukura Forest, the PCA condensed the same six variables into three PCs with an Eigenvalue > 1.0, cumulating 73% of the total variance. PC1 received high factor loadings from forest cover, distance to forest edge and distance to the nearest tourist trail (Table 2), indicating that dense canopy cover was located far from the forest edge but close to tourist trails. PC2 received high factor loadings from the human encounter rate and altitude (Table 2), suggesting high elevation areas encountered more human activities. PC3 received high factor loadings from forest cover and distance to settlement (Table 2), suggesting that areas with dense forest cover were located far from the next settlement.
Occupancy modelling
Seven and 15 species-specific occupancy models were initially conducted for each species detected in Gishwati and Mukura Forest, respectively. Models were ranked according to their Akaike’s Information Criterion (AIC) value and model weight, and models with an ∆AIC < 2 were considered as competitive to the top model (∆AIC = 0; Supplementary Information Table S2, S3). The top-ranking model for L'Hoest's monkey in Gishwati Forest was determined as the best fit model (∆AIC = 0; model weight = 0.55; R-square index: 0.37; Supplementary Information Table S2). MacKenzie and Bailey goodness-of-fit test revealed the global model to have no lack of fit and no overdispersion (chi-squared test: χ2 = 2231.21, P = 0.24, overdispersion parameter ĉ = 1.02). For all other species, a model averaging procedure was applied to all subset models with a ∆AIC < 2 (Supplementary Information Table S2, S3). Due to the overall low detectability, we omitted occupancy analysis of serval cat and African palm civet in Gishwati Forest, and L'Hoest's monkey, serval cat, genet species, side-stripped jackal and the squirrel species in Mukura Forest. The detectability of L'Hoest's monkey and squirrel species in Gishwati Forest, was positively influenced by the site-specific covariate PC2 (high-positive factor loadings from distance to tourist trail, human encounter rate, and altitude), indicating higher detectability in areas far from the nearest tourist trail, at high altitudes and with high presence of humans (Table 3). The detectability of all other species was not affected by any environmental or anthropogenic PC (Table 3). Moreover, the occupancy of all species was not affected by any environmental or anthropogenic PC (Table 3). The estimated detectability and occupancy, their standard errors and the 95% confidence intervals for each species are presented in Table 3.
Discussion
Compared to the neighbouring Nyungwe NP (30 mammal species; Chao 2008) and Mgahinga NP (27 mammal species; Erickson Wilson 1995), Gishwati–Mukura NP revealed a low species richness of medium- to large-sized, ground-dwelling mammals. Out of a total of 32 expected species, only eight (2 primates, 4 carnivores, 2 rodents) were recorded in Gishwati Forest, and six (1 primate, 3 carnivores, 2 rodents) in Mukura Forest, neither including any ungulate species nor their large predators (Table 1). We attribute this result to the fact that Gishwati–Mukura NP lies in one of the most densely populated areas of Rwanda, where a growing human population has led to unsustainable agricultural practices, reduced crop yields and thus starvation (Kisioh 2015). Forest-adjacent communities were driven to seek out alternative livelihoods, resulting into increasing pressure on natural forests resources such as land for agriculture, bushmeat, or other types of resource extraction (Plumptre and Bizumuremyi 1996; Kisioh 2015). The growing off-take of larger herbivores, such as duikers (Cephalophus spp.), bush pig (Potamochoerus larvatus), or forest buffalo (Syncerus caffer nanus), resulted eventually into their local extinction and inevitably led to the loss of their predators. However, this does not explain the absence of meso-predators such as the Africa civet, honey badger (Mellivora capensis) or mongooses (Herpestidae) although they were likely to occur in the National Park (Erickson Wilson 1995; Chao 2008; Kisioh 2015; RDB 2015; REM 2015). The absence of some meso-predators might be attributed to the possibility that some generalist species may be more prevalent in anthropogenically shaped habitats such as secondary forest, forest plantations or pastures and farmland which were not included in our study and may have, therefore, biased our results.
Interestingly, two threatened primate species, i.e., the vulnerable L'Hoest's monkey and the endangered Eastern chimpanzee (Ukizintambara et al. 2019; Plumptre et al. 2016) were detected with high relative abundance in Gishwati Forest (2.21 and 0.54, respectively; Fig. 4), but with extremely low abundance in Mukura Forest (0.03 and 0, respectively; Fig. 4). Given the absence of ungulates and their predators due to illegal hunting, it was surprising that chimpanzees persisted—at least in Gishwati Forest (approximately 30 individuals; Kisioh 2015). This might be explained by the continuous conservation efforts in the protected area, but also by a taboo which prevents the Banyarwanda from hunting and consuming primate meat (O’Brien et al. 2014). The estimated occupancy of Eastern chimpanzee in Gishwati Forest was 0.31 (range: 0.15–0.54; Table 3) which was well below that established for neighbouring Nyungwe NP (0.69; Moore et al. 2020) or that reported from the Taï NP in Ivory Coast (0.5–1.0; Kalan et al. 2015). The detectability and occupancy of chimpanzees were not affected by any environmental or anthropogenic variable, indicating that chimpanzees were widely and equally distributed across Gishwati Forest, despite a strong human impact on other wildlife species. It, therefore, appears that the flagship species is well protected in Gishwati–Mukura NP—at least in Gishwati Forest—and that Rwanda met the 2020 National Biodiversity Strategy and Action Plan target, i.e., to prevent the extinction and to improve the conservation status of this endangered primate in the National Park (RoR 2016). One could further argue that the higher richness and relative abundance of ground-dwelling mammals in Gishwati Forest, compared to that observed in Mukura Forest, could be attributed to lower poaching rates and the presence of chimpanzees, suggesting that the flagship species concept in Gishwati Forest was—at least to a certain degree—effective. By contrast, in Mukura Forest more ground-dwelling mammal species became extinct since here the flagship species concept did not fit (since time immemorial chimpanzees did not occur in Mukura Forest, although it can be assumed that chimpanzees once occurred in the area, i.e., long before the flagship species concept was formulated).
A similar situation was observed for L'Hoest's monkey, beside chimpanzee the only ground-dwelling primate surviving in Gishwati–Mukura NP. The estimated occupancy of L'Hoest's monkey was 1.00 (Table 3), suggesting that the species appears to be common in Gishwati Forest. However, this result had a low confidence and should thus be viewed with caution. The detectability of L'Hoest's monkey was positively related to the distance of the nearest tourist trail, to altitude and to human encounter rate, implying that these primates occur in remote areas at high altitudes, but with high human presence. Since human disturbance by local communities (i.e., mainly hunting, tree cutting, and gathering firewood; RDB 2015), is high throughout the protected area, avoiding humans becomes increasingly difficult, at least during daytime. At night, local farmers reported increased crop raiding by ground-dwelling primates, asserting that about 10–20% of their crops were raided by either primate species (Mc Guinness and Taylor 2014). Given this considerable loss of income, it appears even more surprising that both ground-dwelling primates were so well protected, while other crop-raiding species, such as ungulates, were completely extirpated, which might be also linked to cultural values, such as the taboo of consuming primates.
The most shocking result of our study was the non-detection, of larger herbivore species, such as duikers or pigs. The role of large herbivores as browsers, seed dispersers and producers of food for higher trophic levels, makes them a key part of the ecosystem and changes in their abundance or occupancy have severe knock-on effects on other ecological functions within the ecosystem (Kumpel et al. 2008; Effiom et al. 2013). The loss of large- to medium–sized herbivores will lead to reduced seed dispersal of large-seeded, slow-growing, and canopy-forming tree species (Harrison 2011; Abernethy et al. 2013) and may thus cause changes to fruit availability, the carbon storage capacity of the forest, and will eventually impede forest biodiversity and the ecosystem’s resilience to flood and fire (Chanthorn et al. 2019). During the Clinton Global Initiative meeting in September 2008, Rwanda’s President Kagame stated: “We are creating a 'forest of hope' that transcends the restoration of biodiversity—it is about the people of Gishwati and improving their lives in harmony with nature. We are determined to reverse the history of human-induced environmental abuse in the Gishwati area.” Following the presidential appeal and the declaration of the Gishwati–Mukura NP in February 2016, the Gishwati Area Conservation Program, the ‘Great Ape Trust of Iowa’ and ‘Earthpark’ lined up to make the National Park a forest of hope for the endangered chimpanzee. The Eastern chimpanzee was proclaimed the flagship species of the newly established National Park, and it was anticipated that through the continued conservation of chimpanzees and their habitat other wildlife would also benefit. However, this was not the case since both, large carnivores, and their prey species, encountered a continued population decline or were unable to recover. Instead of supporting the recovery of all forest organisms through earnest law-enforcement—i.e., the complete deterrence of illegal hunting—the prioritization of the flagship species (and other primates) resulted in the neglect of other mammalian wildlife, leading eventually into the demise of entire functional guilds. Given this, the new National Park should be rather labelled a ‘depleted forest’ (sensu Bruce et al. 2017), or when considering only Mukura Forest, classified as an ‘empty forest’ (sensu Redford 1992; Wilkie et al. 2011; Harrison 2011). Both, theory (Wiens et al. 2008) and empirical research (Ozaki et al. 2006; Laub and Budy 2014) proposed that it might be dubious to consider a single species representing the habitat requirements of all sympatric organisms, especially if the conservation goal is solely targeted at preserving one high-profile species like the chimpanzee (Lambeck 1997; Caro and Doherty1999). Despite the extensive use of the flagship species concept in conservation, its usefulness is subject to extensive debate (Andelman and Fagan 2000; Roberge and Angelsteam 2004; Shen et al. 2020; McGowan et al. 2020), often described as being successful (Collins et al. 2011; Li and Pimm 2016; Thornton et al. 2016), but also branded to be futile (Caro et al. 2004; Ozaki et al. 2006). Such an ambiguity was refuted by our study, clearly demonstrating that the use of chimpanzee as a flagship species for the newly established Gishwati–Mukura NP was ineffective.
Furthermore, our study revealed side-stripped jackal, serval cat and servaline genet to be the most detected and the most widely distributed carnivore species in the park. Other anticipated carnivore species (e.g., leopard, Panthera pardus or African golden cat, Caracal aurata; Musabyimana 2014; RoR 2014; RDB 2015; Kisioh 2015) were not detected at all. The relatively high detectability (0.17, 95% CI 0.09–0.32) and occupancy (0.23, 95% CI 0.10–0.44) of jackals in Gishwati–Mukura NP and the absence of any environmental or anthropogenic impact on their distribution (Table 3), suggests that jackals are moderately abundant, benefiting from the human presence in and around the National Park. This finding is in line with previous studies on black-backed jackal (Canis mesomelas) in Botswana (Gusset et al. 2009; Van der Weyde et al. 2018) and Namibia (Fink et al. 2020), which described distance to human settlements and the availability of anthropogenic discard to be the best predictors of their occupancy and/or detectability. Furthermore, our study revealed the occupancy of servaline genet to reach from 0.26 to 0.97 (Table 3), but no relationship between detectability or occupancy and any environmental or anthropogenic variable was unravelled (Table 3), suggesting that human disturbance occurs in large parts of the park and that servaline genets benefit—or at least do not suffer—from human activities in the park. Findings reported for large-spotted genets (Genetta tigrina) from South African farmlands (occupancy: 0.72–0.73; Ehlers Smith et al. 2018) or common genets (Genetta genetta) from Kenyan rangelands (occupancy: 0.6–0.8; Schuette et al. 2013)—where occupancies were similar to those observed in the study area—suggest that genets benefit from the human presence and the loss of large predators. Generally, the loss of large predators leads to further knock-on effects in the trophic cascade which in turn promotes the revival of small- to medium-sized predators and allows them to disperse and flourish in a variety of habitats—a phenomenon known as the meso-predator release (Crooks and Soulé 1999; Schuette et al. 2013; Wang et al. 2015). This phenomenon may also explain the situation in Gishwati–Mukura NP, although other reasons could account for the high abundance of small- to medium-sized predators, e.g., the fact that they are generalist species with a wider array of food sources, as opposed to large carnivores that are more specialised and thus predisposed to early extinction. In contrast to the detectability revealed for side-striped jackal and servaline genet, the detectability of serval cat was expected to be increased in remote, densely vegetated areas, far from human settlements (Kane 2014). Such a relationship was confirmed by the occupancy model for serval cat in Gishwati Forest (Table 3), highlighting the rather timid character of this feline species. However, due to the small sample size and thus a low detectability, the estimated occupancy was insufficient to allow a robust conclusion.
Beside the squirrel species, another opportunistic rodent, i.e., Emin's giant pouched rat, was widely distributed in Gishwati (occupancy: 0.44–0.79; Table 3) and Mukura Forest (occupancy: 0.01–1.00; Table 3). These figures were twice as high as occupancies reported for Nyungwe NP (0.19–0.47), where distribution declined between 4 and 27% from 1997 to 2011 (O'Brien et al. 2014), but like the occupancy (0.53–0.85) observed in the Udzungwa Mountains in Tanzania (Oberosler et al. 2017). In our study, detectability and occupancy were expected to be high in areas close to the forest edge or even close to human settlements since this omnivorous generalist is known as an anthropophilous species, i.e., a species that lives near, and benefits from, human beings and their activities (Kingdon et al. 2013; Bruce et al. 2017; Oberosler et al. 2017). However, the occupancy models revealed that neither environmental nor anthropogenic variables had a significant effect on detectability and occupancy (Table 3), suggesting that giant pouched rats are not affected by humans since humans are encountered throughout the study area (i.e., a high human encounter rate). Meso-predators, such as genets, serval cats and jackals, are the main predators of pouched rats (Kingdon et al. 2013) and the high abundance of pouched rats might be thus indicative for a declining meso-predator population, yet another reason to classify the National Park as ‘depleted’ or even ‘empty’.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Abernethy KA, Coad L, Taylor G, Lee ME, Maisels F (2013) Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos Trans R Soc Lond B Biol Sci 368:20120303. https://doi.org/10.1098/rstb.2012.0303
Ltl Acorn (2019) LtlAcorn Ltl-6510 Series User Manual. Ltl Acorn, Shenzhen, China. Available at: C:/Users/scrip/Downloads/ltl-acorn-Ltl-6511M-user-manual.pdf
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Amin R, Andanje SA, Ogwonka B, Ali AH, Bowkett AE, Omar M, Wacher T (2015) The northern coastal forests of Kenya are nationally and globally important for the conservation of Aders’ duiker Cephalophus adersi and other antelope species. Biodiver Conserv 24:641–658. https://doi.org/10.1007/s10531-014-0842-z
Andelman SJ, Fagan WF (2000) Umbrellas and flagships: efficient conservation surrogates or expensive mistakes? PNAS 97:5954–5959. https://doi.org/10.1073/pnas.100126797
Barakabuye N, Mulindahabi F, Plumptre AJ, Kaplin B, Munanura I, Ndagijimana D, Ndayiziga O (2007) Conservation of chimpanzees in the Congo-Nile divide. Report for the US Fish and Wildlife Service. Wildlife Conservation Society, New York
Barton K, Barton MK (2020) Package ‘MuMIn’. https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf. Accessed 21 Jan 2021
Van Berkel T (2014) Camera trapping for wildlife conservation: Expedition field techniques. Geography Outdoors, Royal Geographical Society, London
Bro-Jørgensen J, Mallon DP (2016) Antelope conservation: from diagnosis to action. John Wiley & Sons, London
Bruce T, Wacher T, Ndinga H, Bidjoka V, Meyong F, Ngo Bata M, Olson D (2017) Camera-trap survey for larger terrestrial wildlife in the Dja Biosphere Reserve. Zoological Society of London (ZSL) & Cameroon Ministry of Forests and Wildlife (MINFOF), Yaoundé
Brunner E, Munzel U (2000) The nonparametric Behrens-Fisher problem: asymptotic theory and a small-sample approximation. Biom J 42:17–25. https://doi.org/10.1002/(SICI)1521-4036(200001)42:1%3c17::AID-BIMJ17%3e3.0.CO;2-U
Caro T, Doherty GO (1999) On the use of surrogate species in conservation biology. Conserv Biol 13:805–814
Caro T, Engilis A Jr, Fitzherbert E, Gardner T (2004) Preliminary assessment of the flagship species concept at a small scale. Anim Conserv 7:63–70. https://doi.org/10.1017/S136794300300115X
Chancellor RL, Langergraber K, Ramirez S, Rundus AS, Vigilant L (2012a) Genetic sampling of unhabituated chimpanzees (Pan troglodytes schweinfurthii) in Gishwati Forest Reserve, an isolated forest fragment in western Rwanda. Int J Primatol 33:479–488. https://doi.org/10.1007/s10764-012-9591-6
Chancellor RL, Rundus AS, Nyandwi S (2012b) The influence of seasonal variation on chimpanzee (Pan troglodytes schweinfurthii) fallback food consumption, nest group size, and habitat use in Gishwati, a montane rainforest fragment in Rwanda. Int J Primatol 33:115–133. https://doi.org/10.1007/s10764-011-9561-4
Chancellor RL, Rundus AS, Nyandwi S (2016) Chimpanzee seed dispersal in a montane forest fragment in Rwanda. Am J Primatol 79:e22624. https://doi.org/10.1002/ajp.22624
Chanthorn W, Hartig F, Brockelman WY, Srisang W, Nathalang A, Santon J (2019) Defaunation of large-bodied frugivores reduces carbon storage in a tropical forest of Southeast Asia. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-46399-y
Chao N (2008) Bird & mammal lists-Nyungwe National Park. Wildlife Conservation Society. https://library.wcs.org/doi/ctl/view/mid/33065/pubid/DMX1332400000.aspx. Accessed 17 Nov 2020
Collins MB, Milner-Gulland EJ, Macdonald EA, Macdonald DW (2011) Pleiotropy and charisma determine winners and losers in the REDD+ game: all biodiversity is not equal. Trop Conserv Sci 4:261–266. https://doi.org/10.1177/194008291100400304
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566. https://doi.org/10.1038/23028
Dickson KB, Middleton JFM, Clarke JI, Gardiner RKA, Kröner A, Mabogunje AL, McMaster DN, Nicol DSHW, Smedley A, Steel RW (2020) Africa. Encyclopaedia Britannica, UK. https://www.britannica.com/place/Africa. Accessed 21 Jan 2021
Dorst J, Dandelot P (1970) A field guide to the larger mammals of Africa. Collins, London
East R (1999) African antelope data base 1998. IUCN/SSG Antelope Specialist Group, IUCN, Gland, Switzerland
Effiom EO, Nunez-Iturri G, Smith HG, Ottosson U, Olsson O (2013) Bushmeat hunting changes regeneration of African rainforests. Proc R Soc B 280:20130246. https://doi.org/10.1098/rspb.2013.0246
Ehlers Smith YC, Ehlers Smith DA, Ramesh T, Downs CT (2018) Forest habitats in a mixed urban-agriculture mosaic landscape: patterns of mammal occupancy. Landsc Ecol 33:59–76. https://doi.org/10.1007/s10980-017-0580-1
Emery Thompson M, Wrangham RW (2013) Common or robust chimpanzee. In: Kingdon J, Happold D, Hoffmann M, Butynski T, Happold M, Kalina J (eds) Mammals of Africa, Volumes II. Bloomsbury Publishing, London, pp 55–64
Erickson Wilson S (1995) Ugandan National Parks - Bird and Mammal Check List. National Biodiversity Data Bank, Institute of Environment and Natural Resources, Makerere University, Kampala
Fink S, Chandler R, Chamberlain M, Castleberry S, Glosenger-Thrasher S (2020) Distribution and Activity Patterns of Large Carnivores and Their Implications for Human-Carnivore Conflict Management in Namibia. Hum Wildl Interact 14:16
Fiske I, Chandler R (2019) Overview of unmarked: an R package for the analysis of data from unmarked animals. R package version 0.13–1. https://cran.r-project.org/web/packages/unmarked/unmarked.pdf. Accessed 17 May 2020
Gastwirth JL, Gel YR, Hui WW, Lyubchich V, Miao W, Noguchi K, Lyubchich MV (2020) Package ‘lawstat’. https://cran.r-project.org/web/packages/lawstat/lawstat.pdf.
Gotelli NJ, Colwell RK (2011) Estimating species richness. In: Magurran AE, McGill BJ (eds) Biological diversity: Frontiers in measurement and assessment. Oxford University Press, Oxford, pp 39–54
Gusset M, Swarner MJ, Mponwane L, Keletile K, McNutt JW (2009) Human–wildlife conflict in northern Botswana: livestock predation by endangered African wild dog Lycaon pictus and other carnivores. Oryx 43:67–72. https://doi.org/10.1017/S0030605308990475
Halthenorth T, Diller H (1977) Field Guide to the Mammals of Africa including Madagascar. Collin, London
Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman SV, Goetz SJ, Loveland TR, Kommareddy A (2013) High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 342: 850–853. https://earthenginepartners.appspot.com/science-2013-global-forest,
Harrison RD (2011) Emptying the forest: hunting and the extirpation of wildlife from tropical nature reserves. Biosci 61:919–924. https://doi.org/10.1525/bio.2011.61.11.11
Hassan R, Scholes R, Ash N (2005) Millenium Ecosystem Assessment: Ecosystems and Human Wellbeing, Volume I, Current State and Trends. Island Press, Washington
Huggel A (2012) Exiv2 software tool. https://www.exiv2.org/index.html. Accessed 6 Nov 2020
Hunter L, Bowland J (2013) Serval. In: Kingdon J, Happold D, Hoffmann M, Butynski T, Happold M, Kalina J (eds) Mammals of Africa, Volumes V. Bloomsbury Publishing, London, pp 180–186
Kalan AK, Mundry R, Wagner OJ, Heinicke S, Boesch C, Kühl HS (2015) Towards the automated detection and occupancy estimation of primates using passive acoustic monitoring. Ecol Indic 54:217–226. https://doi.org/10.1016/j.ecolind.2015.02.023
Kane MD (2014) Estimating population size, density, and occupancy of lions (Panthera leo), leopards (P. pardus), and servals (Leptailurus serval) using camera traps in the Niokolo Koba National Park in Senegal, West Africa. Memoire de Master. Virginia Tech University, Blacksburg, Virginia
Kanyamibwa S (2013) Albertine Rift Conservation Status Report. Albertine Rift Conservation Series No 1. Albertine Rift Conservation Society (ARCOS Network), Kampala, Uganda
Kassambara A, Mundt F (2017) Extract and Visualize the Results of Multivariate Data Analyses, Version 1.0.3. R Package ‘factoextra’. https://mran.revolutionanalytics.com/snapshot/2016-11-01/web/packages/factoextra/factoextra.pdf. Accessed 17 Nov 2020
Kindt R, van Breugel P, Lillesø J-PB, Minani V, Ruffo CK, Gapusi J, Jamnadass R, Graudal L (2014) Potential natural vegetation of eastern Africa, Volume IX. Atlas and tree species composition for Rwanda. Department of Geosciences and Natural Resource Management, University of Copenhagen
Kingdon J, Happold D, Hoffmann M, Butynski T, Happold M, Kalina J (2013) Mammals of Africa, Volumes I-VI. Bloomsbury Publishing, London
Kisioh M (2015) Gishwati Forest Reserve Three Years Interim Management Plan 2015–2018. Forest of Hope Association (FHA), Rwanda Development Board (RDB) and Rwanda Natural Resources Authority (RNRA), Kigali, Rwanda
Kumpel NF, Milner-Gulland EJ, Rowcliffe JM, Cowlishaw G (2008) Impact of gun-hunting on diurnal primates in continental Equatorial Guinea. Int J Primatol 29:1065–1082. https://doi.org/10.1007/s10764-008-9254-9
Lambeck RJ (1997) Focal species: a multi-species umbrella for nature conservation. Conserv Biol 11:849–856. https://www.jstor.org/stable/2387320
Laub BG, Budy P (2014) Assessing the likely effectiveness of multispecies management for imperiled desert fishes with niche overlap analysis. Conserv Biol 29:1153–1163. https://doi.org/10.1111/cobi.1245
Li BV, Pimm SL (2016) China’s endemic vertebrates sheltering under the protective umbrella of the giant panda. Conserv Biol 30:329–339. https://doi.org/10.1111/cobi.12618
Macdonald DW, Burnham D, Hinks AE, Wrangham R (2012) A problem shared is a problem reduced: seeking efficiency in the conservation of felids and primates. Folia Primatol 83:171–215. https://doi.org/10.1159/000342399
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. JABES 9:300–318. https://doi.org/10.1198/108571104X3361
Mallinson JC (1991) ‘Flagship’ species aiding the conservation of animals and associated habitat. Unpublished report, 46th annual conference of International Union of Directors of Zoological Gardens, Singapore
Mazerolle MJ (2019) AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf. Accessed 22 Nov 2020
Mc Guinness S, Taylor D (2014) Farmers’ perceptions and actions to decrease crop raiding by forest-dwelling primates around a Rwandan forest fragment. Hum Dimens Wildl 19:179–190. https://doi.org/10.1080/10871209.2014.853330
McGowan J, Beaumont LJ, Smith RJ, Chauvenet ALM, Harcourt R, Atkinson SC, Mittermeier JC, Esperon-Rodriguez M, Baumgartner JB, Beattie A, Dudaniec RY, Grenyer R, Nipperess DA, Stow A, Possingham HP (2020) Conservation prioritization can resolve the flagship species conundrum. Nat Commun 11:994. https://doi.org/10.1038/s41467-020-14554-z
Mittermeier RA (1986) Primate conservation priorities in the Neotropical region. In: Benirschke E (ed) Primates—The Road to Self-Sustaining Populations. Springer, New York, pp 221–240
Mittermeier RA (1988) Primate diversity and the tropical forest. In: Wilson EO (ed) Biodiversity. National Academy Press, Washington DC., pp 145–154
Moore JF, Nyiratuza M, Akampurira E, O’Brien GO (2018) Sightings of Lestrade’s duiker in Rwanda and Uganda. Gnusletter 35:17–20
Moore JF, Pine WE, Mulindahabi F, Niyigaba P, Gatorano G, Masozera MK, Beaudrot L (2020) Comparison of species richness and detection between line transects, ground camera traps, and arboreal camera traps. Anim Conserv 23:561–572. https://doi.org/10.1111/acv.12569
Musabyimana T (2014) The Landscape Approach to Forest Restoration and Conservation (LAFREC) Project In Rwanda (Gishwati and Mukura Forest Reserves), Social Assessment, Final Report. Rwanda Environmental Management Authority (REMA), Kigali
Nagelkerke NJD (1991) A note on a general definition of the coefficient of determination. Biometrika 78:691–692. https://doi.org/10.1093/biomet/78.3.691
Oberosler V, Groff C, Iemma A, Pedrini P, Rovero F (2017) The influence of human disturbance on occupancy and activity patterns of mammals in the Italian Alps from systematic camera trapping. Mamm Biol 87:50–61. https://doi.org/10.1016/j.mambio.2017.05.005
O’Brien TG, Ahumada J, Akampurila E, Beaudrot L, Boekee K, Brncic T, Hickey J, Jansen PA, Kayijamahe C, Moore J, Mugerwa B (2020) Camera trapping reveals trends in forest duiker populations in African National Parks. Remote Sens Ecol Conserv 6:168–180. https://doi.org/10.1002/rse2.132
O'Brien TG, Mulindahabi F, Ntare N, Cipolletta C (2014) Trends in Populations of Birds and Mammals in Nyungwe National Park, Southwest Rwanda. Wildlife Conservation Society, New York, USA
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) Package ‘vegan’. Community ecology package, version, 2.5–6. https://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 17 Nov 2020
Ordway EM (2015) Political shifts and changing forests: effects of armed conflict on forest conservation in Rwanda. Glob Ecol Conserv 3:448–460. https://doi.org/10.1016/j.gecco.2015.01.013
Ozaki K, Isono M, Kawahara T, Iida S, Kudo T, Fukuyama K (2006) A mechanistic approach to evaluation of umbrella species as conservation surrogates. Conserv Biol 20:1507–1515. https://doi.org/10.1111/j.1523-1739.2006.00444.x
Plumptre AJ, Davenport TRB, Behangana M, Kityo R, Eilu G, Ssegawa P, Ewangoa C, Meirte D, Kahindo C, Herremans M, Peterhanse JK, Pilgrim JD, Wilson M, Languy M, Moyer D (2007) The biodiversity of the Albertine Rift. Biol Conserv 34:178–194. https://doi.org/10.1016/j.biocon.2006.08.021
Plumptre AJ, Bizumuremyi JB (1996) Ungulates and hunting in the Parc National des Volcans, Rwanda: The effects of the Rwandan civil war on ungulate populations and the socioeconomics of poaching. Unpublished report, Wildlife Conservation Society
Plumptre AJ, Behangana M, Ndomba E, Davenport T, Kahindo C, Kityo R, Ssegawa P, Eilu G, Nkuutu D, Owiunji I (2003) The biodiversity of the Albertine Rift. Albertine Rift Technical Reports No. 3, Wildlife Conservation Society, New York
Plumptre AJ, Hart JA, Hicks TC, Nixon S, Piel AK, Pintea L (2016) Pan troglodytes ssp. schweinfurthii (errata version published in 2016). The IUCN Red List of Threatened Species 2016: e.T15937A102329417. https://dx.doi.org/https://doi.org/10.2305/IUCN.UK.2016-2.RLTS. T15937A17990187.en. Accessed on 21 January 2021
RDB, Rwanda Development Board (2015) Gishwati-Mukura National Park Guidebook. Government of Rwanda, RDB, Kigali
Redford KH (1992) The Empty Forest. Biosci 42:412–422. https://doi.org/10.2307/1311860
REMA, Rwanda Environment Management Authority (2015) Rwanda: State of Environment and Outlook Report 2015. Government of Rwanda, REMA, Kigali
Revelle W, Revelle MW (2015) Package ‘psych’. The comprehensive R archive network. http://kambing.ui.ac.id/cran/web/packages/psych/psych.pdf. Accessed 17 Nov 2020
Roberge JM, Angelstam P (2004) Usefulness of the umbrella species concept as a conservation tool. Conserv Biol 18:76–85. https://doi.org/10.1111/j.1523-1739.2004.00450.x
RoR, Republic of Rwanda (2014) Fifth National Report to the Convention on Biological Diversity. Kigali: Republic of Rwanda. http://www.cbd.int/doc/world/rw/rw-nr-05-en.pdf. Accessed 17 Nov 2020
RoR, Republic of Rwanda (2016) National Biodiversity Strategy and Action Plan (2016–2020). https://www.cbd.int/doc/world/rw/rw-nbsap-v2-en.pdf. Accessed 17 Nov 2020
Rovero F, Zimmermann F, Berzi D, Meek P (2013) “Which camera trap type and how many do I need?” A review of camera features and study designs for a range of wildlife research applications. Hystrix 24:148–156. https://doi.org/10.4404/hystrix-24.2-8789
Rovero F, Owen N, Jones T, Canteri E, Iemma A, Tattoni C (2017) Camera trapping surveys of forest mammal communities in the Eastern Arc Mountains reveal generalized habitat and human disturbance responses. Biodivers Conserv 26:1103–1119. https://doi.org/10.1007/s10531-016-1288-2
Safari B (2012) Trend analysis of the mean annual temperature in Rwanda during the last fifty-two years. J Environ Prot 3:538–551
Schuette P, Wagner AP, Wagner ME, Creel S (2013) Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol Conserv 158:301–312. https://doi.org/10.1016/j.biocon.2012.08.008
Shen X, Li S, McShea WJ, Wang D, Yu J, Shi X, Dong W, Mi X, Ma K (2020) Effectiveness of management zoning designed for flagship species in protecting sympatric species. Conserv Biol 34:158–167. https://doi.org/10.1111/cobi.13345
Spinu V, Grolemund G, Wickham H, Lyttle I, Costigan I, Law J, Mitarotonda D, Larmarange J, Boiser J, Lee HC (2020) Package ‘lubridate’. Make Dealing with Dates a Little Easier, version 1.7.9. https://cran.r-project.org/web/packages/lubridate/lubridate.pdf. Accessed 17 Nov 2020
Theobald DM (2013) A general model to quantify ecological integrity for landscape assessments and US application. Landscape Ecol 28(7):1859–1874. https://doi.org/10.1007/s10980-013-9941-6
Thornton D, Zeller K, Rondinini C, Boitani L, Crooks K, Burdett C, Rabinowitz A, Quigley H (2016) Assessing the umbrella value of a range-wide conservation network for jaguars (Panthera onca). Ecol Appl 26:1112–1124. https://www.jstor.org/stable/24818150
Tobler MW, Carrillo-Percastegui SE, Pitman RL, Mares R, Powell G (2008) An evaluation of camera traps for inventorying large-and medium-sized terrestrial rainforest mammals. Anim Conserv 11:169–178. https://doi.org/10.1111/j.1469-1795.2008.00169.x
Ukizintambara T, Olupot W, Hart J (2019) Allochrocebus lhoesti. The IUCN Red List of Threatened Species 2019: e.T4220A92345122. https://doi.org/10.2305/IUCN.UK.2019-Chimp
UNEP-WCMC, United Nations Environment Program-World Conservation Monitoring Centre, (2020) Protected Area Profile for Rwanda from the World Database of Protected Areas. https://www.protectedplanet.net. Accessed 21 Jan 2021
Van der Weyde LK, Mbisana C, Klein R (2018) Multi-species occupancy modelling of a carnivore guild in wildlife management areas in the Kalahari. Biol Conserv 220:21–28. https://doi.org/10.1016/j.biocon.2018.01.033
Wang Y, Allen ML, Wilmers CC (2015) Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biol Conserv 190:23–33. https://doi.org/10.1016/j.biocon.2015.05.007
Wiens JA, Hayward GD, Holthausen RS, Wisdom MJ (2008) Using surrogate species and groups for conservation planning and management. Bioscience 58:241–252. https://doi.org/10.1641/B580310
Wilkie DS, Bennett EL, Peres CA, Cunningham AA (2011) The empty forest revisited. Ann N Y Acad Sci 223:120–128. https://doi.org/10.1111/j.1749-6632.2010.05908.x
Wrangham RW, Hagel G, Leighton M, Marshall AJ, Waldau P, Nishida T (2008) The Great Ape World Heritage Species Project. In: Stoinski TS, Steklis HD, Mehlman PT (eds) Conservation in the 21st Century: Gorillas as a Case Study, Springer, New York, pp. 282–295). https://doi.org/10.1007/978-0-387-70721-1_14
Acknowledgements
We thank the Rwanda Development Board for permitting research in Gishwati–Mukura NP, especially Musana Abel (Research and Monitoring Warden) for his, and his team’s assistance in the field. Financial support came from a Rufford Small Grant (Rufford Ref. 20936-1). We thank Anthony Rendall and an anonymous reviewer for their constructive feedback on the manuscript. Finally, we would like to thank the late Prof Dr Ann Apio from the University of Rwanda for her continued contributions to conservation in Rwanda and her practical support rendered to this study.
Funding
Financial support was provided by a Rufford Small Grant (Rufford Ref. 20936-1).
Author information
Authors and Affiliations
Contributions
PS: statistical analysis; PU: field work and data collection; TW, PS: manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study since camera trapping was considered a non-invasive method for collecting data on wildlife species. Permission to conduct this study in Gishwati–Mukura NP was granted by the Rwanda Development Board—Tourism & Conservation Section.
Consent to participate
All authors declare to have participated in this study.
Consent for publication
All authors agree on the publication of the data and results presented in this article.
Additional information
Handling Editor: Emma Vitikainen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, P., Umuntunundi, P. & Wronski, T. Species richness, relative abundance and occupancy of ground-dwelling mammals denote the ineffectiveness of chimpanzee as flagship species. Mamm Biol 102, 1835–1850 (2022). https://doi.org/10.1007/s42991-022-00289-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-022-00289-5