Abstract
Salt-sensitive crop varieties suffer from oxidative stress as a consequence of osmotic and ionic stresses in plants under salinity stress. This study is aimed at identifying the effects of riboflavin (RIB) application on uplifting rice growth under salinized soil condition. Two-week-old seedlings of IR29 (a salt-sensitive variety) were supplemented with 0.5 μM of RIB, and 50 mM of NaCl was supplied for 2 weeks, inducing salinized soil conditions. The results indicated that RIB pretreatment (RP) seedlings possessed higher plant biomass, and lower electrolyte leakage ration (ELR), hydrogen peroxide (H2O2) and malondialdehyde (MDA) concentrations, higher chlorophyll, magnesium (Mg), and iron (Fe) concentrations in the leaf blades, a higher proline concentration, and a lower Na+ concentration in the leaf blades. To further understand the mechanisms behind the difference in plant growth between the RP and non-RP seedlings, molecular analysis revealed that RP seedlings upregulated OsNHX1 and OsHKT1;5 expressions were observed in the roots of RP seedlings, regulating Na+ uptake through the transpiration stream and reducing Na+ concentration in the leaf blades. Collectively, these results suggest that RP is a potent method for improving plant growth under salinized soil conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The accumulation of greenhouse gases, carbon dioxide (CO2), and methane in the atmosphere has led to increased planetary heat-trapping and global warming resulting in ongoing climate change and led to more frequent and worsened abiotic stresses (Eckardt et al. 2023; Verslues et al. 2023). In addition, the manner in which plants react to these stresses can be directly affected by rising CO2 levels. Excessive soluble salts and exchangeable sodium are present in salinity-affected soils, which affect plant root systems and metabolism (Anwar and Kim et al. 2020). Salinity stress initially begins with osmotic stress, which inhibits water uptake, followed by ionic stress caused by the overaccumulation of Na+ (Munns and Tester 2008). These stresses inevitably lead to oxidative damage due to excessive accumulation of ROS and lipid peroxidation (Roy et al. 2014; Hasanuzzaman et al. 2021). ROS is important signaling molecules involved in stress responses, and plants have a variety of mechanisms that enable them to perceive and transduce external signals to trigger adaptive responses (Miller et al. 2010). Furthermore, it is widely acknowledged that Na+ overaccumulation interferes with cell wall integrity, inhibits enzymatic activity, decreases photosynthetic efficiency, and disturbs ion balance (Miyamoto et al. 2015; Colin et al. 2023).
Salinity stress hinders plant growth and reduces crop yield and quality of the staple food, rice (Oryza sativa L.), which is consumed by two-thirds of the world's population (Horie et al. 2012). Rice can withstand salinity stress at a threshold soil electrical conductivity (EC) of 3 dS m−1, and on average, a 12% yield loss occurs with every dS m−1 increase in soil EC (Machado and Serralheiro 2017). The indica rice cultivar IR29, known as a salt-sensitive and high-yielding variety, has been predominantly utilized as a model for researching salinity stress in rice (Kruasuwan et al. 2023). Several attempts have been made to enhance rice salinity tolerance and improve rice growth in higher EC soil conditions (Linh et al. 2012). Therefore, salt-sensitive plant varieties are commonly pretreated to ameliorate salinity stress using organic and inorganic compounds, biostimulants, and nutrients that have been reported to trigger different salinity stress tolerance mechanisms (Mekawy et al. 2018; Mohsin et al. 2020; Desoky et al. 2018). As it is an unsophisticated and effective application, it can be recommended to farmers. Effective strategies to withstand salinity stress involve the selecting/breeding crop genotypes with better performance on salinized soil and managing the soil properties by adding optimal nutrients or agent that uplift the salinity stress tolerance in plant (Zhao et al. 2021; Ahmad et al. 2023). Although rice breeding is a potent method for mitigating salinity stress, the main barriers to this are the complexities of the multigenetic traits of salinity and the limitations on the use of transgenic plants in some countries (Qin et al. 2020).
Salinity stress impairs plant growth by affecting the intricate relationships between nutrient uptake and accumulation, hormonal imbalances, and oxidative stress (Anwar and Kim 2020). To combat ROS and prevent the cells from oxidative damage, rice plants have developed various enzymatic and non-enzymatic detoxification systems in response to salinity, including catalase, glutathione reductase, phenolic compounds, and compatible solutes (Sairam and Tyagi 2004). Tartary buckwheat responses to salinized soil by increasing the osmolyte concentrations (Zhang et al. 2023). Compatible solutes play a pivotal role as signaling molecules in maintaining cell turgidity, preventing oxidative damage to cell compartments, and increasing the osmotic pressure (Tang et al. 2011). Proline is a compatible solute required for osmotic adjustment, and osmolyte profiles may differ between species under salinity stress (Van Zelm et al. 2020). Proline is important for protecting cells from ROS overproduction under salinity stress. Exogenous proline application induces upregulation of P5C5 expression and increases proline concentrations in cucumber (Cucumis sativus L.) that may regulate cytokinin metabolism to mitigate salinity stress (Zhu et al. 2020). In addition, exogenous proline reduces oxidative stress damages in rice by stimulating antioxidant enzymes under salinity stress (Hasanuzzaman et al. 2014). Proline has thus been identified as an osmoprotectant (Szabados and Savouré 2010), a potent non-enzymatic antioxidant (Rejeb et al. 2014), and ROS detoxifier (Alia et al. 2001). Exogenous ABA induces expression of OsP5CS1 which in turn increases proline concentration under salinity stress (Sripinyowanich et al. 2013). Maintaining the favorable element concentrations improves salinity stresses tolerance in plants (Mohsin et al. 2020; Chuamnakthong et al. 2019). Mg is an important mineral for the growth of plants. Approximately 75% of the magnesium found in leaf blades is engaged in metabolic activities, whereas 15% to 20% of the total magnesium is associated with chlorophyll pigments (Pogłodziński et al. 2021). Furthermore, even a slight Mg deficiency affects the overall plant growth and plant susceptibility to external challenges stressors by interfering with their biochemical and physiological functions (Senbayram et al. 2015). Salicylic acid pretreatment enhances potassium (K), calcium (Ca), Fe, and proline concentrations in the leaf blades of a salt-sensitive tomato variety (Souri and Tohidloo 2019).
RIB is a water-soluble Vitamin B2 consisting of flavin adenine dinucleotide and flavin mononucleotide and is involved in various redox processes that affect plant defense responses (Abbas and Sibirny 2011). RIB pretreatment in tobacco (Nicotiana benthamiana) decreased H2O2 and thiobarbituric acid reactive substance concentrations, which were correlated with an increase in antioxidant enzymes, resulting in drought stress alleviation in soil-based experiments (Deng et al. 2014). Salinity stress alleviation by reducing lipid peroxidation and Na+ uptake has also been studied in tomatoes (Lycopersicon esculentum Mill.) using exogenous ascorbic acid (Shalata and Neumann 2001). In our previous study, RIB seed priming triggered the upregulation of the OsNHXs family, alleviating salinity stress in the Koshihikari salt-sensitive rice variety grown hydroponically (Jiadkong et al. 2022). However, the mechanisms of RIB-pretreated plants under salinized soil conditions in different rice varieties are not completely understood yet. Due to the differences in RIB application, growth medium, and rice cultivar compared to the previous studies, it is valuable to elucidate the response of RP in soil-based conditions. This prompted us to further analyze the effects of direct RIB application and changes caused in the physiological and biochemical properties of the salt-sensitive rice variety (IR29) under salinized soil conditions. In the current study, we demonstrated that the mechanism of direct RIB application under salinized soil conditions is related to improving the rate-limiting step of proline biosynthesis, which in turn increases proline concentration in the shoots to minimize oxidative stress. In addition, the upregulation of OsIRT2 and OsMGT1 expressions in the roots was congruent with the higher Fe and Mg concentrations and the upregulation of OsNHX1 and OsHKT1;5 expressions resulted in high Na+ concentration in the roots and low Na+ concentrations in the leaf blades. Notably, RP may be recommended to farmers and applied to salinized fields.
Materials and methods
Seed and soil preparation and plant growth conditions
The rice seeds (O. sativa L. cv. IR29) were obtained from the Plant Nutritional Physiology Laboratory of Hiroshima University. After sterilizing the seed surface with 5% sodium hypochlorite for 30 min, the seeds were washed thoroughly under running water for 10 min. Seeds were then soaked in tap water and incubated at 30 °C. After 24 h, the water was renewed, and the seeds were re-soaked in tap water for an additional 24 h. Five kilograms of commercial soil (the amount of nitrogen, phosphorus, and K in each container is 0.2, 0.25, and 0.325 g, respectively) were placed in four containers (13.3 L, 386 L × 256 W × 135 H). Germinated seeds were sown in the soil, watered to 5.2 kg weight, and maintained under identical conditions. A week after sowing, water was maintained 2 cm from the top of the containers (up to 5.7 kg) and remained in this condition throughout the experiment. Two-week-old seedlings were divided into two groups: RP, 10 mM RIB was applied directly to make 0.5 μM RIB in each treatment (RIB, RIB + NaCl), and non-pretreatment (NP) was used as the control (control, NaCl). After 24 h of pretreatment, seedlings from the first group were grown under controlled conditions with soil and tap water, whereas the salinity stress group was grown in salinized soil induced with 50 mM NaCl solution. NaCl solution was added every 3 days for 2 weeks. Seedlings were harvested when salinity stress damage was evident. The growth chamber was maintained at the following conditions: 70% relative humidity at 28 °C in the dark for 8 h and 25 °C in the light for 16 h and 400/0 μmol m−2 s−1 (day/night) photosynthetic photon flux density.
Plant dry weight
Four-week-old seedlings were harvested, and the roots, leaf sheaths, and leaf blades were separated. The roots were gently washed with deionized water. To determine the plant dry weight, the separated seedlings were dried in an oven at 70 °C for 72 h. The remaining fresh seedlings from each treatment were immediately frozen in liquid nitrogen and stored at − 80 °C until they were used for biochemical analyses.
Percentage of ELR, MDA, and H2O2 concentration analysis
The third leaf from the top was cut and placed in polypropylene tubes containing 30 ml of deionized water to determine the ELR. The tubes were then covered with plastic caps and agitated gently for 24 h. An electrical conductivity meter (CM31P; Kyoto Electronics, Kyoto, Japan) was used to measure the initial electrical conductivity (EC1) of the medium. The samples were autoclaved at 121 °C for 20 min to completely deactivate the tissues and release all electrolytes. The samples were then cooled to room temperature, and the final electrical conductivity (EC2) was determined. ELR was calculated using the following formula: \({\text{ELR }}\left( \% \right)\, = \,\left( {{\text{EC1}}/{\text{EC2}}} \right)\, \times \,{1}00\).
To determine MDA concentration in the leaf blades, 100 mg was homogenized in 5 mL of extraction buffer (10 mM HEPES, pH 7, 15.0% [w/v], trichloroacetic acid, 0.375% [w/v] thiobarbituric acid, 0.25 N HCl, 0.04% [v/v] butylated hydroxyl toluene, and 2.0% [v/v] ethanol) and incubated at 95 °C for 30 min, as previously described (Assaha et al. 2015). The reaction was terminated by placing the mixture in an ice bath. A UV-1850 spectrophotometer was used to measure the absorbance of the supernatant at 532 nm and 600 nm after centrifuging the mixture at 10,000 g for 20 min. According to Dionisio-Sese and Tobita (1998), an extinction coefficient of 155 mM per cm was used to calculate the MDA concentration.
To determine H2O2 concentration, frozen leaf blades (100 mg) were homogenized in 4 mL of cold acetone. The homogenate was centrifuged at 8000 g for 15 min at 4 °C. 100 L of each homogenate sample was added to 1 mL of reaction buffer (0.25 mM FeSO4, 0.25 mM (NH4)2SO4, 25 mM H2SO4, 125 M xylenol orange, and 10 mM sorbitol), and the mixture was left to stand for 1 h at room temperature. At 560 nm, H2O2 concentrations were measured using a spectrophotometer. A standard was used to calculate H2O2 concentration (Suharsono et al. 2002).
Proline concentration
Leaf blades (400 mg) were homogenized in 5 mL of 3% (w/v) sulfosalicylic acid, and the extract was then centrifuged at 10,000 g for 5 min at 4 °C. Later, 2 mL of the supernatants were added into 2 mL each of acid ninhydrin and acetic acid in the glass tubes and were then incubated in the water bath for 1 h at 100 °C. The samples were cooled in an ice bath, and toluene (4 mL) was added and vigorously mixed for 20 s for chromophore development. The absorbance of the chromophores was measured at 520 nm using a spectrophotometer. Proline standards were used to estimate the free proline concentration in roots and leaf blades (Bates et al. 1973).
Chlorophyll concentration
The dissected second leaf was weighed (100 mg) and immersed in a glass bottle containing 10 ml N, N-dimethyl formamide (DMF). The bottles were closed tightly with a plastic cap, covered with aluminum foil, and kept at room temperature for 24 h. Chlorophyll content was measured at 646.8 nm and 663.8 nm using a spectrophotometer. Total chlorophyll concentration was calculated based on the FW described by (Porra et al. 1989; Wellburn et al. 1994).
Macro- and micro-element concentration
The dried leaf blades, leaf sheaths, and roots were homogenized using a freeze crusher (μT-48, TAITEC) and weighed to 100 mg in 50-mL polypropylene tubes. This protocol has been previously described (Jiadkong et al. 2022; Wheal et al. 2011). The concentrations of Na+, K+, phosphorus (P), magnesium (Mg), calcium (Ca), iron (Fe), and manganese (Mn) in roots, leaf sheaths, and leaf blades were analyzed using inductively coupled plasma optical emission spectrophotometry (ICP-OES).
Na+ transporter genes expression analysis
Leaf blades, leaf sheaths, and roots (200 mg) were homogenized to extract the total RNA from both NP and RP seedlings. The protocol previously described was followed (Jiadkong et al. 2022). The relative expression levels of each gene were examined in the NP seedlings under control conditions. Table 1 presents a detailed description of the primers used in this study.
Statistical analyses
Three biological replicates were used for each analysis. Statistical significance was calculated using a one-way analysis of variance (ANOVA) with IBM SPSS Statistics 23.0. The statistical significance of differences between datasets and treatment was compared using Duncan’s multiple comparison tests which were considered statistically significant at P ≤ 0.05.
Results
Effect of RIB direct application on plant growth
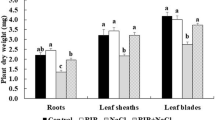
Plants possess diverse survival mechanisms under unfavorable conditions that alter the balance of energy use in them. Thus, it is essential to increase the ability of plants to grow under salinity stress, as the soil salinity tends to increase annually. Our study directly applied RIB to a soil-based experiment using commercial soil and salinity stress induced by 50 mM NaCl for 2 weeks. The seedlings were harvested when salinity damage was evident. Under control conditions, no yellowish leaves were observed in NP and RP seedlings. In contrast, NP seedlings exhibited brownish, smaller leaves, and shorter roots compared to RP seedlings under salinity stress (Fig. 1A). Under control conditions, the NP and RP seedlings showed no significant differences in plant dry weight (Fig. 1B). However, under salinity stress, RP seedlings possessed significantly higher leaf sheath and leaf blade dry weights than NP seedlings (Fig. 1B).
RP rescued rice seedlings from wilting and death under salinity stress. Image of seedlings growth (A), (B), and plant dry weight (C). Two-week-old rice seedlings were pretreated with 0.5 μM RIB (RIB, RIB + NaCl) or without (control, NaCl) for 24 h and then subjected either to control or salinity stress conditions. Values are means ± SE, n = 3. The different letter represents a significant difference (P ≤ 0.05)
Percentage of ELR, H2O2, and MDA concentrations
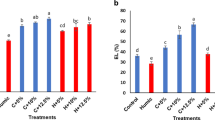
Table 2 indicates that ELR remained unchanged between the NP and RP seedlings under control conditions. A significantly higher percentage of ELR was observed in the NP seedlings than in the RP seedlings under salinity stress. H2O2 is the key ROS generated during oxidative stress and is a precursor of lipid peroxidation. The H2O2 and MDA concentrations showed a trend similar to that of the ELR (Table 2). This implies that NP seedlings suffered more cellular damage from salinity stress than RP seedlings.
Proline concentration and proline biosynthesis-related genes
In our study, proline concentration was analyzed based on the reaction of proline with the acid ninhydrin. Figure 2 shows that no significant difference was observed in proline concentration in the roots and shoots of NP and RP seedlings under control conditions. Proline concentration in the shoots of NP and RP seedlings under salinity stress was significantly higher than that in NP seedlings under control conditions (Fig. 2). However, a significant increase was observed in the shoots of RP seedlings compared to those of NP seedlings under salinity stress (Fig. 2). To gain further insight into proline biosynthesis in RP seedlings under salinity stress, the expression profiles of the proline biosynthesis-responsive genes OsP5CS1 and OsP5CS2 were analyzed in the roots, leaf sheaths, and leaf blades. OsP5CS1 was significantly upregulated in the roots, leaf sheaths, and leaf blades of RP seedlings compared with those of NP seedlings under salinity stress (Fig. 3A, B, C). OsP5CS2 was upregulated in the leaf sheaths of RP seedlings compared to that in NP seedlings under salinity stress (Fig. 3D). A similar trend was observed in the leaf blades of RP seedlings (Fig. 3E). However, the upregulation of OsP5CS2 was not observed in the roots (data not shown).
RP enhanced proline concentration under salinity stress. Two-week-old rice seedlings were pretreated with 0.5 μM RIB (RIB, RIB + NaCl) or without (control, NaCl) for 24 h and then subjected either to control or salinity stress conditions. Values are means ± SE, n = 3. The different letter represents a significant difference (P ≤ 0.05)
RP upregulated proline biosynthesis-responsive genes under salinity stress. The upregulation of OsP5CS1 in the roots (A), leaf sheaths (B), and leaf blades (C). The upregulation of OsP5CS2 in the leaf sheath (D) and leaf blades (E). Two-week-old rice seedlings were pretreated with 0.5 μM RIB (RIB, RIB + NaCl) or without (control, NaCl) for 24 h and then subjected either to control or salinity stress conditions. Values are means ± SE, n = 3. The different letter represents a significant difference (P ≤ 0.05)
Chlorophyll concentration
Rice grown under control conditions had more green leaves and higher chlorophyll concentrations (Figs. 1A and 4). Significantly higher Chlb and Chla+b concentration was observed in the leaf blades of RP seedlings, and no significant differences were found in Chla under salinity stress (Fig. 4). In contrast, NP and RP seedlings reduced Chlb and Chla+b concentration by 66%, 36%, and 51%, 17%, respectively, in comparison with the NP seedlings under control conditions (Fig. 4).
RP improved chlorophyll concentration under salinity stress. Two-week-old rice seedlings were pretreated with 0.5 μM RIB (RIB, RIB + NaCl) or without (control, NaCl) for 24 h and then subjected either to control or salinity stress conditions. Values are means ± SE, n = 3. The different letter represents a significant difference (P ≤ 0.05)
Element concentration
Figure 5A shows that RP seedlings possessed higher P concentrations in the leaf sheaths than NP seedlings under control conditions. A similar trend was observed in the leaf blades under salinity stress (Fig. 5A). The Mg concentration in the leaf sheaths, roots, and leaf blades of the RP seedlings under control conditions was significantly higher than that in the NP seedlings (Fig. 5B). While a significantly higher Mg concentration of the roots and leaf blades was observed in RP seedlings compared to NP seedlings under salinity stress (Fig. 5B). Under salinity stress, Ca concentration was also found to be significantly higher in the roots, leaf sheaths, and leaf blades of RP seedlings than in those of NP seedlings (Fig. 5C). The Mn concentration in the leaf blades of RP seedlings was significantly higher than that of NP seedlings under salinity stress (Fig. 5D). The Fe concentration greatly increased in the roots, regardless of the treatments and conditions (Fig. 5E). However, a significant decrease in the Fe concentration was observed in the leaf blades of NP seedlings under salinity stress (Fig. 5E). To further explore the relationship between the ion transporter genes and RP in rice seedlings under salinity stress, the expression level of OsMGT1, OsYSL15, and OsIRT2 in the roots was analyzed. Under salinity stress, RP seedlings significantly upregulated OsMGT1 and OsIRT2 expression in the roots (Fig. 6A, C). Whereas the upregulation of OsYSL15 expression was observed in the roots of NP seedlings under salinity stress (Fig. 6B).
RP increased element concentrations under salinity stress. The concentration of P (A), Mg (B), Ca (C), Mn (D), and Fe (E). Two-week-old rice seedlings were pretreated with 0.5 μM RIB (RIB, RIB + NaCl) or without (control, NaCl) for 24 h and then subjected either to control or salinity stress conditions. Values are means ± SE, n = 3. The different letter represents a significant difference (P ≤ 0.05)
RP upregulated Mg and Fe transporter genes under salinity stress. The upregulation of OsMGT1 (A), OsYSL15 (B), and OsIRT2 (C) in the roots. Two-week-old rice seedlings were pretreated with 0.5 μM RIB (RIB, RIB + NaCl) or without (control, NaCl) for 24 h and then subjected either to control or salinity stress conditions. Values are means ± SE, n = 3. The different letter represents a significant difference (P ≤ 0.05)
Na+, K+ concentration and Na+/K+ and the relative expression of Na+ transporter genes
The Na+ concentration significantly increased under salinity stress compared to that in NP and RP seedlings under control conditions (Fig. 7A). However, the Na+ concentration in the RP seedlings was significantly lower in the leaf sheath and leaf blades and higher in the roots than in the NP seedlings (Fig. 6A). In contrast, a significantly higher K+ concentration was observed in the roots of RP seedlings than in those of NP seedlings under salinity stress (Fig. 7B). No significant difference was observed in the K+ concentration in the leaf sheaths and leaf blades under salinity stress (Fig. 7B). These results inevitably led to a significantly lower Na+/K+ ratio in the leaf sheaths and leaf blades of RP seedlings than in NP seedlings under salinity stress (Fig. 7C), suggesting that RP seedlings may have regulated Na+ accumulation in the shoots. Significantly higher Na+ concentrations were found in the roots of RP seedlings than in those of NP seedlings (Fig. 7A). It is suspected that Na+ transporter genes are positively regulated in RP seedlings. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to determine the expression of Na+ transporter genes. Figure 7A, B showed that the expression of OsNHX1 and OsHKT1;5 significantly upregulated in the roots of RP seedlings in comparison with the NP seedlings under salinity stress which are responsible for Na+ compartmentalization and Na+ retrieval, respectively (Assaha et al. 2017). It is possible that the contributions of these two Na+ transporter genes led to higher Na+ concentrations in the roots, preventing plant metabolic disturbances in the shoots. The proposed mechanisms of RIB-pretreated seedlings illustrated in Fig. 8.
RP maintained favorable Na+ (A), K+ (B) concentrations, and Na+/K+ ratio (C). The upregulation of OsNHX1 (D) OsHKT1;5 (E) under salinity stress. Two-week-old rice seedlings were pretreated with 0.5 μM RIB (RIB, RIB + NaCl) or without (control, NaCl) for 24 h and then subjected either to control or salinity stress conditions. Values are means ± SE, n = 3. The different letter represents a significant difference (P ≤ 0.05)
Discussion
The consequences of salinity stress in plants include metabolic malfunction, irregular cell division and expansion, reduced photosynthetic activity, increased ion toxicity, and enzymatic disorders that eventually lead to cell death (Munns and Tester 2008; Assaha et al. 2017). ROS detoxification and ion balance are key indicators of plant survival under salinity stress. To utilize salinized soil, it is important to understand how pretreatment ameliorates salinity stress in plants. In our attempt to understand the role of RP in salinized soil conditions, we found that RP seedlings improved plant biomass, proline concentration, and essential element concentrations involved in photosynthesis. Simultaneously, RP seedlings showed reduced lipid peroxidation and Na+ accumulation in the shoots and retained lower ELR and higher chlorophyll concentrations under salinized soil conditions.
ROS, including H2O2, superoxide radicals, and hydroxyl radicals, is typically formed in the apoplast and is constitutive components of lipid peroxidation (Mittler et al. 2004). H2O2 concentration correlates with MDA concentration, which is an indicator of oxidative stress under biotic and abiotic stresses (Miller et al. 2010). A previous study reported that salinity stress causes cellular damage owing to an increase in H2O2 and MDA concentrations and ELR in Populus cathayana Rehder (Yang et al. 2009). Electrolyte leakage was used to quantify the amount of damage inflicted on plant tissues under unfavorable conditions (Demidchik et al. 2014). The salt-tolerant rice variety exhibited higher plant biomass and lower ELR and Na+ concentrations in the leaf blades under salinity stress (Wangsawang et al. 2018). As shown in Table 2, lower H2O2 and MDA concentrations and ELR were observed in RP seedlings under salinity stress, implying less cellular damage in RP seedlings under salinity stress. Simultaneously, proline has been reported to be a non-enzymatic antioxidant that limits the oxidative stress caused by ROS overproduction (Alia et al. 2001; Rejeb et al. 2014). P5CS upregulation is one of the mechanisms used to overcome osmotic stress in barley (Ueda et al. 2004). Plants use glutamate, which is converted to proline by two successive reductions catalyzed by Δ1-pyrroline-5-carboxylate synthetase (P5CS) and Δ1-pyrroline-5-carboxylate reductase (P5CR), as the primary pathway for proline biosynthesis during osmotic stress (Szabados and Savouré 2010). P5CS, the rate-limiting enzyme in proline biosynthesis, may increase in response to stress, resulting in proline accumulation (Sharma and Verslues 2010). This is consistent with our findings of higher proline concentrations and upregulation of OsP5CS1 and OsP5CS2 expressions (Fig. 3A–E). This implies that proline acts as a non-enzymatic antioxidant that reduces oxidative stress in RP seedlings under salinity stress.
Furthermore, ROS decreases membrane fluidity and selectivity by causing membrane lipid peroxidation and chlorophyll degradation (Verma and Mishra 2005). Our results indicate a significant increase in Chlb and Chla+b in the RP seedlings under salinity stress (Fig. 4), which agrees with the results of Mekawy et al. (2018) on apigenin-pretreated rice seedlings under salinity stress. Salinity stress affects the light-absorbing structures and governs the state transition of photosynthesis (Chen and Hoehenwarter 2015). Porphyrin formation is limited by salinity stress, which may decrease the production of proteins that bind to chlorophyll (Abdelkader et al. 2007). The main factors involved in the reduced chlorophyll concentration are not only the inhibition of chlorophyll synthesis but also the stimulation of its degradation by the enzyme chlorophyllase (Santos 2004). However, either slow synthesis or fast breakdown suggests the existence of a photoprotection mechanism that minimizes light absorbance by reducing the chlorophyll content (Elsheery and Cao 2008). Notably, the retention of higher chlorophyll concentrations may be related to the reduced oxidative damage in RP seedlings under salinity stress.
Kabata-Pendias (2011) reported that N, P, K, Ca, sulfur (S), Mg, Mn, copper (Cu), and Fe are crucial for physiological processes and plant growth. However, the availability of these elements depends on the soil pH, hydrolytic acidity, granulometric composition, and organic matter content (Kalaji et al. 2018). To further understand this phenomenon in rice, we investigated the P, Mg, Ca, Mn, and Fe concentrations in the roots, leaf sheaths, and leaf blades of NP and RP seedlings under control and salinity stress conditions. Plant growth and development are intertwined with the physiological responses that result from the ion accumulation; macro and micronutrients are required for plants to adapt to salinity stress (Wang et al. 2003). The accumulation of P, Mg, Mn, and Fe plays a crucial role in the chlorophyll components and photosynthetic reactions (Taiz et al. 2015; Ebrahimi et al. 2023). Cobalamin (Vitamin B12) contributes to photosynthesis as a ring-contracted modified tetrapyrrole containing cobalt (Osman et al. 2021). Significantly higher chlorophyll concentrations are correlated with higher Mg, Mn, and Fe concentrations in the leaf blades of RP seedlings under salinity stress (Figs. 4 and 5B, D, E). RP improves photosynthesis by increasing the concentration of related elements. Two distinct roles of Ca have been well studied: a structural/apoplastic role and a signaling role that initiate plant responses to environmental stimuli (Taiz et al. 2015). ROS and Ca signal work together to maintain cellular pH and ion homeostasis in the early stage of salinity stress in which 3′5′-cyclic guanosine monophosphate triggers Ca import, reduces Na+ influx, and reduces K+ efflux (Van Zelm et al. 2020). MgSO4 and CaSO4 applications enhanced plant biomass and chlorophyll concentration in the red clover (Trifolium pratense) and tall fescue (Festuca arundinacea) under long-term salinity stress (Sharavdorj et al. 2022) Fig. 5C shows that RP seedlings under salinity stress had higher Ca concentrations in the leaf blades, implying that Ca may have played a signaling role in maintaining a favorable ion balance. Despite the abundance of Fe in soils, it is mostly found in its oxidized and insoluble form, Fe3+, under aerobic conditions which is generally inaccessible to plants (Ishimaru et al. 2006), which is consistent with our results that the Fe concentration in the roots greatly increased, regardless of the conditions and treatments (Fig. 5E). The significantly higher concentration of Mg and Fe prompted us to further analyze the upregulation of ion transporter genes. The expression of OsMGT1 and OsIRT2 was upregulated in the roots of RP seedlings (Fig. 6A, C) which is correlated with the higher Mg and Fe concentrations in the leaf blades of RP seedlings under salinity stress (Fig. 5B, E). OsMGT1 is a plasma membrane-localized protein facilitating Mg uptake in plants (Chen et al. 2017). Rice plants have a distinct Fe2+ absorption mechanism which associated with the expression of OsIRT1 and OsIRT2 upregulation (Ishimaru et al. 2006). Wang et al. (2019) reported that OsIRT1 expression was downregulated and OsIRT2 expression was upregulated in Nippobare rice cultivar implying its role in Fe uptake under flooded conditions. These results suggest that RP seedlings improved the Mg and Fe uptake which, in turn, increased Mg, Fe, and chlorophyll concentrations under salinized soil condition.
The benefit of Na+ at low concentrations is that it improves water use efficiency, and stomatal diffusion augments water uptake efficiency (Mateus et al. 2019). Sriskantharajah et al. (2020) reported that 1 mM NaCl was a potent concentration for acclimatizing rice to salinity stress. However, Na+ overaccumulation causes detrimental cell growth. Under salinity stress, the RP seedlings accumulated lower Na+ and higher K+ concentrations in their shoots, which led to higher plant biomass (Figs. 1B and 4A). Suppression of Na+ enhanced apoplastic flow to reduce Na+ uptake has been observed in rice plants under salinity stress (Sobahan et al. 2009). Lower maintenance of the Na+/K+ ratio involves the regulation of Na+ and K+ transporter genes, which are critical determinants of salt tolerance (Assaha et al. 2017). This suggests that RP seedlings may regulate the Na+ transporter to reduce Na accumulation in the shoots resulting in a lower Na+/K+ ratio in the shoots. It is suggested that RP seedlings may regulate the Na+ transporter to reduce Na accumulation in the shoots resulting in a lower Na+/K+ ratio in shoots. Upregulation of OsNHX1 and OsHKT1;5 expressions was observed in RP seedlings under salinity stress (Fig. 7D, E). High Na+ concentrations and the upregulation of OsNHX expression are commonly observed together, contributing to Na+ compartmentalization from the cytosol to vacuoles without detrimental effects on plant growth (Rodríguez-Rosales et al. 2009). RIB seed priming activates the expression OsNHXs family in the roots, resulting in high Na+ concentration in the roots of the Koshihikari rice-sensitive cultivar under hydroponic conditions (Jiadkong et al. 2022). OsHKT1;5 plays a key role in retrieving Na+ from the transpiration stream to the xylem parenchyma and is localized in the plasma membrane (Golldack et al. 2002). The reduced Na+ concentration in young leaf blades was attributed to the upregulation of OsHKT1;5 expression in rice (Kobayashi et al. 2017). The upregulation of OsHKT1;5 expression was correlated with the upregulation of OsMGT1 expression, which restricts Na+ uptake in the shoots (Chen et al. 2017). This agrees with our finding that higher Mg concentrations and OsMGT1 and OsHKT1;5 expressions were observed in RP seedlings under salinity stress (Figs. 5B, 6A and 7E). This implies not only the high Na+ concentration in the roots is caused by the OsNHX1 and OsHKT1;5 expressions, which prevented toxic effects on cellular molecules in the leaf sheaths and blades of RP seedlings. But also, RP seedlings induced the expression of OsMGT1 to uptake more Mg and trigger the expression of OsHKT1;5 in the roots alleviating salinity stress.
Conclusion
The direct application of RIB as a pretreatment for rice seedlings under salinity stress resulted in substantial protection from oxidative and ionic stress injuries. It is worth noting that the mechanisms of RP seedlings under salinized soil conditions upregulate the expression of the responsive genes in the rate-limiting step of proline biosynthesis, resulting in an enhancement of proline concentration in the leaf blades, which acts as a non-enzymatic antioxidant. Also, RP seedlings upregulate the expression of Mg and Fe transporter genes, inducing the Mg and Fe concentrations in the leaf blades to retain a higher chlorophyll concentration. Furthermore, RP seedlings upregulate the expression of OsNHX1 and OsHKT1;5 to positively manage Na+ overaccumulation by accumulating Na+ in the roots instead of translocating to the leaf blades to maintain regular enzyme activities. However, further elucidation is needed to ascertain whether the initial trends observed in this study are repeatable at the field scale and therefore could be advised for soil amendment under salinity stress.
References
Abbas CA, Sibirny AA (2011) Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. Microbiol Mol Biol Rev 75:321–360. https://doi.org/10.1128/mmbr.00030-10
Abdelkader AF, Aronsson H, Sundqvist C (2007) High salt stress in wheat leaves causes retardation of chlorophyll accumulation due to a limited rate of protochlorophyllide formation. Physiol Plant 130:157–166. https://doi.org/10.1111/j.1399-3054.2007.00885.x
Ahmad I, Zhu G, Zhou G, Younas MU, Suliman ME, Liu J, Zhuyi M, Gabralla EIS (2023) Integrated approaches for increasing plant yield under salt stress. Front Plant Sci. https://doi.org/10.3389/fpls.2023.1215343
Alia MP, Matysik J (2001) Effect of proline on the production of singlet oxygen. Amino Acids 21:195–200. https://doi.org/10.1007/s007260170026
Anwar A, Kim JK (2020) Transgenic breeding approaches for improving abiotic stress tolerance: recent progress and future perspectives. Inter J Mol Sci 21:2695. https://doi.org/10.3390/ijms21082695
Assaha DVM, Liu L, Mekawy AMM, Ueda A, Nagaoka T, Saneoka H (2015) Effect of salt stress on Na accumulation, antioxidant enzyme activities and activity of cell wall peroxidase of huckleberry (Solanum scabrum) and eggplant (Solanum melongena). Int J Agric Biol 17:1149–1156. https://doi.org/10.17957/ijab/15.0052
Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509. https://doi.org/10.3389/fphys.2017.00509
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Chen Y, Hoehenwarter W (2015) Changes in the phosphoproteome and metabolome link early signaling events to rearrangement of photosynthesis and central metabolism in salinity and oxidative stress response in Arabidopsis. Plant Physiol 169(4):3021–3033. https://doi.org/10.1104/pp.15.01486
Chen ZC, Yamaji N, Horie T, Che J, Li J, An G, Ma JF (2017) A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physio 174:1837–1849. https://doi.org/10.1104/pp.17.00532
Chuamnakthong S, Nampei M, Ueda A (2019) Characterization of Na+ exclusion mechanism in rice under saline-alkaline stress conditions. Plant Sci 287:110171. https://doi.org/10.1016/j.plantsci.2019.110171
Colin L, Ruhnow F, Zhu JK, Zhao C, Zhao Y, Persson S (2023) The cell biology of primary cell walls during salt stress. Plant Cell 35:201–217. https://doi.org/10.1093/plcell/koac292
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65:1259–1270. https://doi.org/10.1093/jxb/eru004
Deng B, Jin X, Yang Y, Lin Z, Zhang Y (2014) The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production. Plant Growth Regul 72:269–277. https://doi.org/10.1007/s10725-013-9858-8
Desoky ESM, Merwad ARM, Rady MM (2018) Natural biostimulants improve saline soil characteristics and salt stressed-sorghum performance. Commun Soil Sci Plant Anal 49:967–983. https://doi.org/10.1080/00103624.2018.1448861
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
Ebrahimi P, Shokramraji Z, Tavakkoli S, Mihaylova D, Lante A (2023) Chlorophylls as natural bioactive compounds existing in food by-products: a critical review. Plants 12:1533. https://doi.org/10.3390/plants12071533
Eckardt NA, Ainsworth EA, Bahuguna RN, Broadley MR, Busch W, Carpita NC, Castrillo G, Chory J, DeHaan LR, Duarte CM, Henry A (2023) Climate change challenges, plant science solutions. Plant Cell 35:24–66. https://doi.org/10.1093/plcell/koac303
Elsheery NI, Cao KF (2008) Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol Plant 30:769–777. https://doi.org/10.1007/s11738-008-0179-x
Golldack D, Su H, Quigley F, Kamasani UR, Muñoz-Garay C, Balderas E, Popova OV, Bennett J, Bohnert HJ, Pantoja O (2002) Characterization of a HKT-type transporter in rice as a general alkali cation transporter. Plant J 31:529–542. https://doi.org/10.1046/j.1365-313X.2002.01374.x
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res Int. https://doi.org/10.1155/2014/757219
Hasanuzzaman M, Raihan MRH, Masud AAC, Rahman K, Nowroz F, Rahman M, Nahar K, Fujita M (2021) Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int J Mol Sci 22:9326. https://doi.org/10.3390/ijms22179326
Horie T, Karahara I, Katsuhara M (2012) Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice 5:11. https://doi.org/10.1186/1939-8433-5-11
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45(3):335–346. https://doi.org/10.1111/j.1365-313x.2005.02624.x
Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–651. https://doi.org/10.1016/j.bbrc.2006.04.140
Jiadkong K, Nampei M, Wangsawang S, Ueda A (2022) Riboflavin Seed priming activates OsNHXs expression to alleviate salinity stress in rice seedlings. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10768-1
Kabata-Pendias A (2011) Trace elements in soils and plants/fourth editions. CRC Taylor and Francis Group, Boca Raton, 505
Kalaji HM, Bąba W, Gediga K, Goltsev V, Samborska IA, Cetner MD, Dimitrova S, Piszcz U, Bielecki K, Karmowska K, Dankov K (2018) Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth Res 136:329–343. https://doi.org/10.1007/s11120-017-0467-7
Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T, Nayef MA (2017) OsHKT1; 5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J 91:657–670. https://doi.org/10.1111/tpj.13595
Kruasuwan W, Lohmaneeratana K, Munnoch JT, Vongsangnak W, Jantrasuriyarat C, Hoskisson PA, Thamchaipenet A (2023) Transcriptome landscapes of salt-susceptible rice cultivar IR29 associated with a plant growth promoting endophytic streptomyces. Rice. https://doi.org/10.1186/s12284-023-00622-7
Linh LH, Linh TH, Xuan TD, Ham LH, Ismail AM, Khanh TD (2012) Molecular breeding to improve salt tolerance of rice (Oryza sativa L.) in the Red River Delta of Vietnam. Int J Plant Genom. https://doi.org/10.1155/2012/949038
Machado RMA, Serralheiro RP (2017) Soil salinity: effect on vegetable crop growth. Management practices to revent and mitigate soil salinization. Horticulturae 3:30
Mateus NDS, Ferreira EDO, Arthur Junior JC, Domec JC, Jordan-Meille L, Gonçalves JDM, Lavres J (2019) The ideal percentage of K substitution by Na in Eucalyptus seedlings: evidences from leaf carbon isotopic composition, leaf gas exchanges and plant growth. Plant Physiol Biochem 137:102–112. https://doi.org/10.1016/j.plaphy.2019.02.006
Mekawy AMM, Abdelaziz MN, Ueda A (2018) Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiol Biochem 130:94–104. https://doi.org/10.1016/j.plaphy.2018.06.036
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Miyamoto T, Ochiai K, Nonoue Y, Matsubara K, Yano M, Matoh T (2015) Expression level of the sodium transporter gene OsHKT2; 1 determines sodium accumulation of rice cultivars under potassium-deficient conditions. J Soil Sci Plant Nutr 61:481–492. https://doi.org/10.1080/00380768.2015.1005539
Mohsin SM, Hasanuzzaman M, Parvin K, Fujita M (2020) Pretreatment of wheat (Triticum aestivum L.) seedlings with 2, 4-D improves tolerance to salinity-induced oxidative stress and methylglyoxal toxicity by modulating ion homeostasis, antioxidant defenses, and glyoxalase systems. Plant Physiol Biochem 152:221–231. https://doi.org/10.1016/j.plaphy.2020.04.035
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Osman D, Cooke A, Young TR, Deery E, Robinson NJ, Warren MJ (2021) The requirement for cobalt in vitamin B12: a paradigm for protein metalation. Biochim Biophys Acta Mol Cell Res 1868:118896. https://doi.org/10.1016/j.bbamcr.2020.118896
Pogłodziński R, Barłóg P, Grzebisz W (2021) Effect of nitrogen and magnesium sulfate application on sugar beet yield and quality. Plant Soil Environ 67(9):507–513
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerg 975:384–394. https://doi.org/10.1016/S0005-2728(89)80347-0
Qin H, Li Y, Huang R (2020) Advances and challenges in the breeding of salt-tolerant rice. Int J Mol Sci 21:8385. https://doi.org/10.3390/ijms21218385
Rejeb KB, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284. https://doi.org/10.1016/j.plaphy.2014.04.007
Rodríguez-Rosales MP, Gálvez FJ, Huertas R, Aranda MN, Baghour M, Cagnac O, Venema K (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4:265–276. https://doi.org/10.4161/psb.4.4.7919
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26:115–124. https://doi.org/10.1016/j.copbio.2013.12.004
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr sci 407–421
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Horti 103:93–99. https://doi.org/10.1016/j.scienta.2004.04.009
Senbayram M, Gransee A, Wahle V, Thiel H (2015) Role of magnesium fertilisers in agriculture: plant–soil continuum. Crop Pasture Sci 66(12):1219. https://doi.org/10.1071/cp15104
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211. https://doi.org/10.1093/jexbot/52.364.2207
Sharavdorj K, Byambadorj SO, Jang Y, Cho JW (2022) Application of magnesium and calcium sulfate on growth and physiology of forage crops under long-term salinity stress. Plants 11(24):3576. https://doi.org/10.3390/plants11243576
Sharma S, Verslues PE (2010) Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ 33:1838–1851. https://doi.org/10.1111/j.1365-3040.2010.02188.x
Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2022) The heterotrimeric G protein α subunit acts upstream of the small GTPase Rac in disease resistance of rice. PNAS 99(20):13307–13312. https://doi.org/10.1073/pnas.192244099
Sobahan MA, Arias CR, Okuma E, Shimoishi Y, Nakamura Y, Hirai Y, Mori IC, Murata Y (2009) Exogenous proline and glycinebetaine suppress apoplastic flow to reduce Na+ uptake in rice seedlings. Biosci Biotechnol Biochem 73:2037–2042. https://doi.org/10.1271/bbb.90244
Souri MK, Tohidloo G (2019) Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem Biol Technol Agric. https://doi.org/10.1186/s40538-019-0169-9
Sripinyowanich S, Klomsakul P, Boonburapong B, Bangyeekhun T, Asami T, Gu H, Buaboocha T, Chadchawan S (2013) Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): the role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ Exp Bot 86:94–105. https://doi.org/10.1016/j.envexpbot.2010.01.009
Sriskantharajah K, Osumi S, Chuamnakthong S, Nampei M, Amas JC, Gregorio GB, Ueda A (2020) Contribution of two different Na+ transport systems to acquired salinity tolerance in rice. Plant Sci 297:110517. https://doi.org/10.1016/j.dib.2020.106023
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Taiz L, Zeiger E, Møller IM, Murphy A (2015) Plant physiology and development, 6th edn. Sinauer Associates Incorporated
Tang ZH, Liu Y, Guo X, Zu Y (2011) The combined effects of salinity and nitrogen forms on Catharanthus roseus: the role of internal ammonium and free amino acids during salt stress. J Plant Nutr Soil Sci 174:135–144. https://doi.org/10.1002/jpln.200900354
Ueda A, Kathiresan A, Inada M, Narita Y, Nakamura T, Shi W, Takabe T, Bennett J (2004) Osmotic stress in barley regulates expression of a different set of genes than salt stress does. J Exp Bot 55:2213–2218. https://doi.org/10.1093/jxb/erh242
Ueda A, Yahagi H, Fujikawa Y, Nagaoka T, Esaka M, Calcaño M, González MM, Hernandez Martich JD, Saneoka H (2013) Comparative physiological analysis of salinity tolerance in rice. Soil Sci Plant Nutr 59(6):896–903. https://doi.org/10.1080/00380768.2013.842883
Van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71:403–433. https://doi.org/10.1146/annurev-arplant-050718-100005
Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol 162:669–677. https://doi.org/10.1016/j.jplph.2004.08.008
Verslues PE, Bailey-Serres J, Brodersen C, Buckley TN, Conti L, Christmann A, Dinneny JR, Grill E, Hayes S, Heckman RW, Hsu PK (2023) Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. Plant Cell 35:67–108. https://doi.org/10.1093/plcell/koac263
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218(1):1–14. https://doi.org/10.1007/s00425-003-1105-5
Wang P, Yamaji N, Inoue K, Mochida K, Ma JF (2019) Plastic transport systems of rice for mineral elements in response to diverse soil environmental changes. New Phytol 226(1):156–169. https://doi.org/10.1111/nph.16335
Wangsawang T, Chuamnakthong S, Kohnishi E, Sripichitt P, Sreewongchai T, Ueda A (2018) A salinity-tolerant japonica cultivar has Na+ exclusion mechanism at leaf sheaths through the function of a Na+ transporter OsHKT 1; 4 under salinity stress. J Agron Crop Sci 204:274–284. https://doi.org/10.1111/jac.12264
Wellburn RW (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wheal MS, Fowles TO, Palmer LT (2011) A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal Methods 3:2854–2863. https://doi.org/10.1039/C1AY05430a
Yang F, Xiao X, Zhang S, Korpelainen H, Li C (2009) Salt stress responses in Populus cathayana Rehder. Plant Sci 176:669–677. https://doi.org/10.1016/j.plantsci.2009.02.008
Zhang X, He P, Guo R, Huang K, Huang X (2023) Effects of salt stress on root morphology, carbon and nitrogen metabolism, and yield of Tartary buckwheat. Sci Rep. https://doi.org/10.1038/s41598-023-39634-0
Zhao D, Gao S, Zhang X, Zhang Z, Zheng H, Rong K, Zhao W, Khan SA (2021) Impact of saline stress on the uptake of various macro and micronutrients and their associations with plant biomass and root traits in wheat. Plant Soil Environ 67(2):61–70
Zhu Y, Jiang X, Zhang J, He Y, Zhu X, Zhou X, Gong H, Yin J, Liu Y (2020) Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol Biochem 156:209–220. https://doi.org/10.1016/j.plaphy.2020.09.014
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 20KK0129 and JST the establishment of university fellowships toward the creation of science technology innovation, and Grant Number JPMJFS2129. Seeds of IR29 were kindly provided by the International Rice Research Institute.
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Contributions
The authors have made the following declarations about their contributions. Conception and design of the experiments were contributed by KJ and AU. Performing the experiments and collecting data were involved by KJ. Analysis and data processing were performed by KJ. Writing and manuscript processing was done by KJ, AU.
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest to declare.
Additional information
Communicated by Tibor Janda.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiadkong, K., Ueda, A. Effects of riboflavin application on rice growth under salinized soil conditions. CEREAL RESEARCH COMMUNICATIONS (2024). https://doi.org/10.1007/s42976-024-00504-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42976-024-00504-8