Abstract
Purpose
Heat acclimation (HA) kinetics often necessitates that the intervention is conducted in the weeks immediately preceding athletic competitions, potentially interfering with a training taper. Therefore, we investigated the efficacy of a mixed-method HA protocol, superimposed over planned external training loads, during the 3-weeks prior to the 2022 U23 World Triathlon Championships.
Methods
Six international triathletes completed 8 pre-competition HA sessions (5 active: running/cycling, 3 passive: hot water immersion [HWI]), across 2-weeks. Outdoor high-intensity training sessions were followed by 30–60 min HWI, whilst low-intensity cycling/running sessions were completed in a hot, humid environmental chamber. To assess heat adaptations, participants completed three 25 min heat stress tests (HST) involving iso-speed treadmill running (session 1 = HST1, session 5 = HST2, and session 8 = HST3). Physiological, haematological and wellbeing monitoring were conducted throughout HA.
Results
Reduced heart rate (~ − 6 beats/min) was observed within HST3 (P = 0.01, ηp2 = 0.64), versus HST1 and HST2. No changes in core temperature were observed across HSTs (P = 0.055, ηp2 = 0.44). Sweat sodium concentration was lower by HST2 at the arm (− 23 ± 16 mmol/L, P = 0.02) and back (− 27 ± 17 mmol/L, P = 0.01). White blood cell count reduced from baseline to the end of HA (P = 0.02, ηp2 = 0.27), but no changes were found in any other haematological markers (all P > 0.05). Perceptual wellbeing measures did not change across HA (all P > 0.05).
Conclusion
By HST3, seven prior mixed-method HA sessions improved markers of heat adaptation (exercising HR and sweat concentration) within international triathletes. Mixed-method HA may be implemented without modifying training load, with no apparent detrimental effects on athlete health or training stress markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elite athletes habitually undertake complex training programmes, whereby physical preparation is balanced against technical and tactical preparation priorities. Physical training demands are typically monitored through internal (i.e. heart rate [HR], rating of perceived exertion [RPE]) and external training loads (i.e. duration, distance) [1, 25, 26]. In endurance sports, weekly external training volumes may exceed 20 h [8]. Whilst multisport events (e.g. pentathlon, heptathlon, triathlon), must incorporate multiple exercise modalities within this training volume. Evidently, allocation of training time is challenging for elite athletes and sessions may integrate concurrent physical, technical, tactical and skill-orientated training objectives [45].
Prior to competing in hot [16, 31], and sometimes temperate environments [35, 37], heat acclimation and acclimatization form a central focus of many elite athlete’s preparation [15, 50]. The prevalence of heat acclimation/acclimatization training reflects the greater performance and physiological effects of heat adaptation, compared with acute heat alleviation interventions [30], such as body pre-cooling [29]. A particular characteristic of heat acclimation (HA) is that phenotypic adaptations both induce and decay across a typical training mesocycle (i.e. weeks) [11]. The HA phenotype is characterised by adaptations including decreased resting and exercising, core (TCORE) and skin (TSKIN) temperatures, changes in sweat rate and composition, and a reduction in exercising heart rate (HR), which is likely due to plasma volume (PV) expansion [53] The majority of HA evidence originates from programmes comprising 5–10 days of 60–90 min exercise training sessions [16, 48, 56]. Whilst HA demonstrates effectiveness as a performance ergogen across several sports, the implementation alongside other training priorities is evidently challenging. Moreover, in endurance sports such as triathlon, completing HA in the weeks preceding competition likely coincides with carefully planned training taper phases [43, 44]. Therefore, contemporary perspectives are exploring HA prescription methods that mitigate some of these programming challenges [6].
Recent recommendations suggest acclimating ‘early’ (i.e. > 4-weeks prior to competition) and then undertaking subsequent regular maintenance thermal exposures (i.e. training sessions where individuals experience considerable thermal stimuli) [16]. This enables greater programming flexibility and training of concurrent physical and technical objectives, in the weeks preceding a competition [16, 49]. Despite negating disruption to a training taper phase, maintenance sessions necessitate a greater number of total training hours being devoted to heat preparation, compared with a traditional, concentrated programme. Other emerging approaches enabling greater flexibility around training programming include; passive heating protocols (either standalone, or post-exercise) [34, 40, 54], training twice on non-consecutive days [59, 62], completing regular training whilst overdressing [23, 60], or a combination thereof, known as mixed-methods HA [52]. These emerging approaches afford athletes the benefit of eliciting increased heat strain, without replacing planned training sessions or imposing additional external training load. Passive heat exposures demonstrate efficacy for inducing HA in sporting populations [22]. However, the ability to familiarise with representative pacing and thermal/exertional perception, that play an important role in endurance performance outcomes [27], may be limited by purely passive approaches. Thus, superimposing active and passive HA on programmed training is likely both pragmatic and efficacious for a variety of sporting scenarios, where modification to a periodised training programme may be undesirable [40, 52].
Notwithstanding the addition of heat stress to an athlete’s training, the programming of elite endurance athlete’s training already requires a delicate balance between providing stimuli for adaptation and mitigating the risk of ‘overreaching’ and ‘overtraining’. Symptoms of both may include altered immune status, such as decreases in neutrophil function, serum and salivary immunoglobulin concentrations and white-blood cell count, in athletes [39]. Therefore, the addition of heat stress represents an additive stressor for athletes, which independently can provide a further immune challenge [21, 42]. Laboratory-based HA does not appear to compromise immune, stress or inflammatory biomarkers following short- (4 days [58]; 5 days [62] and medium-term time scales, for both passive (9 days [33]) and active protocols (7 days [20]; 8 days [10]; 10 days [62]). However, these data predominantly originate from moderately trained populations (i.e. Tier 2 [41]) who undertake modest weekly training loads. Therefore, a need exists to examine biomarker responses in elite endurance athletes (i.e. ≥ Tier 4) undertaking a short-term HA intervention, in conjunction with representatively high training loads.
The aim of this study was to investigate the effect of a mixed-method HA protocol consisting of post-training passive heat stress, and low-intensity exercise in hot-humid conditions, on the physiological and health responses of six elite international triathletes. The present study occurred in the 3-weeks preceding the U23 World Triathlon Championships and no modifications were made to the planned external training loads. The intervention sought to maintain planned external training volume, and it was hypothesised that superimposing greater internal training load via HA would lead to heat adaptation, but not present undesirable physiological responses as individuals prepared for competition in heat stress.
Methods
Participants
Six international triathletes volunteered to participate in the study (3 male: 23 ± 1 years, 181 ± 8 cm, 67.0 ± 7.2 kg, sum of 7 skinfold 36.5 ± 12.8 mm, body fat percentage 16.8% ± 3.3%, \(\dot {\rm V}\)O2max 69.7 ± 8.9 mL/kg/min; 3 female: 24 ± 5 years, 167 ± 6 cm, 55.8 ± 4.8 kg, 50.7 ± 4.1 mm, 22.1% ± 3.1%, 63.6 ± 2.8 mL/kg/min). Five athletes were preparing for 2022 U23 World Championships (Abu Dhabi, United Arab Emirates, air temperature: 25.1 °C, water temperature: 28.9 °C [66]). One athlete was preparing for the 2022 Asia Triathlon Cup (Ipoh, Malaysia air temperature: 27 °C, relative humidity: 79% [Integrated Surface Database, National Oceanic and Atmospheric Administration] [47]). No athletes had previously undertaken HA. Two female athletes reported the onset of menses eleven days after the study began and therefore commenced the HA protocol within the luteal phase and ended it within the follicular phase. The third female athlete self-reported as amenorrhoeic. All participants had regularly competed in international and continental triathlon competitions for at least > 3 years. Athletes were based at the regional high-performance centre, where they typically trained for ~ 20–30 h per week (~ 6 h swimming, ~ 6 h running, ~ 10 h cycling, ~ 2 h strength, ~ 3 h recovery). Athletes provided written, informed consent and all procedures complied with the Declaration of Helsinki regarding human experimentation. Institutional ethical approval was not sought, as all measurements contained within the study were taken as part of routine physical monitoring, with training occurring as part of player’s employment obligations [65].
Experimental Design
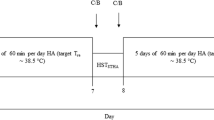
In the 3-weeks preceding the competitions, all participants undertook eight HA sessions (i.e. ‘medium-term’ HA: 5 active [running/cycling], 3 passive [post-exercise hot water immersion, HWI]). Exercise heat stress tests (HST) occurred at the start of the 1st, 5th and 8th HA sessions. Therefore, we report the effect of seven HA sessions (i.e. ‘short-term’ HA), although participants completed this 8th session, as part of competition preparation. HSTs involved 25 min of iso-speed running in the environmental chamber, at the start of the respective ~ 60 min HA sessions (HA session 1 = HST1, session 5 = HST2 and session 8 = HST3, thereby corresponding to the start, middle and end of the HA programme, respectively) (Fig. 1). Participants completed four HA sessions in week 1 and four sessions in week 2, before travelling to the venues 1-week in advance of competition (Fig. 1). During the World Championships, male athletes’ finishing positions were between 14th and 40th, from 67 starters. Females finished between 18th and 30th, from 39 starters. Given the specifics of the U23 World Championships (i.e. age-restricted competition), it was not feasible to recruit a representative control group. Likewise, a repeated-measures design would not account for this being the major event of the calendar around which training is periodised. Our sampling approach was therefore pragmatic, recruiting all individuals eligible for these competitions within the regional training centre. HA sessions were integrated into the existing training programme, such that planned external training load remained unaltered (Table 1). To achieve this, outdoor high-intensity training sessions were followed by 30–50 min HWI (water temperature ~ 39 °C), whilst low-intensity running/cycling sessions were relocated to a thermostatically controlled environmental chamber (running; 35 °C, 50% relative humidity [R.H.], cycling; 39 °C, 50% R.H., Welltech Instruments, Hong Kong China). Outdoor environmental conditions in Hong Kong during the intervention were between 19.7 and 28.5 °C (mean daily average air temperature 24.1 °C) and 80.5% R.H.
Heat Acclimation Protocol
Running speed during each 25 min HST was set to individual’s ‘easy’ training zone (11.1 ± 0.7 km/h), representing an exercise intensity below the first lactate threshold (i.e. the first deviation from ‘baseline’ or ‘steady-state’ blood lactate concentration responses). Training zones were established during regular (normothermic) laboratory testing, which had occurred within the preceding 2 months for all athletes. During session 1, 5 min after the completion of HST1, athletes completed 6 × 80 m striding efforts on the treadmill, before resting in the chamber for the remaining time (~ 15 min). Athletes self-selected the speed, which was above 17.5 km/h for all. During sessions 5 and 8, participants completed the HST and then rested in the hot environment, exercising in 5 min blocks where required (i.e. to maintain TCORE at 38.5 °C). Session 4 involved athletes completing 4 × 5 min intervals at an intensity corresponding to the second lactate threshold (‘LT2’). Athletes had 90 s rest between running bouts and then rested in the hot environment (36.9 °C, 42% R.H.) until 50 min (i.e. 25 min active, 25 min passive). During session 6, participants completed the final 60 min of a scheduled 3 h ‘easy’ outdoor cycling session, in the environmental chamber (39.0 °C, 42% R.H.). For HA sessions 2 and 7, participants completed high-intensity interval training on an outdoor athletics track, and then undertook HWI (39.9 ± 0.92 °C) as soon as practically possible (< 5 min later). During HA session 3, participants completed an outdoor, cycling and running session (~ 2.5 h), before HWI afterwards. HWI duration progressed from 30 to 60 min during the HA intervention. This provided an initially conservative, but progressive thermal stimulus through the training block.
Physiological Measures and Equipment
During all HA sessions, physiological and perceptual data were recorded at 5 min intervals. TCORE was monitored using ingestible gastrointestinal temperature capsules (precision 0.1 °C, recording every min [BodyCap, Hérouville-Saint-Clair, France]). For morning sessions, capsules were provided the day before, with instructions to swallow it immediately before bed. Two hours prior to attending the laboratory, staff met with athletes to verify that the pill was still in the body. A second pill was activated and provided if the pill had been passed. This was necessary on five occasions, prior to the three HST tests (out of a possible 18). For afternoon training, the capsule was swallowed approximately 6–8 h in advance. Body temperature was also measured at the tympanic membrane (TTYMP) using an infrared thermometer (Thermoscan 7, Braun Instruments, Frankfurt, Germany). Measurements of TTYMP were recorded every 5 min throughout all sessions, aside of when participants were running. Hydration status was assessed 30 min prior to all chamber sessions, using a pocket refractometer (PAL-10S, Atago, USA). If dehydrated (urine specific gravity > 1.020), athletes consumed 500–600 mL of water or sports drink before the run. No athletes drank during the run. During all sessions, HR was monitored using OH1 arm sensors (Polar, Kempele, Finland), or via chest strap and watch (Garmin Forerunner 955, Kansas, USA). Individual athletes were consistent in their use of the same device and position across all HA sessions. Sweat sodium (Na+) and potassium (K+) concentrations were sampled in duplicate from the upper back and arm, during HST1, HST2 and HST3 (Fig. 1). Sterilised absorbent patches were placed on clean skin prior to entering the chamber. Samples were removed using sterile tweezers, with the experimenter wearing a surgical mask and gloves. Patches were placed into new plastic syringes, expelled onto the measurement surface and analysed immediately using portable LAQUAtwin Na-11 and K-11 pocket meters (Horiba, Kyoto, Japan).
Haematology and Biomarkers
Haematological monitoring occurred in duplicate prior to HA and then three times per week during HA (Fig. 1). Fingertip capillary blood samples were collected in 300-μl Lithium Heparin microvettes for creatine kinase and cortisol measurements. Samples were then centrifuged at 4700 r/min for 5 min, before being analysed using a biochemistry and immunoassay analyser (Abbott Architect ci4100, Abbott Illinois, USA). Further fingertip blood samples were collected to assess haematology parameters (urea, white blood cell [WBC] count, red blood cell [RBC] count, haemoglobin [Hb] concentration, haematocrit [Hct], mean corpuscular volume [MCV], platelet count [PLT], testosterone concentration) in 200-μL K3 EDTA microvettes. Samples were collected whilst participants were sat in an upright position, on eight occasions (Fig. 1). Whole blood samples were assessed using an automated haematology analyser (XT2000i, Sysmex, Kobe, Japan). Manufacturer stated reliability for all parameters were between 4.5% and 12.0%. All analyses were conducted within 60 min of the samples being collected. Blood samples were collected at standardised times, generally between 11 a.m. and 12 p.m., for all participants, at least 60 min after any training sessions. Estimated plasma volume (PV) changes were indicated using established formulae [12].
Perceptual Responses
RPE [4] and both thermal comfort and thermal sensation [14], were monitored every 5 min throughout all sessions. At the same time as haematological sampling were performed, athletes rated the following perceptual wellbeing responses; sleep quality, muscle soreness, psychological stress, training demands and appetite using a four-point scale, with 1 being the ‘best’ and 4 being the ‘worst’ they have previously experienced. Athletes were also asked to self-report sleep duration during this visit.
Statistical Analysis
Data are reported as mean ± standard deviation (SD). Prior to statistical analysis, all data were checked for normality of distribution using Kolmogorov–Smirnov test and where this requirement was not met, non-parametric tests applied. Training data are reported descriptively. Changes in heat adaptation during the HST were analysed using repeated measures ANOVA (HST*time), where data at multiple time points were recorded, with Bonferroni post-hoc. Where singular data occurred, paired samples t-tests were used. Perceptual data during the HST were analysed using a non-parametric approach (Friedman’s ANOVA). Haematological and self-reported wellbeing data were analysed from two perspectives; (i) using the two ‘baseline’ values against the two ‘final’ values, using linear mixed models, (ii) using linear regression utilising all data points across the training period, to determine the relationship between each outcome variable over time. Partial eta-squared (ηp2) was used as an index of the effect size within ANOVA models if significant differences were found. Analyses were conducted within SPSS statistical software (IBM Inc, USA), with P < 0.05. Where possible, outcome data were interpreted against absolute and relative reliability of the measure, to help interpret meaningfulness [28]. These calculations were; Pearson’s correlation coefficient (r), typical error (TE: as calculated from the SD of the mean difference for each pair of trials using the formula: TE = SD(diff)/√2) and, the limits of agreement (LoA: with 95% lower and upper confidence intervals [CI]) [3]. For haematological parameters, TE was calculated from the difference between the two baseline values, whilst duplicate measures of sweat concentration were used to calculate TE. Agreement from Pearson’s correlation coefficients were interpreted based upon: ‘Negligible’ = 0.0–0.3, ‘Low’ = 0.3–0.5, ‘Moderate’ = 0.5–0.7, ‘High’ = 0.7–0.9, and, ‘Very high’ = 0.9–1.0 [2]. Typical error and associated coefficient of variation (TE CV%) were categorised as: ‘Poor’ = > 10%, ‘Moderate’ = 5%–10%, and, ‘Good’ = < 5% [64]. On one occasion when TCORE was not measured directly, TTYMP was used with an individual-specific correction applied based upon the TCORE-TTYMP regression equation obtained during that individual’s previous HSTs (on average 6 data points per athlete).
Results
Training Data
Training data throughout the study are reported in Table 1. Between sessions 1–7, the average total time whereby TCORE exceeded 38.5 °C for each athlete was 116 ± 49 min (females 143 ± 38 min, males 88 ± 49 min). This equated to 17 ± 14 min per session. Across all sessions (i.e. including HST3 and following 30 min exposure which represents an eight HA session), the average total time with TCORE > 38.5 °C for each athlete increased to 123 ± 57 min (females; 155 ± 50 min, males; 90 ± 49 min). However, the average duration > 38.5 °C per session remained similar (15 ± 14 min). Across all eight sessions, time > 38.5 °C was greatest in HWI sessions (total 75 ± 24 min, n = 3) versus active chamber sessions (total 48 ± 35 min, n = 5).
Heat Adaptation
Heat adaptation variables during the HST are displayed in Fig. 2. A reduction in exercising HR was observed within HST3 (P = 0.01, ηp2 = 0.64), as compared to HST1 and HST2. However, no statistical changes in exercising TCORE were seen across any HST (P = 0.055, ηp2 = 0.44). Similarly, no differences were found in resting TCORE (HST1 37.1 ± 0.2 °C, HST2 37.0 ± 0.4 °C, HST3 37.0 ± 0.2 °C, P = 0.60). Reduced Na+ concentration at the arm (P = 0.02) and back (P = 0.01), were found during HST2, versus HST1, while sweat Na+ concentration at the back was also lower in HST3 versus HST1 (P = 0.04). Increased sweat K+ concentration occurred at the arm (P = 0.01) and back (P < 0.01) by HST3 versus HST2. Changes were also identified in K+ concentration in HST3 versus HST1 (arm P = 0.04, back P < 0.01). Reliability data for sweat Na+ and K+ concentrations are displayed in Table 2. No differences were found in RPE, thermal sensation or thermal comfort across HSTs (all P > 0.05).
Panel of physiological markers during the HA training intervention. A core temperature over time during each HST, B peak core temperature at the end of each HST, C change in core temperature during each HST, D heart rate over time during each HST, E peak heart rate at the end of each HST, F change in heart rate during each HST, G arm sweat sodium concentration, H back sweat sodium concentration, I arm sweat potassium concentration, J back sweat potassium concentration. Coloured bar represents group mean (red = HST1, yellow = HST2, blue = HST3). Coloured shapes represent individual athletes, circles represent male athletes, squares represent female athletes. * denotes a difference from HST1 within datapoint (P < 0.05), ^ denotes difference from HST2 within datapoint (P < 0.05)
Athlete Monitoring – Haematology, Biomarkers and Perceptual Wellbeing
No changes were found in creatine kinase, cortisol, urea, RBC count, Hb concentration, Hct, PV, PLT, testosterone concentration or the testosterone: cortisol ratio (all P > 0.05, Fig. 3). A reduction in WBC count was detected between baseline (7.3 ± 1.8 109/L) and end (6.0 ± 1.0 109/L, P = 0.02, ηp2 = 0.27). An effect was also observed via regression (P = 0.01, β = − 0.117), although the mean difference (− 1.36 109/L) was smaller than the calculated TE (1.44 109/L). An increase in MCV was found from baseline (87.7 ± 3.2 fL) to end (88.4 ± 3.3 fL, P = 0.01, ηp2 = 0.33, regression: P = 0.047, β = 0.042). Perceptual wellbeing measures (i.e., sleep quality [pre 2.0 ± 0.8, post 1.8 ± 0.6], muscle soreness [pre 2.3 ± 0.5, post 2.2 ± 0.4], psychological stress [pre 1.7 ± 0.7, post 1.9 ± 0.5], training demands [pre 1.6 ± 0.5, post 1.8 ± 0.6], appetite [pre 1.6 ± 0.7, post 1.8 ± 0.6] and sleep duration [pre 7:47 ± 1:01 h, post 8:26 ± 1:08 h]) all did not change during HA (all P > 0.05).
Panel of measured biomarkers during the HA training intervention. A creatine kinase, B cortisol, C testosterone, D testosterone to cortisol ratio, E urea, F platelet count, G white blood cell count (WBC), H mean corpuscular volume (MCV), I mean corpuscular haemoglobin concentration (MCHC). Start average of 1st and 2nd measurement (Day-3 and -2), Mid average of 4th and 5th measurement (Day 3 and 5), End average of 9th and 10th measurement (Day 7 and 9). Grey bars represent group mean. Coloured shapes represent individual athletes, circles represent male athletes, squares represent female athletes. * denotes difference within End timepoint compared to Start and Mid (P < 0.05)
During HA, there was stronger agreement between TCORE and TTYMP at rest (17 observations, TCORE = 37.0 (0.3)°C, TTYMP = 36.8 (0.4)°C, r = 0.62, TE = 0.20 °C, LoA = 0.55 °C [− 0.34 to 0.76] and immediately after treadmill running in the hot environment (32 observations, TCORE = 38.3 (0.4)°C, TTYMP = 37.5 (0.4)°C, r = 0.60, TE = 0.26 °C, LoA = 0.72 °C [0.04–1.48]), than during HWI (90 observations, TCORE = 38.6 (0.6)°C, TTYMP = 38.2 (0.9)°C, r = 0.61, TE = 0.50 °C, LoA = 1.37 °C [− 0.99 to 1.76]). Overall, the agreement across all situations was relatively strong (139 observations, TCORE = 38.3 (0.8)°C, TTYMP = 38.9 (0.9)°C, r = 0.73, TE = 0.44 °C, LoA = 1.22 °C [− 0.77 to 1.67]).
Discussion
This study investigated the physiological responses and health effects of a mixed-method HA programme, integrated into the preparation of six elite triathletes preparing for the U23 World Championships (n = 5) and the Asia Triathlon Cup (n = 1). Planned external training loads remained unchanged throughout the lead-in to the Championships. Evidence of partial heat adaptation was observed by way of reduced exercising HR during HST3 (Fig. 2) and lower sweat Na+ concentrations from HST2 (Fig. 3). Exercising TCORE was lower in four out of six athletes in HST3, which also aligns with partial adaptation (Fig. 2). Concurrently, no adverse effects on haematological immune and health markers were observed (Fig. 3), nor were detrimental athlete perceptual responses identified following the superimposition of heat stress to planned training. This indicates the addition of HA to an existing training programme was well-tolerated and provides meaningful change in a population of elite athletes. These partials adaptations serve as a reference for designing HA interventions in other similarly high-demanding elite endurance training programmes.
The mean reduction in exercising HR (~ − 6 beats/min) appears highly meaningful in this population [58] and is comparable to prior research implementing medium-term HA [56]. In the context of elite endurance athletes, such a reduction represented the breadth of a HR training zone for most of this cohort (e.g. training zone of 154–160 beats/min). This has the potential to change the interpretation of internal training load, before and after HA (i.e. run in HR zone 3, is now in zone 2). For example, quantitative training load metrics based on HR data, such as TRIMP [7] or acute: chronic ratio [46] may be altered following HA. Therefore, the consideration of both internal and external load appears pertinent, around HA interventions [26, 51]. Indeed, an alternative emerging approach may be the internal: external training load ratio, providing greater flexibility in training session content, so long as there is consistency in the training monitoring metrics [51]. Reduced exercise HR following HA is usually attributed to an expansion of blood PV, although changes in cardiac contractility and sudomotor function may also contribute [53]. However, we did not observe any changes in PV. Similarly, no statistically significant reduction in TCORE was found at the group level. However, the group mean reduced by 0.22 °C (at rest) and 0.20 °C (exercising, at end of HST) between HST1 and HST3. A physiologically meaningful reduction in exercising or resting TCORE following HA has been proposed as being > 0.2 °C [56, 62]. Interestingly, four athletes displayed a reduction in exercising TCORE of this magnitude or greater though it is acknowledged that the change in menstrual cycle phase across two participants during the analysis timeframe means typical deductive reasoning as to the effects of HA on TCORE reduction is not possible. We also found positive reductions in sweat Na+ concentration following HST2 (arm: − 23 mmol/L, back: ~ − 27 mmol/L), which were maintained at HST3 (arm: − 20 mmol/L, back: − 22 mmol/L) compared to HST1. This change in sweat Na+ retention during HA is in agreement with previous literature (− 20 mmol/L) [9, 62, 63] and exceeded our measurement error (Table 2), thus appears meaningful and has the potential to attenuate the negative implications of reduced systemic sodium during competition. These changes are indicative of improved reabsorption ability of the eccrine sweat glands [5]. A modest increase was observed in sweat K+ concentration (HST2 arm: + 0.6 mmol/L, back: + 0.4 mmol/L, HST3 arm: + 1.9 mmol/L, back: + 2.1 mmol/L). This may be linked to a reduction in sweat Na+, but has also been suggested to reflect the accumulation of K+ within the sweat patch following an increased local sweat rate [36]. As we did not measure sweat rate, we could not verify this mechanism.
In the context of the haematological and biomarker dataset (Fig. 3), changes following the superimposition of heat to the planned training would have indicated a systemic perturbation that may represent ‘overtraining’ or compromised immune status [39]. An 18% reduction in WBC was observed. Leukopenia can be associated with an increased risk of infection in athletes undertaking high training loads [19], however all athlete’s final WBC measures (~ 6.0 109/L) remained within a normal range for athletes (5.7–7.4 109/L) [24]. Accordingly, the superimposition of heat to the planned training was not considered to negatively influence WBC. Across the intervention, no significant, nor meaningful changes were observed in the other biomarkers assessed e.g. urea, RBC count, MCV, PLT, cortisol and testosterone. This supports the conclusion that the superimposition of heat did not push the athletes towards a state of ‘overtraining’ or compromise these selected markers, which can characterise immune status. For example, no observable reduction in testosterone, nor an increased testosterone: cortisol ratio, which may occur in response to training stress [39]. In agreement with our hypothesis, the mixed-method HA programme does not therefore appear to have compromised immune function in these athletes. Taken together, these real-world, elite athlete haematological and biomarker training data align with laboratory data from active and passive HA studies collected on sub-elite and active individuals [10, 33, 58, 62]. Overall, the addition of heat stress to an existing training programme resulted in encouraging, albeit partial heat adaptation, within international endurance athletes.
The individual responses that we observed could be reflective of differences in HA state between individuals [61]. Indeed, athletes may already operate at a greater HA state than untrained individuals and require a greater HA stimulus to reduce TCORE e.g., running at a higher intensity than implemented during HSTs and/or greater total volume. We also acknowledge the potential for measurement bias in HST1, given one of these two participants who did not demonstrate a reduced TCORE took a second pill earlier in the morning having passed the original through the night. As such, fluids consumed prior to the HST may still influence the derived temperature [57].
Inducing heat adaptation without modifying prescribed training load represents an exciting development in HA prescription. Our HA session volume (4 sessions per week, 7 prior to HST3) may be considered modest, compared with related literature (13 sessions in 18 days [52]; 8 sessions in 8 days [55]) or laboratory-derived guidelines (> 6 days [16]). Similarly, the duration of HA sessions (max 60 min) is considerably shorter than many controlled hyperthermia protocols (90 min [17]) and below the historical recommendation to attain 60 min above a ‘target’ TCORE [13]. As a result, average time > 38.5 °C was 15–17 min, which compares with 63 min in a prior short-term laboratory based HA study with trained runners [31]. It is important to note that the mixed-method approach we adopted was designed to support concurrent training aims. Therefore, session design and external training intensities were not prescribed solely targeting specific physiological responses (e.g. exceed 38.5 °C as soon as possible [18]). Rather, the external training demands of sessions were predetermined, as the coach sought to train towards multiple objectives across the three disciplines. Adding a further stressor to a high-volume (> 20 h) endurance training programme logically raises the risk of training maladaptation, such as overreaching. Thus, we adopted a pragmatic and conservative approach to progressively introduce the HA stimulus, avoiding high-intensity training in the heat and utilising initially shorter (30 min) HWI sessions. Interestingly, our shorter active HA sessions and lower weekly total heat exposure than prevailing HA guidelines [16, 48, 56], are comparable to that which has recently been successfully applied over a chronic timeline (5 × 50 min over 5 weeks) in highly trained endurance athletes [38]. Given the modest nature of our HA prescription, these data serve as a reference for short-term HA that achieves partial heat adaptation in elite endurance athletes across 2-weeks, without compromising biomarker status or overreaching. The absence of negative interactions with ongoing training, wellbeing and immune markers, indicates merit in extending our progressive prescription model across a longer timescale.
Our data demonstrate the relevance of a practitioner ‘toolbox’ approach, whereby partial HA can be achieved through a variety of pragmatic techniques (active and passive), so long as the primary potentiating stimuli are achieved (i.e. targeting prolonged, elevated TCORE and profuse sweating) [16]. Interestingly, time TCORE remained > 38.5 °C was greatest in HWI sessions (total 75 ± 24 min) compared to active chamber sessions (total 48 ± 35 min). This is a consequence of the HWI sessions following outdoor high-intensity training. Thus, TCORE is already elevated and HWI primarily served to maintain thermal strain. Conversely, active sessions were predominantly completed from a rested state, where a larger heat gain was required to achieve this level of thermal stress. These are important observations helping to guide integrated HA prescription into high-volume training programmes comprising 15–20 sessions per week (Table 1). Our findings have relevance for the application of HA across a number of endurance sports, and provide encouraging evidence for other sports such as team-sports, whereby heat stress may not be a primary determinant of fatigue, but a performance decrement is observed relative to normothermic conditions [32].
Although we monitored heat adaption across predefined training sessions, this resulted in heat adaptation being assessed in the low-intensity domain during modest levels of thermal and cardiovascular stress, potentially masking the magnitude of adaptations. This approach was necessitated by a need for conservative exercise prescription, given these athletes had not undertaken HA previously and were preparing for important upcoming competitions. Therefore, planned external training loads remained unchanged during our intervention, with pre-existing training sessions relocated to the hot environment. Enhancing thermal strain during future HA interventions is now possible for this cohort with a view to increasing the magnitude of adaptation. Hydration variability within the range described as euhydrated and variability of blood sampling times (i.e. ± 60 min) may also conceal true magnitude of some haematological changes. For example, some athletes may not have been sufficiently rehydrated following training, which could help explain the absence of a detected change in PV. Nevertheless, consistency of sampling in such a busy training week is difficult to achieve. Thus, the true magnitude of adaptation may have been greater than we report, given the challenges of control in an applied environment. Practitioners should also note the circumstance with poorest agreement between TCORE and TTYMP was during passive heating. This is an observation which has recently been made when using a portable steam sauna [64], whereby similar to HWI, an external source of heat interacts across the majority of the skin surface area excluding the measurement site of TTYMP. Therefore, we advocate the development of individual- and situation-specific regression equations (e.g. immediately post exercise only or high/low levels of heat stress), given resting data demonstrated stronger agreement than post-exercise or during HWI.
Conclusion
Superimposing mixed-method HA on top of an existing training programme was well-tolerated by elite endurance athletes and provided partial heat adaptation in this population. This intervention serves as a reference for pragmatic HA strategies that other elite endurance athletes may utilise, when undertaking similarly highly demanding training programmes. Heat adaptation can be achieved through numerous approaches that are complimentary and not dichotomous to concurrent training.
Data Availability
Data may be made available upon request to the corresponding author.
References
Akubat I, Taylor AR, Sanders D, Myers T, Abt G, Taylor C. The dose-response relationship between training load and aerobic fitness in academy rugby union players. Int J Sports Physiol Performance. 2017;13(2):163–9.
Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. J R Stat Soc Ser D (The Stat). 1983;32(3):307–17. https://doi.org/10.2307/2987937.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Borg G, Löllgen H. Borg’s perceived exertion and pain scales. Dtsch Z Sportmed. 2001;52(9):252.
Buono MJ, Kolding M, Leslie E, Moreno D, Norwood S, Ordille A, Weller R. Heat acclimation causes a linear decrease in sweat sodium ion concentration. J Thermal Biol. 2018;71:237–40. https://doi.org/10.1016/j.jtherbio.2017.12.001.
Casadio JR, Kilding AE, Cotter JD, Laursen PB. From lab to real world: heat acclimation considerations for elite athletes. Sports Med. 2017;47(8):1467–76. https://doi.org/10.1007/s40279-016-0668-9.
Cejuela R, Esteve-Lanao J. Training load quantification in triathlon. J Hum Sport Exerc. 2011;6(2):218–32. https://doi.org/10.4100/jhse.2011.62.03.
Cejuela R, Sellés-Pérez S. Road to Tokyo 2020 olympic games: training characteristics of a world class male triathlete. Front Physiol. 2022;13(April):1–10. https://doi.org/10.3389/fphys.2022.835705.
Chinevere TD, Kenefick RW, Cheuvront SN, Lukaski HC, Sawka MN. Effect of heat acclimation on sweat minerals. Med Sci Sports Exer. 2008;40(5):886–91.
Costello JT, Rendell RA, Furber MJW, Massey HC, Tipton MJ, Young JS, Corbett J. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine. 2018;110:277–83. https://doi.org/10.1016/J.CYTO.2018.01.018.
Daanen HAM, Racinais S, Périard JD. Heat acclimation decay and re-induction: a systematic review and meta-analysis. Sports Med. 2018;48(2):409–30. https://doi.org/10.1007/s40279-017-0808-x.
Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol (Bethesda, Md). 1985;37(2):247–8.
Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization to heat in man by controlled elevation of body temperature. J Physiol. 1963;166(3):530–47.
Gagge A, Stolwijk J, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res. 1969;2(3):209–29.
Galan-Lopez N, Esh CJ, Leal DV, Gandini S, Lucas R, Garrandes F, Bermon S, Adami PE, Kajeniene A, Hosokawa Y, Chrismas BCR, Stevens CJ, Taylor L. Heat preparation and knowledge at the world athletics race walking team championships muscat. Int J Sports Physiol Perform. 2023;18(8):813–24. https://doi.org/10.1123/ijspp.2022-0446.
Gibson OR, James CA, Mee JA, Willmott AGB, Turner G, Hayes M, Maxwell NS. Heat alleviation strategies for athletic performance: a review and practitioner guidelines. Temperature. 2019;7(1):3–36.
Gibson OR, Mee JA, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J Therm Biol. 2015;49–50:55–65.
Gibson OR, Willmott AGB, James CA, Hayes M, Maxwell NS. Power relative to body mass best predicts change in core temperature during exercise-heat stress. J Strength Condition Res. 2017;31(2):403–14.
Gleeson M. Biochemical and immunological markers of overtraining. J Sports Sci Med. 2002;1(2):31–41.
Guy JH, Pyne DB, Deakin GB, Miller CM, Edwards AM. Acclimation training improves endurance cycling performance in the heat without inducing endotoxemia. Front Physiol. 2016;7:318. https://doi.org/10.3389/fphys.2016.00318.
Hailes WS, Slivka D, Cuddy J, Ruby BC. Human plasma inflammatory response during 5 days of exercise training in the heat. J Thermal Biol. 2011;5(36):277–82.
Heathcote SL, Hassmén P, Zhou S, Stevens CJ. Passive heating: reviewing practical heat acclimation strategies for endurance athletes. Front Physiol. 2018;9:1851.
Henderson MJ, Chrismas BCR, Stevens CJ, Novak A, Fransen J, Coutts AJ, Taylor L. Additional clothing increases heat load in elite female Rugby sevens players. Int J Sports Physiol Perform. 2021;16(10):1424–31. https://doi.org/10.1123/IJSPP.2020-0620.
Horn PL, Pyne DB, Hopkins WG, Barnes CJ. Lower white blood cell counts in elite athletes training for highly aerobic sports. Eur J Appl Physiol. 2010;110(5):925–32.
Impellizzeri FM, Marcora SM, Coutts AJ. Internal and external training load: 15 years on. Int J Sports Physiol Perform. 2019;14(2):270–3. https://doi.org/10.1123/ijspp.2018-0935.
James CA, Dhawan A, Jones T, Pok C, Yeo V, Girard O. Minimal agreement between internal and external training load metrics across a 2-wk training microcycle in elite squash. J Sports Sci Med. 2021;20(1):101–9. https://doi.org/10.52082/jssm.2021.101.
James CA, Hayes M, Willmott AGB, Gibson OR, Flouris AD, Schlader ZJ, Maxwell NS. Defining the determinants of endurance running performance in the heat. Temperature. 2017;4(3):314–29. https://doi.org/10.1080/23328940.2017.1333189.
James CA, Richardson AJ, Watt PW, Maxwell NS. Reliability and validity of skin temperature measurement by telemetry thermistors and a thermal camera during exercise in the heat. J Thermal Biol. 2014;45:141–9. https://doi.org/10.1016/j.jtherbio.2014.08.010.
James CA, Richardson AJ, Watt PW. Physiological responses to incremental exercise in the heat following internal and external precooling. Scand J Med Sci Sports. 2015;25(s1):190–9. https://doi.org/10.1111/sms.12376.
James CA, Richardson AJ, Watt PW, Willmott AGB, Gibson OR, Maxwell NS. Short-term heat acclimation and precooling, independently and combined, improve 5-km time trial performance in the heat. J Strength Condition Res. 2018;32(5):1366–75. https://doi.org/10.1519/JSC.0000000000001979.
James CA, Richardson AJ, Willmott AGB, Watt PW, Gibson OR, Maxwell NS. Short-term heat acclimation improves the determinants of endurance performance and 5-km running performance in the heat. Appl Physiol Nutrit Metab. 2017;42(3):285–94. https://doi.org/10.1139/apnm-2016-0349.
James CA, Willmott AGB, Dhawan A, Stewart C, Gibson OR. Increased air temperature decreases high-speed, but not total distance, in international field hockey. Temperature. 2021;9(4):357–72. https://doi.org/10.1080/23328940.2021.1997535.
Kanikowska D, Sato M, Sugenoya J, Iwase S, Shimizu Y, Nishimura N, Inukai Y. No effects of acclimation to heat on immune and hormonal responses to passive heating in healthy volunteers. Int J Biometeorol. 2012;56(1):107–12.
Kirby NV, Lucas SJE, Armstrong OJ, Weaver SR, Lucas RAI. Intermittent post-exercise sauna bathing improves markers of exercise capacity in hot and temperate conditions in trained middle-distance runners. Eur J Appl Physiol. 2021;121(2):621–35.
Kirby NV, Lucas SJE, Lucas RAI. Nine-, but not four-days heat acclimation improves self-paced endurance performance in females. Front Physiol. 2019;10:1–12.
Klous L, De Ruiter C, Alkemade P, Daanen H, Gerrett N. Sweat rate and sweat composition during heat acclimation. J Thermal Biol. 2020;93:102697.
Lorenzo S, Halliwill JR, Sawka MN, Minson CT. Heat acclimation improves exercise performance. J Appl Physiol (1985). 2010;109(4):1140–7.
Lundby C, Hamarsland H, Hansen J, Bjørndal H, Berge SN, Hammarström D, Rønnestad BR. Hematological, skeletal muscle fiber and exercise performance adaptations to heat training in elite female and male cyclists. J Appl Physiol. 2023;135(1):217–26.
Mackinnon LT. Special feature for the Olympics: effects of exercise on the immune system: overtraining effects on immunity and performance in athletes. Immunol Cell Biol. 2000;78(5):502–9.
McIntyre RD, Zurawlew MJ, Mee JA, Walsh NP, Oliver SJ. A comparison of medium-term heat acclimation by post-exercise hot water immersion or exercise in the heat: Adaptations, overreaching, and thyroid hormones. Am J Physiol Regul Integr Comp Physiol. 2022;323(5):R601–15. https://doi.org/10.1152/ajpregu.00315.2021.
McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, Sheppard J, Burke LM. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17(2):317–31. https://doi.org/10.1123/ijspp.2021-0451.
Mitchell JB, Dugas JP, McFarlin BK, Nelson MJ. Effect of exercise, heat stress, and hydration on immune cell number and function. Med Sci Sports Exerc. 2002;34(12):1941–50.
Mujika I. Tapering for triathlon competition. J Hum Sport Exerc. 2011;6(2):264–70.
Mujika I. Olympic preparation of a world-class female triathlete. Int J Sports Physiol Perform. 2014;9(4):727–31. https://doi.org/10.1123/IJSPP.2013-0245.
Mujika I, Halson S, Burke LM, Balagué G, Farrow D. An integrated, multifactorial approach to periodization for optimal performance in individual and team sports. Int J Sports Physiol Perform. 2018;13(5):538–61. https://doi.org/10.1123/ijspp.2018-0093.
Murray NB, Gabbett TJ, Townshend AD, Blanch P. Calculating acute:chronic workload ratios using exponentially weighted moving averages provides a more sensitive indicator of injury likelihood than rolling averages. Br J Sports Med. 2017;51(9):749–54. https://doi.org/10.1136/BJSPORTS-2016-097152.
National Oceanic and Atmospheric Administration. Integrated Surface Database – Ipoh, Perak, Malaysia historical data. 2023. https://www.wunderground.com/. Accessed 20 Oct 2023.
Périard JD, Racinais S, Timpka T, Dahlström Ö, Spreco A, Jacobsson J, Bargoria V, Halje K, Alonso JM. Strategies and factors associated with preparing for competing in the heat: a cohort study at the 2015 IAAF World Athletics Championships. Br J Sports Med. 2017;51(4):264–270.
Périard JD, Eijsvogels TMH, Daanen HAM. Exercise under heat stress: thermoregulation, hydration, performance implications, and mitigation strategies. Physiol Rev. 2021;101(4):1873–979. https://doi.org/10.1152/physrev.00038.2020.
Pryor JL, Johnson EC, Roberts WO, andPryor, R.R. Application of evidence-based recommendations for heat acclimation: Individual and team sport perspectives. Temperature. 2019;6(1):37–49.
Ramos JAP, Brade CJ, Ducker KJ, Landers GJ, Girard O. The internal-to-external load ratio: a tool to determine the efficacy of heat acclimation/acclimatization using self-paced exercise. Front Sports Active Living. 2022;3:2021–3. https://doi.org/10.3389/fspor.2021.830378.
Ruddock AD, Thompson SW, Hudson SA, James CA, Gibson OR, Mee JA. Combined active and passive heat exposure induced heat acclimation in a soccer referee before 2014 FIFA World Cup. Springerplus. 2016;5(1):617. https://doi.org/10.1186/s40064-016-2298-y.
Sawka MN, Leon LR, Montain SJ, Sonna LA. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Comprehensive Physiol. 2011;1(4):1883–928.
Stanley J, Halliday A, D’Auria S, Buchheit M, Leicht AS. Effect of sauna-based heat acclimation on plasma volume and heart rate variability. Eur J Appl Physiol. 2015;115(4):785–94.
Stephenson BT, Tolfrey K, Goosey-Tolfrey VL. Mixed active and passive heart rate-controlled heat acclimation is effective for paralympic and able-bodied triathletes. Front Physiol. 2019;10:1214. https://doi.org/10.3389/fphys.2019.01214.
Tyler CJ, Reeve T, Hodges GJ, Cheung SS. The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med. 2016;46(11):1699–724.
Wilkinson DM, Carter JM, Richmond VL, Blacker SD, Rayson MP. The effect of cool water ingestion on gastrointestinal pill temperature. Med Sci Sports Exerc. 2008;40(3):523–8. https://doi.org/10.1249/MSS.0b013e31815cc43e.
Willmott AGB, et al. Short-term heat acclimation prior to a multi-day desert ultra-marathon improves physiological and psychological responses without compromising immune status. J Sports Sci. 2017;35(22):2249–56. https://doi.org/10.1080/02640414.2016.1265142.
Willmott AGB, Gibson OR, Hayes M, Maxwell NS. The effects of single versus twice daily short term heat acclimation on heat strain and 3000m running performance in hot, humid conditions. J Thermal Biol. 2016;56:59–67. https://doi.org/10.1016/j.jtherbio.2016.01.001.
Willmott AGB, Gibson OR, James CA, Hayes M, Maxwell NS. Physiological and perceptual responses to exercising in restrictive heat loss attire with use of an upper-body sauna suit in temperate and hot conditions. Temperature. 2018;5(2):162–74. https://doi.org/10.1080/23328940.2018.1426949.
Willmott AGB, Hayes M, Dekerle J, Maxwell NS. The reliability of a heat acclimation state test prescribed from metabolic heat production intensities. J Thermal Biol. 2015;53:38–45. https://doi.org/10.1016/j.jtherbio.2015.08.008.
Willmott AGB, Hayes M, James CA, Dekerle J, Gibson OR, Maxwell NS. Once- and twice-daily heat acclimation confer similar heat adaptations, inflammatory responses and exercise tolerance improvements. Physiol Rep. 2018;6(24):e13936. https://doi.org/10.14814/phy2.13936.
Willmott AGB, Holliss R, Saynor Z, Corbett J, Causer AJ, Maxwell NS. Heat acclimation improves sweat gland function and lowers sweat sodium concentration in an adult with cystic fibrosis. J Cystic Fibrosis. 2021;20(3):485–8. https://doi.org/10.1016/J.JCF.2020.07.013.
Willmott AGB, James CA, Hayes M, Maxwell NS, Roberts J, Gibson OR. The reliability of a portable steam sauna pod for the whole-body passive heating of humans. J Thermal Biol. 2023;118:103743. https://doi.org/10.1016/j.jtherbio.2023.103743.
Winter EM, Maughan RJ. Requirements for ethics approvals. J Sports Sci. 2009;27(10):985–985. https://doi.org/10.1080/02640410903178344.
World Triathlon. 2022 World Triathlon Championship Finals Abu Dhabi. 2022. https://www.triathlon.org/events/event/2022_world_triathlon_championship_finals_abu_dhabi. Accessed 9 Dec 2023.
Funding
Open access funding provided by Hong Kong Baptist University Library.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
James, C.A., Willmott, A.G.B., Lee, C.W.D. et al. Mixed-Method Heat Acclimation Induces Heat Adaptations in International Triathletes Without Training Modification. J. of SCI. IN SPORT AND EXERCISE (2024). https://doi.org/10.1007/s42978-024-00278-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42978-024-00278-9