Abstract
The taxonomic and metabolic diversity of prokaryotes and their adaptability to extreme environmental parameters have allowed extremophiles to find their optimal living conditions under extreme conditions for one or more environmental parameters. Natural habitats abundant in extremophilic microorganisms are relatively rare in Hungary. Nevertheless, alkaliphiles and halophiles can flourish in shallow alkaline lakes (soda pans) and saline (solonetz) soils, where extreme weather conditions favor the development of unique bacterial communities. In addition, the hot springs and thermal wells that supply spas and thermal baths and provide water for energy use are suitable colonization sites for thermophiles and hyperthermophiles. Polyextremophiles, adapted to multiple extreme circumstances, can be found in the aphotic, nutrient-poor and radioactive hypogenic caves of the Buda Thermal Karst, among others. The present article reviews the organization, taxonomic composition, and potential role of different extremophilic bacterial communities in local biogeochemical cycles, based on the most recent studies on extremophiles in Hungary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life on Earth is based on a diverse and continually changing world of prokaryotes that has existed for more than 3.5 billion years. These microscopic organisms have now inhabited every potential habitat. The modern prokaryotic world exists within a wide range of geological–physical–chemical environments, as microorganisms have adapted to extreme geophysical (e.g., temperature and pressure) and geochemical (e.g., salinity, pH, oxygen deficiency, and redox potential) parameters, as well (Madigan and Marrs 1997; Pikuta et al. 2007; Gupta et al. 2014; Coker 2019; Shu and Huang 2022). The so-called extremophiles or polyextremophiles (Bacteria, Archaea and Eukarya) can live their entire life cycle under extreme conditions with respect to one or more environmental parameters well beyond the human scale (Rothschild and Mancinelli 2001; Pakchung et al. 2006). These microorganisms are usually classified according to the specific environmental factors (temperature, chemistry, salinity, etc.) that is required for their optimal growth (Madigan and Marrs 1997; Rothschild and Mancinelli 2001; Merino et al 2019). Thermophiles or hyperthermophiles are microorganisms that prefer extremely high temperature, while psychrophiles require low temperature. Alkaliphiles need highly alkaline environment, while acidophiles prefer highly acidic one. Halophiles are microorganisms that adapt to high salt concentration.
There are many special habitats on Earth that are unlivable from a human perspective, but to which microorganisms have adapted throughout the evolution. Habitats abundant in extremophiles include, for example, volcanic vents, ice sheets covering Arctic seas or lakes, glaciers, soils, and wetlands with pH ≤ 2 or pH ≥ 10, or deep seas with high hydrostatic pressure (Schmid et al. 2020; Shu and Huang 2022). Although Hungary, located in the northern temperate zone, the Carpathian Basin, is not a typical unique environment, it still has several extreme habitats (e.g., alkaline and saline lakes and soils, deep-drilled thermal wells, and active hypogene karst caves).
One of the importance of studying extremophiles and polyextremophiles is to understand the limits of life on Earth. The research of extremophiles has extended the environmental boundaries within which life has been proved to exist (Madigan and Marrs 1997; Rothschild and Mancinelli 2001; Harrison et al. 2013; Merino et al. 2019; Madigan et al. 2021; Shu and Huang 2022). In addition, knowledge of extremophiles can provide a basis for research into extraterrestrial life forms defined by the laws of physics and chemistry as we know them (Cavicchioli 2002; Cayol et al. 2015; Coker 2019).
Research on the genetic and metabolic diversity of extremophiles and polyextremophiles, their adaptation mechanisms to extreme and/or highly changing environmental parameters, and their interactions with their environment has contributed to our understanding of their role in the biogeochemical cycles from local to global scales (Sorokin et al. 2014; Martínez-Espinosa 2020; González and Terrón 2021). Furthermore, not only the extremophiles themselves, or the macromolecules participating in their structure and function, but also their metabolites can be unique. This allows them to fulfill specific roles in their natural environment and for the benefit of humanity (e.g., extremozymes, extremolytes, secondary metabolites, pigments, and other bioactive compounds). The multiple uses of extremophiles (e.g., antibiotics, antitumors and extremolytes in medicine, phytases and phosphatases in food technology, proteases and lipases in biofuel production, carotenoids in cosmetic industry, xenobiotic degrading enzymes in bioremediation technologies, plant growth inducers in agriculture, etc.) are now unquestionably part of our everyday life (Raddadi et al. 2015; Coker 2016; Rampelotto 2016; Dumorné et al. 2017).
Nowadays, several studies have reported on methodological developments for the more in-depth investigation of extremophiles (Rainey and Oren 2006; Schultz et al 2023). These include various culture-independent procedures, such as metagenomics (Sime-Ngando et al. 2011; Vester et al 2015), proteomics (Burg et al. 2011), RNA stable isotope probing (Graef et al 2011), microarray for gene regulation studies (Campanaro et al 2011), quantitative PCR to measure the abundance of extremophiles (Mayumi et al 2011; Westerholm et al 2011), and development of gene transfer systems for manipulating extremophiles (Cavicchioli et al. 2011; Calo et al 2011; Köcher et al 2011; Taylor et al 2011). In addition, the improvement of classical cultivation techniques opens new possibilities for the detection of previously uncultivated extremophiles and for exploring their metabolic potential. These include, e.g., using media with relatively low nutrient concentrations, prolonging the incubation time, adding known signal molecules to the media to facilitate communication between bacteria, adding antibiotics to inhibit fast-growing microorganisms, changing the gelling agent in solid media, using different concentration of oxygen and other gases, and using cell-targeting methods (Alain and Querellou 2009; Vester et al 2015; Schultz et al 2023).
In view of the increased interest in extremophiles in recent decades, this review presents our knowledge of extremophilic microbial communities occurring in specific habitats of the Carpathian Basin (Hungary), regarding their potential future applications.
Alkaliphiles and halophiles
Effects of extreme weather conditions on the diversity of bacterial communities in shallow soda ponds and an alkaline–saline oxbow lake
The best-studied soda lakes are found in the Great Rift Valley in East Africa (Kenya, Tanzania, Uganda, Ethiopia) and are the most stable natural alkaline habitats on Earth. There are soda lakes in the temperate climate zone in North America (USA), Australia, Asia (Turkey, Russia, Central Asia), and even in Europe (Hungary, Austria, Serbia) (Jones and Grant 1999; Boros 2013; Cayol et al. 2015). In the Carpathian Basin, the small alkaline and saline water bodies (area 1–200 ha) were formed at the end of the Pleistocene and the beginning of the Holocene under specific climatic, geological, and hydrological conditions. They are extremely shallow (water depth < 1 m) and are usually characterized by intermittent (astatic) water circulation. The so-called szék of the Kiskunság National Park (KNP) (e.g., Böddi-szék, Kelemen-szék, Zab-szék) can be classified among the soda pans that dry up once or even more than once a year. The pans are fed by ground water in addition to rainwater. Their water volume decreases significantly in summer due to the strong evaporation, while their salinity increases. The suction effect resulting from evaporation also initiates the upflow of groundwater from the deeper layers, which increases the accumulation of salt on the surface. Compared to other saline waters of the world, the ones of the Carpathian Basin have a relatively low salinity, but a very high degree of alkalinity. These waters consist of four components, in which Na2CO3, NaHCO3, and Ca(HCO3)2 maintain an equilibrium in a dissociated state and CaCO3 in an undissolved state (Boros 1999; Boros and Vörös 2010; Ecsedi and Boros 2013; Felföldi 2020). The salinity of the waters fluctuates between subsaline (0.5–3.0 g l−1) and hypersaline (> 50 g l−1), while the pH varies between values 8–10 (Ecsedi and Boros 2013; Borics et al. 2016).
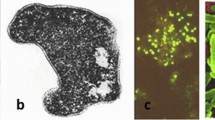
After a longer sunny, dry, warm, and windless period in June 2008, a dual water bloom was observed in the Böddi-szék (46° 46′ N; 19° 08′ E). A surface yellowish-green layer dominated by a green alga (Oocystis submarina Lagerheim) was distinguishable from a purple biogenic layer near the sediment surface in the extremely shallow (5–6 cm depth) water (Fig. 1). The water temperature was 33 °C, the specific electrical conductivity was 15.7 mS cm−1, the pH was 9.7, and the dissolved oxygen content was 32.7 mg l−1 (458%) at the time of sampling according to the on-site measurements (Borsodi et al. 2013). Prior to our observation, a similar phenomenon was reported in Eurasia only in the case of shallow-water Mongolian soda lakes (Sorokin et al. 2004).
During the microbiological examination, a high taxonomic diversity of (poli)extremophilic (alkaliphilic and/or halophilic) bacteria was revealed using a combined cultivation-based and molecular cloning approach (Fig. 1). Green algae and, to a lesser extent, cyanobacteria (with their oxygen-producing photoautotrophic metabolism) were the main primary producers in the upper, yellowish-green, oxygen-rich layer. The low-light and oxygen-depleted lower layer favored the growth of anoxic phototrophic purple sulfur (e.g., Ectothiorhodospira, Thiorhodospira) and non-sulfur bacteria (e.g., Rhodobaca, Rhodobacter) and heliobacteria (e.g., Heliorestis), as well as aerobic anoxygenic phototrophic bacteria (e.g., Erythrobacter). These bacteria provide their energy needs by photolithotrophic oxidation of reduced sulfur compounds and hydrogen or photoorganotrophic oxidation of simple organic substances. In the upper water layer, aerobic chemoorganotrophic alkaliphiles and halophiles (e.g., Alkalimonas, Halomonas, Marinospirillum, Nitrincola, Bacillus, Gracilibacillus) took part in the complete biodegradation of organic carbon compounds produced during primary production (Borsodi et al. 2013). In recent decades, among the bacterial strains isolated from soda pans (e.g., Böddi-szék) of the KNP, several new alkaliphilic species (e.g., Bacillus aurantiacus sp. nov., B. alkalisediminis sp. nov., B. kiskunsagensis sp. nov., Nesterenkonia pannonica sp. nov., Nitrincola alkalilacustris sp. nov.) have been described (Borsodi et al. 2008, 2011, 2017a; b, c). The type strains of the newly described species grew in the pH range of 7.0–12.0, optimally at pH 9.0–10.0, typical of their habitat. In addition, they had a relatively wide salt tolerance, adapting to the varying salt concentrations in the soda pans. They were usually capable of cultivation in a NaCl concentration range of 3–10% (w/v), with optimal growth at 5–7%. These bacteria can also effectively participate in the mineralization processes. In the lower water layer, the biodegradation of complex organic substances began with acidogenesis performed by primary fermenters (e.g., Anoxynatronum, Clostridium, Spirochaeta). This was complemented by the metabolism of sulfate-reducing bacteria (SRB, e.g., Desulfobotulus, Desulfonatronum) that utilize fermentation end products (e.g., organic acids) by partial or complete oxidation. The latter could provide the reduced sulfur compounds required for anoxic phototrophs by dissimilatory reduction of sulfate (Borsodi et al. 2013).
A dual water bloom very similar to the one in Böddi-szék appeared in April 2014, in another soda pan of the KNP (46° 46′ N; 19° 11′ E). The water temperature was 23 °C, the pH was 10.1, and the specific electrical conductivity was 15.5 mS cm−1 at the time of sampling. As before, the upper part of the only 5 cm water layer was dominated by the planktonic green algae Oocystis submarina Lagerheim, while the lower purple-colored, bacteriochlorophyll containing water layer was dominated by representatives of the genera Thiorhodospira and Rhodobaca based on the next-generation sequencing (NGS) results (Korponai et al. 2019).
Purple-colored water blooms like those observed in the soda pans of the KNP were also observed in summer periods, year after year, in a natural oxbow lake (46.9 N; 20.6 E) in the south-eastern region of the Hungarian Great Plain, which is used for the temporary storage of thermal water for energy purposes. Although the physical and chemical properties of the water of the alkaline and saline reservoir (summer temperature 27.6 °C, pH 8.7–9.3, specific electrical conductivity 4.0–5.7 mS cm−1) were similar to those of the soda pans of the KNP, high concentration of aromatic hydrocarbons from the used thermal water (total phenols up to 4.2 mg l−1) was detected in the cooling reservoir. The results of the combined (microscopic, cultivation based, and molecular cloning) methodological approach revealed that in addition to oxygenic photosynthetic cyanobacteria (e.g., Arthrospira, Gloeocapsa), anoxic phototrophic purple sulfur (e.g., Thiolamprovum) and non-sulfur (e.g., Rhodobacter, Rubribacterium) (mostly nitrogen-fixing) bacteria could be responsible for the water blooms in the reservoir. Alkaliphilic and/or halophilic (e.g., Bacillus, Planococcus), syntrophic fermenting (e.g., Smithella, Syntrophobacter), and sulfate-reducing (e.g., Desulfobacterium, Desulfosarcina Desulfovibrio, Desulfonatronum) bacteria capable of aerobically or anaerobically metabolizing aromatic hydrocarbons (e.g., Thauera) were detected in high relative abundance among the chemoorganotrophs involved in the decomposition of organic matter. Their metabolism may have contributed to the significant decrease in the organic pollutant concentrations of the lake water during the storage period (Borsodi et al. 2016).
In conclusion, the similar water blooms observed in various shallow alkaline and saline lakes in Hungary may have been facilitated by high light intensity and relatively high temperatures. Cyanobacteria, in addition to green algae, played a major role in the production of organic matter (carbon dioxide fixation) under aerobic conditions and phototrophic purple sulfur and non-sulfur bacteria under anoxic conditions in these habitats. The oxidation of organic carbon compounds could be carried out by aerobic and anaerobic (fermenting and sulfate-reducing) extremophilic bacteria. The latter may have played an important role in the production of reduced sulfur compounds, which could be utilized by anoxic phototrophs in their sulfur oxidation processes. This suggests the presence of a sulfuretum-type local biological sulfur cycle in all studied water bodies. It is important to note that the diversity studies of water blooms shed light on the significant differences in microbial community composition of the different soda pans. This can be partly due to the different physical (e.g., temperature, specific electrical conductivity) and water chemistry (e.g., salt composition, phenolic concentrations) of the studied water bodies and partly due to differences in the microbiological methods used (e.g., different medium composition, molecular cloning vs. NGS) (Borsodi et al. 2013, 2016; Korponai et al. 2019).
The effect of the drying-wetting cycle on the diversity of extremophilic bacterial communities in the rhizosphere of different alkali vegetation types
Solonetz is the typical genetic soil type, but mosaics of other saline soils (solonchak-solonetz and solonchak) can also occur around Apajpuszta (KNP) (Kuti et al. 2003). Changes in microtopography (up to a few 10 cm) play a decisive role in the differences of the near-surface salt concentration and thus in the development of typical mosaic plant communities (Molnár and Borhidi 2003; Borhidi 2007). There is a very close relationship between the specific characteristics of saline soils (e.g., the length of time they have been covered by water, the amount of salinity near the surface, and the relative height of the land surface) and the natural vegetation. Accordingly, the following zonation can be distinguished from each other, moving away from the water bodies and from the bottom up: Lepidio crassifolii—Champhorosmetum annuae (bare spot), Lepidio crassifolii—Puccinellietum limosae (Puccinellia sward), Artemisio santonici—Festucetum pseudovinae (Artemisia alkali steppe), and Achilleo setacea—Festucetum pseudovinae (Achillea alkali steppe) (Molnár and Borhidi 2003). The extremely variable weather conditions, such as drying-wetting cycles, can cause significant and sometimes dramatic changes in these saline soils. Previous botanical surveys have shown that changes in specific electrical conductivity and soil moisture content have had the strongest influence on the micromosaic structure of alkali vegetation during drying–wetting cycles (Molnár and Borhidi 2003; Zalatnai et al. 2007).
The effect of extreme weather conditions on the metabolic activity and taxonomic diversity of the bacterial communities in the rhizosphere soil of typical Kiskunság saline plant communities was studied in two end states of a natural drying–wetting cycle. The 0–10 cm horizon, considered the most microbiologically active soil horizon, was sampled in June and September 2014 near Apajpuszta (47° 05′ N; 19° 06′ E). At the beginning of summer, the daily mean temperature (25 °C) was 5 °C above the long-term average. After an unusually dry spring, June saw only around 30 mm of rainfall. This was just over half the long-term average, making it the 11th driest June since 1901. The weeks of drought caused the soil surface to dry out and crack. At the end of summer, however, the weather was much wetter than average. September rainfall was almost 180 mm, more than double the long-term average. Due to several days of intense rainfall, the ground surface in the bare spot and in the Puccinellia sward areas was covered with water at a height of about 10–20 cm. The average monthly temperature was 16–18 °C. According to the weather conditions, the soil moisture content during the June drought was on average 0.16 ± 0.02 m/m%, while in September it was 34.12 ± 2.35 m/m% on average. During the extreme drought in June, the soil pH decreased from strongly alkaline to near neutral in the bare spot, Puccinellia sward, Artemisia, and Achillea alkali steppe sampling direction. During the extreme rainfall sampling in September, soil pH values were alkaline at all sampling locations, with much smaller differences between sampling locations compared to June. Specific electrical conductivity values showed the same trend as soil pH, while soil organic matter content values showed the opposite trend (Borsodi et al. 2021).
The bare spots with the highest specific conductivity and pH values (i.e., the most extreme) showed the lowest values of catabolic activity and operational taxonomic unit (OTU) numbers of the soil samples taken near Apajpuszta. These values gradually increased in the direction of the Puccinellia sward, Artemisia, and Achillea alkali steppe samples (i.e., in line with the decrease in extremity). Plant-associated, alkalophilic, and halophilic species of Actinobacteria (e.g., Arthrobacter, Microbacterium, Micrococcus, Nesterenkonia, Streptomyces) and Firmicutes (e.g., Bacillus) with high tolerance and a wide range of metabolic capabilities were identified by cultivation. Using 16S rRNA gene-based amplicon sequencing, significant changes in the relative abundance of phylotypes belonging to the dominant phyla of Proteobacteria, Actinobacteria, Acidobacteria, Gemmatimonadetes and Bacteroidetes were revealed. Representatives of the phyla Acidobacteria and Actinobacteria were the most affected by the drying–wetting cycle in the saline soils of the KNP. Compared to the extremely dry early summer, the relative abundance of the phylum Acidobacteria significantly increased at the extremely wet late summer, while the relative abundance of the phylum Actinobacteria largely decreased. The relative abundance values of the phylum Acidobacteria, however, varied not only with the extreme weather but also with vegetation type; their proportion increased with increasing soil organic matter content and decreasing specific electrical conductivity, i.e., toward bare spot, Puccinellia sward, Artemisia, and Achillea alkali steppe plant communities. The proportion of the phylum Gemmatimonadetes (especially at rewetting) was much higher in the bare spot and Puccinellia sward samples than in the Artemisia and Achillea alkali steppe samples. The number of operational taxonomic units, species richness values, and diversity indices showed a positive correlation with higher plant coverage and lower alkalinity and salinity. These results have shown that different alkali vegetation types, in accordance with the different specific electrical conductivities of the saline soils, had a stronger impact on the metabolic activity and taxonomic composition of bacterial communities than the weather extremities, despite the small geographical distance among the sampling sites (Borsodi et al. 2021).
Thermophiles and hyperthermophiles
Hungary's geothermal potential is exceptional due to the high surface heat flux, which is about one and a half times the world average, and the presence of good aquifers. The temperature of the water stored in the rocks and their pores is on average 60 °C at a depth of 1 km and 110 °C at a depth of 2 km due to the high geothermal gradient. The production of these thermal waters has increased significantly in recent decades. There are currently almost 1000 thermal wells (wells supplying water above 35 °C) in Hungary, mainly in North-East Hungary (Egerszalók, Tura), in the south-eastern part of the Great Plain (Szentes, Hódmezővásárhely, Szeged, Szarvas), as well as in Western Hungary (Zalakaros, Hévíz, Bősárkány, Kapuvár) and Budapest. Of the operating thermal wells, 220 are used for balneological purposes, a further 200 are used for domestic water supply, and around 200 wells, half of which have a temperature above 70 °C, are used for heating purposes in crop or livestock production (Szanyi and Kovács 2010; Szanyi et al. 2021).
The bacterial and archaeal community structures of thermal waters from karstic bedrock with similar temperature (62 °C and 74 °C) and specific electrical conductivity (1080 and 1655 µS cm−1) values, low organic matter content (1.8 mg l−1), and nearly neutral water chemistry (7.0 and 6.2), supplying water to two distant thermal spas were compared (Miseta et al. 2012, 2013; Anda et al. 2015). The thermal water of the Harkány Thermal Spa is bicarbonate (476–622 mg l−1) and high in minerals (730–900 mg l−1), with relatively high concentrations of sulfide (11 mg l−1), sodium (190 mg l−1) and chloride (108 mg l−1). The thermal water of the Széchenyi Thermal Bath (Budapest) contains calcium—magnesium—bicarbonate (156 mg l−1, 35 mg l−1, 555 mg l−1) and relatively large amounts of sodium (176 mg l−1), sulfate (215 mg l−1), and chloride (170 mg l−1). Samples were examined using electron microscopy and molecular cloning methods. Thermal waters supplying the spas were dominated by bacterial phylotypes related to the genera of the Betaproteobacteria. Only representatives of the genus Sulfurihydrogenibium were common among the thermophilic, chemolithotrophic sulfur-oxidizing taxa. These bacteria, through their metabolism, are primarily involved in the sulfur cycle of the thermal water, including the regulation of sulfide concentrations (Miseta et al. 2012, 2013; Anda et al. 2015). In addition to chemolithotrophic sulfur oxidizers (e.g., Thiobacillus), obligate anaerobic heterotrophs (e.g., Bellilinea) and even representatives of species capable of dissimilatory reduction of iron(III) (e.g., Thermus) were identified from the biofilm-forming communities. The presence of the latter was confirmed in SEM images by hollow formations of bacterial origin attached to the surface of calcium carbonate crystals, accumulating iron oxyhydroxide minerals on their filamentous surface. Dominance of thermophilic, chemolitho-autotrophic ammonia-oxidizing phylotypes (e.g., Nitrosocaldus, Nitrososphaera) related to the phylum Thaumarchaeota was observed in both water and biofilm samples, as well (Anda et al. 2015).
The results revealed that the hyperthermophilic bacterial and archaeal community of the thermal waters of deep wells in the Szarvas region differed greatly from that of thermal baths, mainly due to the much higher water temperature (> 90 °C), higher specific electrical conductivity (3770–4270 µS cm−1), and organic matter (total phenol 10 mg l−1) content. Phylotypes of facultative anaerobic nitrate-reducing (e.g., Thauera, Tepidiphilus, Thermanaeromonas) and obligate anaerobic sulfate- and sulfur-reducing (e.g., Desulfotomaculum, Thermodesulfobacterium and Thermofilum) bacteria, also involved in the biotransformation of aromatic hydrocarbons, were detected in these water samples. The presence of methanotrophs (e.g., Methylocystis) as well as thermophilic acetotrophic and hydrogenotrophic methanogens (e.g., Methanosaeta and Methanothermobacter) confirmed the existence of a local biological methane cycle (Németh et al. 2014).
Polyextremophiles
Oligocarbophilic, moderately thermophilic, radiation-resistant, and heavy metal-tolerant bacterial communities of Hungarian hypogenic thermal karst caves
There are more than a hundred caves in the Triassic and Eocene carbonate rocks of the Buda Thermal Karst (BTK) on the north-eastern edge of the Transdanubian Mountains (Leél-Őssy 2017; Leél-őssy and Virág 2018). Many thermal waters of different origins and temperatures come to the surface through wells and springs at the boundaries of the covered and uncovered carbonate aquifers, under the populated area of Budapest (Erőss et al. 2012; Mádl-Szőnyi and Tóth 2015; Déri-Takács et al. 2015; Mádl-Szőnyi et al. 2017). The Molnár János cave belonging to the central discharge area is the largest, still active, hypogenic thermal karst cave in Europe (Leél-Őssy 1995; Goldscheider et al. 2010). The mixing corrosion of upwelling thermal basinal fluids rich in dissolved substances and cold meteoric karst water derived from precipitation led to the formation of spacious cave systems here (Mádl-Szőnyi et al. 2017). Conversely, only deep hydrothermal components were found in the southern discharge area, where the contribution of meteoric waters to karstification processes is negligible. In the lens-shaped, small-scale spring caves, deep basinal fluids and dissolved gases resulted in the formation of very diverse karst formations. Several famous spas (e.g., Rác, Rudas, Gellért) are supplied by thermal springs at the foot of the Gellért Hill (Virág et al. 2013; Mádl-Szőnyi et al. 2017).
At the discharge areas of the BTK, the average water temperature at the time of sampling was 31.0 ± 7.5 °C, the pH was around neutral, and the specific electrical conductivity was 1595.4 ± 331.3 µS cm−1. Sulfate and chloride were the dominant anions, but the values measured in the central area were only half/third of those in the south. Concentrations of ammonium, nitrate, nitrite, and phosphate ions were near or below the detection limit. The amount of total organic carbon was very low, while the amount of total inorganic carbon was significant but highly variable among sampling sites (Borsodi et al. 2018, 2022; Enyedi et al. 2019). The thermal waters of the BTK could be characterized by up to 1000 Bq/L 222Rn activity (Erőss et al. 2012; Enyedi et al. 2019). This detected radon activity is exceptionally high for natural thermal waters worldwide. The elemental analysis showed that the main elements were Fe and Ca, which accumulated in the biofilms as iron oxyhydroxides and calcareous minerals. The element concentrations detected in the biofilm samples (e.g., As, Hg, Pb, Sn, Sr, and Zn) were 1.5–60 times higher than the typical background levels of uncontaminated soils, indicating a high accumulation rate (Dobosy et al. 2016; Enyedi et al. 2019).
The morphological characteristics and taxonomic composition of the microbiota found in different spring cave habitats in the central, and southern discharge areas of the BTK were studied using a combined (electron microscopic, cultivation-based, molecular cloning, and next-generation sequencing) methodological approach (Makk et al. 2016, 2019; Anda et al. 2017, 2020; Borsodi et al. 2018, 2022; Enyedi et al. 2019).
The detailed comparative analysis of biofilm samples collected from cave rock surfaces revealed a highly diverse taxonomic composition dominated by phylotypes belonging to the phyla Proteobacteria, Acidobacteria, Aquificae, Chlorobi, Chloroflexi, Firmicutes, Nitrospirae, Planctomycetes, Parcubacteria, and Thaumarchaeota, using both molecular cloning and pyrosequencing. In the thermal water of BTK, however, a highly simplified community composition, dominated almost exclusively by phylotypes of Betaproteobacteria (e.g., Thiobacillus) was detected. A metabolic network of the biogeochemical cycles of nitrogen, sulfur, and iron could be outlined based on the known metabolic properties of the species most closely related to the detected phylotypes (Fig. 2). Mesophilic or thermophilic, anaerobic sulfate- (e.g., Desulfomonile, Desulfotomaculum, Desulfovibrio, Desulfuromonas, Thermodesulfobium, Thermodesulfovibrio, Thermodesulfobacterium), nitrate- (e.g., Denitratisoma, Sulfuricella, Sulfuritalea, Thiobacillus) and iron(III)-reducing (e.g., Thermolithobacter, Aciditerrrimonas, Geobacter, Georgfuchsia) chemoorganotrophic bacteria, as well as sulfur- (e.g., Sulfuricella, Sulfuritalea, Thiobacillus, Thiobacter), ammonia- (e.g., Nitrosoarchaeum, Nitrosocaldus, Nitrososphaera, Nitrosospira), and nitrite-oxidizing (e.g., Nitrospira) chemolithotrophic bacteria and archaea may be involved in these interspecific cooperations (Anda et al. 2017; Borsodi et al. 2018, 2022).
The presumed role of bacterial genera identified from the Buda Thermal Karst biofilm samples in the biogeochemical cycling of nitrogen, sulfur, and iron (Abbreviations: MJ Molnár János cave, RN Rác Nagy spring, RT Rudas-Török spring cave, DH Diana-Hygieia thermal spring, GOB Gellért spring, VL Városliget, Aq Aquificae, Ac Actinobacteria, F Firmicutes, N Nitrospirae, βP Betaproteobacteria, γP Gammaproteobacteria, δP Deltaproteobacteria, T Desulfobacterota, and TA Thumarchaeota.)
Nearly, a hundred different bacterial species of the phyla Actinobacteria, Firmicutes, Proteobacteria, Bacteriodetes and Deinococcus-Thermus taxa were identified from the rock surface biofilm of the BTK by combining cultivation-based studies with different intensities of gamma irradiation. The irradiated samples were dominated by Gram-positive spore-forming (e.g., Bacillus) or pigment-producing bacterial strains showing resistance to a radiation dose of up to 15 kGy (e.g., Dermacoccus, Kytococcus, Marmoricola, Paracoccus) (Enyedi et al. 2019). Among the isolates, new radiation-resistant bacterial species (Deinococcus budaensis sp. nov. and D. fonticola sp. nov.) were also described, according to polyphasic taxonomic principles (Makk et al. 2016, 2019).
To understand the process of biofilm formation connected to the spring discharge sites, an in situ model system was set up in a southern spring cave of the BTK, where changes in bacterial community composition were monitored under natural conditions for more than a year. Based on the OTU number estimation and diversity index results calculated from the data of the 16S rRNA gene-based pyrosequencing, 9–12 weeks were required for the development of a morphologically complex mature biofilm structure in an environment that was considered relatively stable according to the observed physical and chemical parameters. Although the phylum-level taxonomic diversity reached a maximum already in the third week of biofilm formation, significant changes in the relative abundance of some taxonomic groups were observed throughout the study. This indicated a continuous restructuring of the interactions within the biofilm bacterial community. After 1 year, the biofilm community structure developed on the artificial substrate showed a high degree of similarity to the biofilm formed naturally on the cave rock surface and differed significantly from the composition of the planktonic bacterial communities in the surrounding thermal cave water (Anda et al. 2020).
The high taxonomic diversity revealed from biofilms, as well as the variety of potential microbial metabolic processes, highlights the importance of biogenic karstification processes in these aphotic, very low autochthonous organic carbon, extreme hypogenic cave environments. It should be noted, however, that many of the prokaryotic OTUs involved in the biofilm structure of the BTK have not been identified due to the lack of described known species, which still indicates a largely unknown, hidden taxonomic diversity.
Conclusions for future biology
In the past decades, research on extremophiles, including the results from Hungary, has significantly expanded our previous knowledge of the taxonomic diversity of extremophiles. It should be noted, however, that despite the increasing research efforts in recent years, the ecological and evolutionary processes that determine microbial diversity in extreme habitats are still largely unknown. This can be due to several reasons, such as the extreme accessibility of many extreme environments, the challenges of aseptic sampling, the difficulty of ensuring adequate sample storage and preparation, and the diversity and different selectivity of the methods used in surveys.
Nowadays, the large amount of data generated by modern “omics” methods, which are becoming more and more widespread, can be analyzed in comprehensive mathematical–statistical methods. By applying these cultivation-independent, high-throughput methodological approaches, researchers may be able to explore community network structures that include elements of trophic interactions, community dynamics, and metabolic capabilities of microorganisms. In addition, new isolation and cultivation methods will allow us to exploit the versatile metabolic capabilities of extremophiles and polyextremophiles, e.g., to study the properties of the bioactive compounds or extremozymes they produce and to use them in industrial, biotechnological, and pharmaceutical applications.
References
Alain K, Querellou J (2009) Cultivating the uncultured: limits, advances and future challenges. Extremophiles 13:583–594. https://doi.org/10.1007/s00792-009-0261-3
Anda D, Makk J, Krett G et al (2015) Thermophilic prokaryotic communities inhabiting the biofilm and well water of a thermal karst system located in Budapest (Hungary). Extremophiles 19:787–797. https://doi.org/10.1007/s00792-015-0754-1
Anda D, Krett G, Makk J et al (2017) Comparison of bacterial and archaeal communities from different habitats of the hypogenic Molnár János cave of the Buda thermal karst system (Hungary). J Cave Karst Stud 79:113–121. https://doi.org/10.4311/2015MB0134
Anda D, Szabó A, Kovács-Bodor P et al (2020) In situ modelling of biofilm formation in a hydrothermal spring cave. Sci Rep 10:21733. https://doi.org/10.1038/s41598-020-78759-4
Borhidi A (2007) Magyarország növénytársulásai (Plant cmmunities of Hungary). Akadémiai Kiadó, Budapest
Borics G, Ács É, Boda P et al (2016) Water bodies in Hungary: an overview of their management and present state. Hidrol Közl 96:57–67 (in Hungarian with English abstract)
Boros E (1999) A magyarországi szikes tavak és vizek ökológiai értékelése. Acta Biol Debr Oecol Hung 9:13–80
Boros E (2013) Definitions, types and survey methods of soda pans. In: Boros E, Ecsedi Z, Oláh J (eds) Ecology and management of soda pans in the Carpathian Basin. Hortobágy Environmental Assiciation, Balmazújváros, pp 34–54
Boros E, Vörös L (2010) A magyarországi szikes tavak sótartalma és ionösszetétele. Acta Biol Debr Suppl Oecol Hung 22:37–52 (in Hungarian with English abstract)
Borsodi AK, Márialigeti K, Szabó G et al (2008) Bacillus aurantiacus sp. nov., an alkaliphilic and moderately halophilic bacterium isolated from Hungarian soda lakes. Int J Syst Evol Microbiol 58:845–851. https://doi.org/10.1099/ijs.0.65325-0
Borsodi AK, Pollák B, Zs K et al (2011) Bacillus alkalisediminis sp. nov., an alkaliphilic and moderately halophilic bacterium isolated from sediment of extremely shallow soda ponds. Int J Syst Evol Microbiol 61:1880–1886. https://doi.org/10.1099/ijs.0.019489-0
Borsodi AK, Knáb M, Czeibert K et al (2013) Planktonic bacterial community composition of an extremely shallow soda pond during a phytoplankton bloom revealed by cultivation and molecular cloning. Extremophiles 17:575–584. https://doi.org/10.1007/s00792-013-0540-x
Borsodi AK, Szirányi B, Krett G et al (2016) Changes in the water quality and bacterial community composition of an alkaline and saline oxbow lake used for temporary reservoir of geothermal waters. Env Sci Poll Res 23:17676–17688. https://doi.org/10.1007/s11356-016-6923-7
Borsodi AK, Korponai K, Schumann P et al (2017a) Nitrincola alkalilacustris sp. nov. and Nitrincola schmidtii sp. nov., alkaliphilic bacteria isolated from soda pans, and emended description of the genus Nitrincola. Int J Syst Evol Microbiol 67:5159–5164. https://doi.org/10.1099/ijsem.0.002437
Borsodi AK, Szili-Kovács T, Schumann P et al (2017b) Nesterenkonia pannonica sp. nov., a novel alkaliphilic and moderately halophilic actinobacterium. Int J Syst Evol Microbiol 67:4116–4120. https://doi.org/10.1099/ijsem.0.002263
Borsodi AK, Tóth E, Aszalós JM et al (2017c) Bacillus kiskunsagensis sp. nov., a novel alkaliphilic and moderately halophilic bacterium isolated from soda soil. Int J Syst Evol Microbiol 67:3490–3495. https://doi.org/10.1099/ijsem.0.002149
Borsodi AK, Anda D, Makk J et al (2018) Biofilm forming bacteria and archaea in thermal karst springs of Gellért Hill discharge area (Hungary). J Basic Microbiol 58:928–937. https://doi.org/10.1002/jobm.201800138
Borsodi AK, Mucsi M, Krett G et al (2021) Variation in sodic soil bacterial communities associated with different alkali vegetation types. Microorganisms 9:1673. https://doi.org/10.3390/microorganisms9081673
Borsodi AK, Anda D, Szabó A et al (2022) Impacts of different habitats on the composition of bacterial communities at the discharging endpoints of a hypogene thermal karst system. Geomicrobiol J 39:155–165. https://doi.org/10.1080/01490451.2021.2023709
Burg D, Ng C, Ting L, Cavicchioli R (2011) Proteomics of extremophiles. Env Microbiol 13:1934–1955. https://doi.org/10.1111/j.1462-2920.2011.02484.x
Calo D, Guan Z, Eichler J (2011) Glyco-engineering in Archaea differential N-glycosylation of the S-layer glycoprotein in a transformed Haloferax volcanii strain. Microb Biotech 4:461–470. https://doi.org/10.1111/j.1751-7915.2011.00250.x
Campanaro S, Williams TJ, Burg DW et al (2011) Temperature-dependent global gene expression in the Antarctic archaeon Methanococcoides burtonii. Environ Microbiol 13:2018–2038. https://doi.org/10.1111/j.1462-2920.2010.02367.x
Cavicchioli R (2002) Extremophiles and the search for extraterrestrial life. Astrobiology 2:281–292. https://doi.org/10.1089/153110702762027862
Cavicchioli R, Amils R, Wagner D, McGenity T (2011) Life and applications of extremophiles. Env Microbiol 13:1903–1907. https://doi.org/10.1111/j.1462-2920.2011.02512.x
Cayol J-L, Ollivier B, Alazard D et al (2015) The extreme conditions of life on the planet and exobiology. In: Bertrand JC, Caumette P, Lebaron P et al (eds) Environmental microbiology: fundamentals and applications. Springer, Netherlands, Dordrecht, pp 353–394
Coker JA (2016) Extremophiles and biotechnology: current uses and prospects. F1000Res 5:396. https://doi.org/10.12688/f1000research.7432.1
Coker JA (2019) Recent advances in understanding extremophiles. F1000Res 8:1917. https://doi.org/10.12688/f1000research.20765.1
Déri-Takács J, Erőss A, Kovács J (2015) The chemical characterization of the thermal waters in Budapest, Hungary by using multivariate exploratory techniques. Environ Earth Sci 74:7475–7486. https://doi.org/10.1007/s12665-014-3904-3
Dobosy P, Sávoly Z, Óvári M, Mádl-Szőnyi J, Záray G (2016) Microchemical characterization of biogeochemical samples collected from the Buda Thermal Karst System, Hungary. Microchem J 124:116–120. https://doi.org/10.1016/j.microc.2015.08.004
Dumorné K, Córdova DC, Astorga-Eló M, Renganathan P (2017) Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol 27:649–659. https://doi.org/10.4014/jmb.1611.11006
Ecsedi Z, Boros E (2013) Description of the surveyed natural soda pans. In: Boros E, Ecsedi Z, Oláh J (eds) Ecology and management of soda pans in the Carpathian Basin. Hortobágy Environmental Assiciation, Balmazújváros, pp 176–181
Enyedi NT, Anda D, Borsodi AK et al (2019) Radioactive environment adapted bacterial communities constituting the biofilms of hydrothermal spring caves (Budapest, Hungary). J Environ Radioact 203:8–17. https://doi.org/10.1016/j.jenvrad.2019.02.010
Erőss A, Mádl-Szőnyi J, Surbeck H et al (2012) Radionuclides as natural tracers for the characterization of fluids in regional discharge areas, Buda Thermal Karst, Hungary. J Hydrol (amst) 426–427:124–137. https://doi.org/10.1016/j.jhydrol.2012.01.031
Felföldi T (2020) Microbial communities of soda lakes and pans in the Carpathian Basin: a review. Biol Futur 71:393–404. https://doi.org/10.1007/s42977-020-00034-4
Goldscheider N, Mádl-Szőnyi J, Erőss A, Schill É (2010) Review: thermal water resources in carbonate rock aquifers. Hydrogeol J 18:1303–1318. https://doi.org/10.1007/s10040-010-0611-3
González AG, Terrón RP (2021) Importance of extremophilic microorganisms in biogeochemical cycles. GSC Adv Res Rev 9:082–093. https://doi.org/10.30574/gscarr.2021.9.1.0229
Graef C, Hestnes AG, Svenning MM, Frenzel P (2011) The active methanotrophic community in a wetland from the High Arctic. Environ Microbiol Rep 3:466–472. https://doi.org/10.1111/j.1758-2229.2010.00237.x
Gupta GN, Srivastava S, Khare SK, Prakash V (2014) Extremophiles: an overview of microorganism from extreme environment. Int J Agric Env Biotech 7:371. https://doi.org/10.5958/2230-732X.2014.00258.7
Harrison JP, Gheeraert N, Tsigelnitskiy D, Cockell CS (2013) The limits for life under multiple extremes. Trends Microbiol 21:204–212. https://doi.org/10.1016/j.tim.2013.01.006
Jones BE, Grant WD (1999) Microbial diversity and ecology of the soda lakes of East Africa. In: Bell CR, Brylinsky M, Johnson-Green P (eds) Microbial biosystems: new frontiers proceedings of the 8th international symposium on microbial ecology. Halifax, Canada, pp 1–7
Köcher S, Averhoff B, Muller V (2011) Development of a genetic system for the moderately halophilic Halobacillus halophilus: Generation and characterization of mutants defect in the production of compatible solute proline. Environ Microbiol 13:2122–2131. https://doi.org/10.1111/j.1462-2920.2011.02437.x
Korponai K, Szabó A, Somogyi B et al (2019) Dual bloom of green algae and purple bacteria in an extremely shallow soda pan. Extremophiles 23:467–477. https://doi.org/10.1007/s00792-019-01098-4
Kuti L, Tóth T, Kalmár J, Kovács-Pálffy P (2003) Szikes talajok ásványi összetétele és recens ásványképződés Apajpusztán és Zabszék térségében. Agrokém Talajt 52:275–292. https://doi.org/10.1556/Agrokem.52.2003.3-4.3. (in Hungarian with English abstract)
Leél-Őssy S (1995) A budai Rózsadomb és környékének különleges barlangjai. Földtani Közlöny 125:363–432 (in Hungarian with English abstract)
Leél-Őssy S (2017) Caves of the Buda thermal karst. In: Klimchouk A, Palmer N, De Waele A et al (eds) Hypogene karst regions and caves of the world. Cave and Karst Systems of the World. Springer, Cham, pp 279–297
Leél-őssy S, Virág M (2018) Az utóbbi 20 év barlangkutatási eredményei a Budai-hegységben (különös tekintettel a Rózsadomb környékére). Földtani Közlöny 148:45. https://doi.org/10.23928/foldt.kozl.2018.148.1.45. (in Hungarian with English abstract)
Madigan MT, Marrs BL (1997) Extremophiles. Sci Am 276:82–87. https://doi.org/10.1038/scientificamerican0497-82
Madigan MT, Bender KS, Buckley DH et al (2021) Brock biology of microorganisms, 16th edn. Pearson Education, Harlow
Mádl-Szőnyi J, Tóth Á (2015) Basin-scale conceptual groundwater flow model for an unconfined and confined thick carbonate region. Hydrogeol J 23:1359–1380. https://doi.org/10.1007/s10040-015-1274-x
Mádl-Szőnyi J, Erőss A, Tóth Á (2017) Fluid flow systems and hypogene karst of the Transdanubian range, Hungary: with special emphasis on Buda thermal karst. In: Klimchouk A, Palmer A, De Waele J et al (eds) Hypogene karst regions and caves of the world. Cave and Karst Systems of the World. Springer, Cham, pp 267–278
Makk J, Tóth EM, Anda D et al (2016) Deinococcus budaensis sp. nov., a mesophilic species isolated from a biofilm sample of a hydrothermal spring cave. Int J Syst Evol Microbiol 66:5345–5351. https://doi.org/10.1099/ijsem.0.001519
Makk J, Enyedi NT, Tóth E et al (2019) Deinococcus fonticola sp. nov., isolated from a radioactive thermal spring in Hungary. Int J Syst Evol Microbiol 69:1724–1730. https://doi.org/10.1099/ijsem.0.003383
Martínez-Espinosa RM (2020) Microorganisms and their metabolic capabilities in the context of the biogeochemical nitrogen cycle at extreme environments. Int J Mol Sci 21:4228. https://doi.org/10.3390/ijms21124228
Mayumi D, Mochimaru H, Yoshioka H et al (2011) Evidence for syntrophic acetate oxidation coupled to hydrogenotrophic methanogenesis in the high-temperature petroleum reservoir of Yabase oil field (Japan). Environ Microbiol 13:1995–2006. https://doi.org/10.1111/j.1462-2920.2010.02338.x
Merino N, Aronson HS, Bojanova DP et al (2019) Living at the extremes: extremophiles and the limits of life in a planetary context. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00780
Miseta R, Palatinszky M, Makk J et al (2012) Phylogenetic diversity of bacterial communities associated with sulfurous karstic well waters of a Hungarian spa. Geomicrobiol J 29:101–113. https://doi.org/10.1080/01490451.2011.558563
Miseta R, Palatinszky M, Makk J et al (2013) Spatial and temporal changes of bacterial communities inhabiting the well waters of Harkány spa. Acta Microbiol Immunol Hung 60:329–343. https://doi.org/10.1556/AMicr.60.2013.3.8
Németh A, Szirányi B, Krett G et al (2014) Prokaryotic phylogenetic diversity of Hungarian deep subsurface geothermal well waters. Acta Microbiol Immunol Hung 61:363–377. https://doi.org/10.1556/AMicr.61.2014.3.9
Pakchung AAH, Simpson PJL, Codd R (2006) Life on earth. Extremophiles continue to move the goal posts. Environ Chem 3:77. https://doi.org/10.1071/EN05093
Pikuta EV, Hoover RB, Tang J (2007) Microbial extremophiles at the limits of life. Crit Rev Microbiol 33:183–209. https://doi.org/10.1080/10408410701451948
Raddadi N, Cherif A, Daffonchio D et al (2015) Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol 99:7907–7913. https://doi.org/10.1007/s00253-015-6874-9
Rainey FA, Oren A (2006) 1 Extremophile Microorganisms and the Methods to Handle Them. Methods Microbiol 35:1–25. https://doi.org/10.1016/S0580-9517(08)70004-7
Rampelotto PH (ed) (2016) Extremophiles and extreme environments. MDPI, Basel
Rothschild LJ, Mancinelli RL (2001) Life in extreme environments. Nature 409:1092–1101. https://doi.org/10.1038/35059215
Schmid AK, Allers T, DiRuggiero J (2020) Snapshot: Microbial extremophiles. Cell 180:818-818.e1. https://doi.org/10.1016/j.cell.2020.01.018
Schultz J, Modolon F, Peixoto RS, Rosado AS (2023) Shedding light on the composition of extreme microbial dark matter: alternative approaches for culturing extremophiles. Front Microbiol 14:1167718. https://doi.org/10.3389/fmicb.2023.1167718
Shu W-S, Huang L-N (2022) Microbial diversity in extreme environments. Nat Rev Microbiol 20:219–235. https://doi.org/10.1038/s41579-021-00648-y
Sime-Ngando T, Lucas S, Robin A et al (2011) Diversity of virus-host systems in hypersaline Lake Retba, Senegal. Environ Microbiol 13:1956–1972. https://doi.org/10.1111/j.1462-2920.2010.02323.x
Sorokin DY, Gorlenko VM, Namsaraev BB et al (2004) Prokaryotic communities of the north-eastern Mongolian soda lakes. Hydrobiologia 522:235–248. https://doi.org/10.1023/B:HYDR.0000029989.73279.e4
Sorokin DY, Berben T, Melton ED et al (2014) Microbial diversity and biogeochemical cycling in soda lakes. Extremophiles 18:791–809. https://doi.org/10.1007/s00792-014-0670-9
Szanyi J, Kovács B (2010) Utilization of geothermal systems in South-East Hungary. Geothermics 39:357–364. https://doi.org/10.1016/j.geothermics.2010.09.004
Szanyi J, Nádor A, Madarász T (2021) A geotermikus energia kutatása és hasznosítása Magyarországon az elmúlt 150 év tükrében. Földtani Közlöny 151:79–102. https://doi.org/10.23928/foldt.kozl.2021.151.1.79. (in Hungarian with English abstract)
Taylor MP, van Zyl L, Tuffin IM et al (2011) Genetic tool development underpins recent advances in thermophilic whole-cell biocatalysts. Microbiol Biotech 4:438–448. https://doi.org/10.1111/j.1751-7915.2010.00246.x
Vester JK, Glaring MA, Stougaard P (2015) Improved cultivation and metagenomics as new tools for bioprospecting in cold environments. Extremophiles 19:17–29. https://doi.org/10.1007/s00792-014-0704-3
Virág M, Sz L-Ő, Mindszenty A (2013) Szpeleológiai adottságok. A felszín alatti víz oldóhatásának tanúi: A budai barlangok. In: Mindszenty A (ed) Budapest: Földtani értékek és az ember. Városgeológiai tanulmányok. ELTE Eötvös Kiadó, Budapest, pp 105–111 (in Hungarian)
Westerholm M, Dolfing J, Sherry A et al (2011) Quantification of syntrophic acetate-oxidizing microbial communities in biogas processes. Environ Microbiol Rep 3:500–505. https://doi.org/10.1111/j.1758-2229.2011.00249.x
Zalatnai M, Körmöczi L, Tóth T (2007) Community boundaries and edaphic factors in saline-sodic grassland communities along an elevation gradient. Tiscia 36:7–15
Zs M, Borhidi A (2003) Hungarian alkali vegetation: Origins, landscape history, syntaxonomy, conservation. Phytocoenologia 33:377–408. https://doi.org/10.1127/0340-269X/2003/0033-0377
Acknowledgements
Special thanks to my co-authors T. Felföldi, G. Krett, K. Márialigeti, T. Szili-Kovács, B. Szirányi, and E. Tóth for their participation in research on alkalophiles-halophiles, R. Miseta, A. Németh, and M. Palatinszky for their participation in research on thermophiles-hyperthermophiles, and D. Anda, N.T. Lange-Enyedi, J. Makk, and J. Mádl-Szőnyi for their participation in research on polyextremophiles involved in hypogene karstification processes.
Funding
Open access funding provided by Eötvös Loránd University. The research for this review was funded by the National Research, Development and Innovation Office, Hungary (Grants of NKFIH OTKA: T038021, NK101356 and K108572).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borsodi, A.K. Taxonomic diversity of extremophilic prokaryotes adapted to special environmental parameters in Hungary: a review. BIOLOGIA FUTURA (2024). https://doi.org/10.1007/s42977-024-00224-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42977-024-00224-4