Abstract
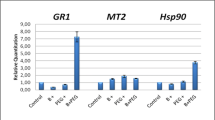
Riboflavin (vitamin B2) is required for normal plant growth and development. Previous studies have shown that riboflavin application can enhance pathogen resistance in plants. Here, we investigated the role of riboflavin in increasing drought tolerance (10 % PEG6000 treatment) in plants. We treated 4 week-old tobacco plants with five different levels of riboflavin (0, 4, 20, 100 and 500 μM) for 5 days and examined their antioxidant responses and levels of drought tolerance. Compared with the controls, low and moderate levels of riboflavin treatment enhanced drought tolerance in the tobacco plants, whereas higher concentrations of riboflavin (500 μM) impaired drought tolerance. Further analysis revealed that plants treated with 500 μM riboflavin accumulated higher levels of ROS (O2 − and H2O2) and lipid peroxide than the control plants or plants treated with low levels of riboflavin. Consistent with this observation, the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) were higher in plants treated with low or moderate (4, 20 and 100 μM) levels of riboflavin compared with the control. We also found that chlorophyll degraded rapidly in control and 500 μM riboflavin-treated plants under drought stress conditions. In addition, the survival times of the riboflavin-treated plants were significantly modified by treatment with reduced glutathione, a well-known ROS scavenger, under drought stress conditions. Thus, riboflavin-mediated ROS production may determine the effects of riboflavin on drought tolerance in tobacco plants.





Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- O2 − :
-
Superoxide anion
- H2O2 :
-
Hydrogen peroxide
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- GR:
-
Glutathione reductase
- TBARS:
-
Thiobarbituric acids reactive substance
- FMN:
-
Flavin monophosphate
- FAD:
-
Flavin adenine dinucleotide
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- AsA:
-
Reduced ascorbate
- DHA:
-
Oxidized ascorbate
References
Azami-Sardooei Z, França SC, De Vleesschauwer D, Höfte M (2010) Riboflavin induces resistance against Botrytis cinerea in bean, but not in tomato, by priming for a hydrogen peroxide-fueled resistance response. Physiol Mol Plant Pathol 75:22–29
Beutler E (1969) Effect of flavin compounds on glutathione reductase activity: in vitro and in vivo studies. J Clin Invest 48:1957–1966
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Conrath U, Pieterse C, Mauch-Mani B (2002) Priming in plant-pathogen interactions. Trends Plant Sci 7:210–216
Dhindsa RS, Dhindsa PP, Thorpe TA (1980) Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Eichler M, Lavi R, Shainberg A, Lubart R (2005) Flavins are source of visible-light-induced free radical formation in cells. Las Surg Med 37:314–319
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium-chloride: simple assay for superoxide dismutase. Anal Biochem 70:616–620
Escobar JA, Rubio MA, Lissi EA (1996) SOD and catalase inactivation by singlet oxygen and peroxyl radical. Free Radic Biol Med 20:285–290
Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR (1998) Relationship between CO 2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol 116:571–580
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Galston AW, Baker RS (1949) Inactivation of enzymes by visible light in the presence of riboflavin. Science 109:485–486
Gay C, Collins J, Gebicki J (1999) Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem 273:149–155
Gechev T, Gadjev I, Van Breusegem F, Inzé D, Dukiandjiev S, Toneva V, Minkov I (2002) Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol Life Sci 59:708–714
Gill S, Tuteja N (2010) Reactive oxygen species and antioxidant mechinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:180–198
Huang SS, Zheng RL (2006) Biphasic regulation of angiogenesis by reactive oxygen species. Pharmazie 61:223–229
Huang R, Choe E, Min DB (2004) Kinetics for singlet oxygen formation by riboflavin photosensitization and the reaction between riboflavin and singlet oxygen. J Food Sci 69:726–732
Jakab G, Ton J, Flors V, Zimmerli L, Metraux J, Mauch-Mani B (2005) Enhancing Arabidopsis salt and drought tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139:267–274
Liang Y, Sun W, Zhu Y, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Lichtenthaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic biomembrane. Meth Enzymol 148:349–382
Liu Z, Zhang X, Bai J, Suo B, Xu P, Wang L (2009) Exogenous paraquat changes antioxidant enzyme activities and lipid peroxidation in drought-stressed cucumber leaves. Scientia Horticul 121:138–143
Mandels GR (1950) The photoinactivation of enzymes by riboflavin. Plant Physiol 25:763–766
Marshall JG, Scarratt JB, Dumbroff EB (1991) Induction of drought resistance by abscisic acid and paclobutrazol in jack pine. Tree Physiol 8:415–421
Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, Sullivan PA (1969) The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Comm 36:891–897
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Mori T, Sakurai M (1995) Effects of riboflavin and increased sucrose on anthocyanin production in suspended strawberry cell cultures. Plant Sci 110:147–153
Moussa HR, Abdel-Aziz SM (2008) Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Aust J Crop Sci 1:31–36
Mu P, Liu Q, Zheng RL (2010) Biphasic regulation of H2O2 on angiogenesis implicated NADPH oxidase. Cell Biol Int 34:1013–1020
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Neumann PM (2008) Coping mechanisms for crop plants in drought-prone environments. Ann Bot 101:901–907
Paarlberg R (2002) The real threat to GM crops in poor countries: consumer and policy resistance to GM foods in rich countries. Food Policy 27:247–250
Porter JR (2005) Rising temperatures are likely to reduce crop yields. Nature 436:174
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced response of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Sandoval FJ, Zhang Y, Roje S (2008) Flavin nucleotide metabolism in plants: monofunctional enzymes synthesize FAD in plastids. J Biol Chem 283:30890–30900
Taheri P, Tarighi S (2010) Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J Plant Physiol 167:201–208
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Yamane K, Rahman S, Kawasaki M, Taniguchi M, Miyake H (2004) Pretreatment with a low concentration of methyl viologen decreases the effects of salt stress on chloroplast ultrastructure in rice leaves (Oryza sativa L.). Plant Prod Sci 7:435–441
Zaka R, Vandecasteele C, Misset M (2002) Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata (Poaceae). J Exp Bot 53:1979–1987
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhang S, Yang X, Sun M, Sun F, Deng S, Dong H (2009) Riboflavin-induced priming for pathogen defense in Arabidopsis thaliana. J Integr Plant Biol 51:167–174
Acknowledgments
This work was supported by the Grant of Scientific Research Fund of Heilongjiang Provincial Key Project GA06C101-01 and Scientific Research Fund of Heilongjiang Land Reclamation Bureau Key Project HNKXIV-06-03B, HNKXIVZD-017 and HNK11A-05-10. We also should give our thanks to Prof. Hansong Dong, the Father of riboflavin in China, for his helps in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, B., Jin, X., Yang, Y. et al. The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production. Plant Growth Regul 72, 269–277 (2014). https://doi.org/10.1007/s10725-013-9858-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9858-8