Abstract
Plant height (PH) and flag leaf (FL) are two crucial agronomic traits for crop yield. A recombinant inbred line (RIL) population from ‘Mian 96-5’ (M96-5) and ‘Guixie 3’ (GX3) was investigated in five environments to study PH and FL characteristics in Chinese southwest wheat. Eight QTLs related to PH were detected on five chromosomes 1D (1), 2D (1), 4B (2), 4D (2), and 5A (2). Of these, Qph.gaas.2D explained phenotypic variance (PVE) of 22.40%, Qph.gaas.4B.1 (PVE = 21.92%), Qph.gaas.4D.1 (PVE = 12.29%), and Qph.gaas.4D.2 (PVE = 13.69%), which were consistent with known Rht8, Rht-B1, and Rht-D1 loci, respectively. The other four QTLs about PH, namely Qph.gaas.1D (212.50–258.36 Mb), Qph.gaas.4B.2 (643.50–646.67 Mb), Qph.gaas.5A.1 (515.06–523.07 Mb), and Qph.gaas.5A.2 (523.07–528.26 Mb), were novel QTLs for reduced wheat PH. Five, four, and five QTLs were related to length, width, and area of flag leaf. Among them, a major QTL (Qfll.gaas.2D, 15.79 ~ 37.04 Mb) was detected on chromosome 2D, where six candidate genes related to the photo-respiration stage of photosynthesis were found. Two important regions on chromosome 4B (25.83 ~ 29.20 Mb and 32.96 ~ 35.63 Mb) were related to the length, width and area of flag leaf at multi-environments, and including genes related to programmed cell death and metabolic regulation of gibberellin in the wheat grain-filling stages. These QTLs would be useful in further studies for decreasing plant height, providing theoretical support for the improvement of wheat plant type and boosting grain yield through pyramiding breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common wheat (Triticum aestivum L.) is a vital crop providing a substantial proportion of the consumed calories and protein (Curtis and Halford. 2014; Chen et al. 2016). Wheat production may increase by more than 70% in the next 30 years to meet the needs of the growing population (Bailey-Serres et al. 2019). Genetic improvement of food production will be one of the main goals of wheat breeding programs to ensure global food security (Kumari et al. 2018; Arif et al. 2022).
Plant height (PH) is one of the most vital and intuitive agronomic traits of wheat, influencing plant shape and yield (Zhai et al. 2016). Appropriately reduced PH decreases lodging and increases grain yield (Tian et al. 2017; Arif et al. 2021). Wheat PH is regulated by multiple genes, and the identification of major dwarfing or semi-dwarfing genes is a vital step, enabling plant breeders to modify wheat PH. The introduction of reduced height (Rht) genes into wheat was a major component of the ‘green revolution’ (Hedden 2003). So far, 25 Rht genes have been identified in wheat (Mo et al. 2018; Agarwal et al. 2020; Kroupin et al. 2021). Of these, Rht1 (Rht-B1b) and Rht2 (Rht-D1b) encode for DELLA protein, which exhibits insensitivity to gibberellins (GAs), are located on chromosomes 4BS and 4DS (Peng et al. 1999; Zhang et al. 2012). Rht8 is sensitive to GAs and located on the 2DS chromosome (Worland and Law 1986). Presently, only a few Rht genes such as Rht-B1b (4BS) (Pearce et al. 2011), Rht-D1b (4DS) (Wu et al. 2011), Rht8 (2DL, encode Ribonuclease H-Like 1/RNase H-like protein) (Chai et al. 2022; Xiong et al. 2022), and Rht24 (6AL, encode TaGA2ox-A9 protein) (Tian et al. 2021) have been cloned. These are extensively used in wheat breeding globally (Guan et al. 2018; Hu et al. 2020). Therefore, Rht gene/QTL must be more efficiently explored to decrease PH, understand the genetic mechanism of wheat PH, and ultimately protect food security.
In addition to these major Rht genes, genetic mapping studies have identified several quantitative trait loci (QTLs) for wheat PH (McIntosh et al. 2017). Using a recombinant inbred line (RIL) from the wheat line ‘20,828’/Chuannong 16, three stable QTLs for PH were detected on the 2D, 4B, and 4D chromosomes, and the contribution rate was 1.96–21.22% (Li et al. 2019). Using recombinant inbred line (RIL) population (shanghai3/catbird), three stable QTLs were detected for PH, flag leaf length, and leaf width, respectively. These QTLs could be identified on chromosomes 6A and 2B, with a contribution rate of 8.10–13.93% in multiple environments (Wang et al. 2019).
Flag leaf (FL) of wheat was regarded as an important functional leaf in the late growth stage of wheat, and one of the main photosynthetic organs in the grain filling stage, which can provide 41–43% of carbohydrates (Ma et al. 2020a, b; Wu et al. 2016). Flag leaf size can be measured by flag leaf length (FLL), flag leaf width (FLW), and flag leaf area (FLA), and flag leaf area is a derived trait that can be defined FLA = FLL × FLW × 0.75 (Yang et al. 2016); and FLs are closely related to yield traits such as 1000-kernel weight in wheat (Pérez-Pérez et al. 2010; Muhammad et al. 2021). The research on FL-related traits of wheat is of great significance to the theory of wheat yield breeding.
At present, many studies have been reported to reveal the inheritance of wheat FL morphology in various genetic populations and environments (Arif et al. 2021), QTLs for FL-related traits were detected on nearly all 21 wheat chromosomes (Hussain et al. 2017). SSR (Simple Sequence Repeat) marker technology was mainly used for the mapping of wheat FL-related QTL. Li et al. (2021) used a RIL population of 207 families constructed by Jingdong 8 and Aikang 58, and detected 10 QTLs that control FL traits. Wang et al. (2019) used a RIL population constructed by common wheat Shanghai 3/Catbird and Naxos, detected two QTLs related to FLs in multiple environments. Lu et al. (2018) used a DH population consisting of 216 families constructed by Nongda 3338 and Jingdong 6, detected 40 FL-related QTLs, among them, four QTLs could be detected in multiple contexts. Ma et al. (2020a) used a RIL population to detect FL-related QTLs in 10 different settings, of which five main QTLs were validated in five additional populations. Yan et al. (2020) used two RIL populations (Lumai 14/Jing 411 and Lumai 14/Shaanhan 8675) to detect multiple FL-related QTLs, one of which (QFLW-6A) was able to significantly increase FLW. Farokhzadeh et al. (2019) used a RIL population derived from SeriM82/Babax to detect 11 QTL loci associated with FL traits under aluminum stress. Liu et al. (2018) used the RIL population constructed by common wheat ND3331 and Tibetan semi-wild wheat Zang1817, and detected multiple QTL loci associated with FLs.
With the development of bioinformatics, single nucleotide polymorphism (SNP) array technology such as wheat 9K, 90K, 820K, 660K, and 55K SNP arrays (Li et al. 2020a; Lv et al. 2021), in addition to the genotyping by sequencing (GBS) technology (Akram et al. 2021) has been increasingly used in wheat genetics research. Using wheat 55K SNP array, a QTL (QSEL.sicau-2CN-5A) on the 5AS chromosome was identified to be a new QTL related to the ear extension length (Li et al. 2020b). With genotyping of 207 RILs using the 55K SNP array, two and three QTLs for PH were identified on chromosomes 4B and 6B, respectively (Xiong et al. 2022). Tu et al. (2021) used a 55K SNP chip to detect QTLs associated with FL size and morphology in seven genetic populations with multiple environments, and confirmed two of them (QFll.sau-SY-2B and QFla.sau-SY-2B) may be new loci. Yao et al. (2020) used a RIL population of 120 lines constructed by Xiaoyan 81 and Xinong 1376, combined with the wheat 90K chip, to detect two QTLs that control the FLs length, among them Qfll.nwsuaf-5A.1 showed stable expression in multiple environments. Lian et al. (2016) used the wheat backbone parent Zhou 8425B and the excellent variety Xiaoyan 81 to construct a RIL population, and used the wheat 90K gene chip technology to detect a number of QTLs related to flag leaf traits, and three QTL-enriched segments were detected on 2A, 5A and 6B chromosomes.
The present study performed a phenotypic investigation of 228 RILs from a cross of Mian 96-5 (M96-5) and Guixie 3 (GX3) in five environments. Wheat 55K array was used to harvest genotyping data. The present study attempted to discover a new gene or QTL to reduce PH, provide theoretical support for the ideal plant type, which will be helpful in high yield breeding in the future.
Materials and methods
Plant materials
The dwarf winter landrace line M96-5 and high line GX3 were used as the parental lines for the present study. The mapping population comprised 228 F2:7 RILs from M96-5 × GX3. The GX3 line is a semi-winter, late maturity, long spikelet line of common wheat, and its entire growth period is estimated 210 days. The M96-5 line has large spikelet with good agronomic traits with an estimated growth period of 190 days. The average GX3 and M96-5 PHs were 129.60 and 74.80 cm, respectively. The RIL population procured from the Chengdu Institute of Biology, Chinese Academy of Sciences (Chengdu, Sichuan Province, China).
Field planting and phenotypic investigation
In field trials, the RIL population (M96-5 × GX3) were planted in October 2018 and 2019 in Guiyang, Guizhou Province (2019GY and 2020GY, 26°29′ N, 106°39′ E, altitude 1175 m), in November 2019 in Anshun, Guizhou Province (2020AS, 26°24′ N, 105°96′ E, altitude 1280 m), and in October 2018 and 2019 in Chengdu, Sichuan Province (2019CD and 2020CD, 30°57′ N, 103°92′ E, altitude 498 m).
These materials were planted in two rows per RIL with two replications, 30 seeds per row of one meter, and 10 lines per block. In the 2-year normal wheat growth period, three well-growing and uniform individual plants were selected for each wheat line (excluding marginal effects). The wheat PH of the two parents and 228 RILs were measured at pre-harvest time in a natural setting and recorded in centimeters from the ground to the tip of the spikes, excluding awns. The length and width of the FLs of the parental and population lines were measured at the grain filling stage of wheat, and five plants were randomly selected from each material for measurement, and the average value was taken as the length and width of each line.
DNA extraction and polymerase chain reaction amplification
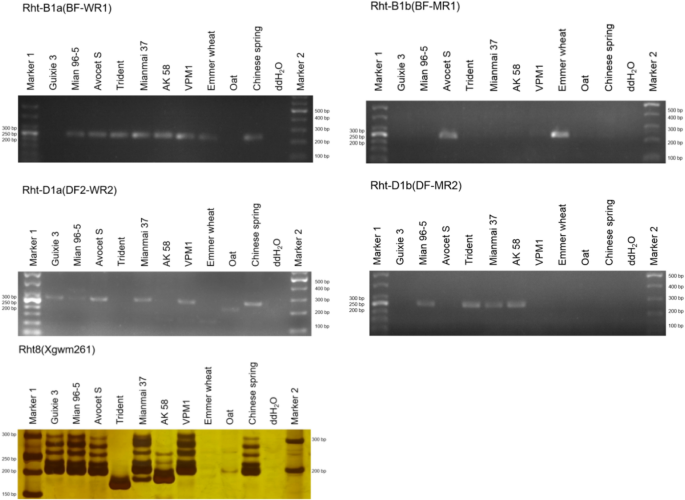
The leaves of the parents and RIL population lines were obtained at the seedling stage, and the DNA of samples was extracted using the plant genomic DNA extraction kit (DP320, Tiangen Biochemical Technology Co., Ltd., Beijing, China). The experiment used a 10 μL polymerase chain reaction (PCR) system, including 1.0 μL of template DNA (50–80 ng μL−1), 1.0 μL of forward and reverse primers (10 μmol μL−1), 5.0 μL of 2 × Taq PCR mix, and 2.0 μL of ddH2O. The PCR reaction program was set as follows: 94 °C pre-denaturation for 5 min; 94 °C denaturation for 40 s; 65.5 °C (Rht-B1a)/64.7 °C (Rht-B1b) /60.0 °C (Rht-D1a)/63.0 °C (Rht-D1b)/55.5 °C (Rht8) annealed for 40 s, extended at 72 °C for 40 s, 35 cycles; extended at 72 °C for 10 min; and stored at 4 °C. The amplified products were detected using 2% agarose gel electrophoresis or silver-stained using 8% non-denaturing polyacrylamide gel electrophoresis (Worland et al. 1998; Ellis et al. 2002) (Table 1).
Genotyping
Genomic DNA was extracted using the cetyl trimethyl ammonium bromide (CTAB) method (Saghai-Maroof et al. 1984). The two parents and the RIL population were genotyped using the 55K SNP array by China Golden Marker (Beijing) Co., Ltd. (Beijing, China). Basic quality control (QC) tests were performed on samples by measuring markers based on genotyping data detection rate, minor allele frequency (MAF), and heterozygosity. The criteria used for sample QC were as follows: DQC > 0.82, detection rate ≥ 85%, and heterozygosity rate ≤ 10%. The criteria for marker QC were as follows: detection rate ≥ 95%, MAF ≥ 5%, heterozygosity rate ≤ 50%, and the number of alleles being 2.
QTL mapping
IciMapping v4.1 software and inclusive composite interval mapping (ICIM) were used for QTL detection. The chromosome step size was 0.1 cM, whereas the stepwise regression probability was P < 0.001. The QTL with a logarithm of odds (LOD) score > 2.5 was detected, and the contribution rate and additive effect of each QTL were calculated. TBtools was used to draw the QTL map (Chen et al. 2022).
Statistical and bioinformatics analyzes
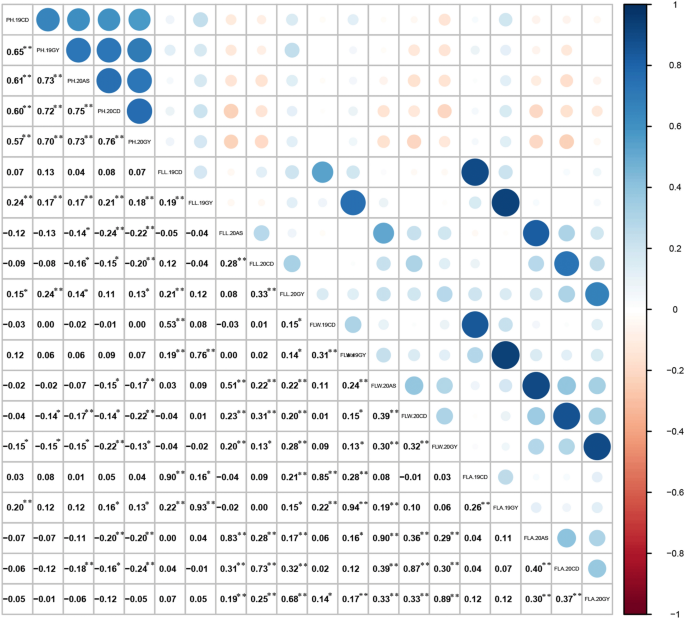
The environments and line × environment interactions were compared using the AOV function in IciMapping v4.1 software (Zeng et al. 2019) to estimate the genetic and environmental effects in each line. The significance was measured using an analysis of variance (ANOVA). The correlation between multiple field conditions was analyzed using the Pearson method in SPSS v20 software. The normal distribution was plotted with Origin 2019b, h = VG/VG + VE (h stands for broad sense heritability, VG stands for genetic variation, VE stands for environmental variation).
Gene annotation information was conveniently obtained from the Triticeae Multi-omics Center (http://202.194.139.32) (Ma et al. 2021), in which genome, transcriptome, proteome, and epigenome dataset resources are provided for common wheat and relatives, including useful tools such as the basic local alignment search tool (BLAST), sequence extraction, and molecular markers and primers (Sun et al. 2020). Based on the alignment results in the Triticeae Multi‐omics Center, unique probes with reliable physical positions in the 55K SNP array were extracted. Using a web platform (Triticeae Gene Tribe, http://wheat.cau.edu.cn/TGT/) to conduct online GO analysis (Chen et al. 2020).
Results
Phenotype
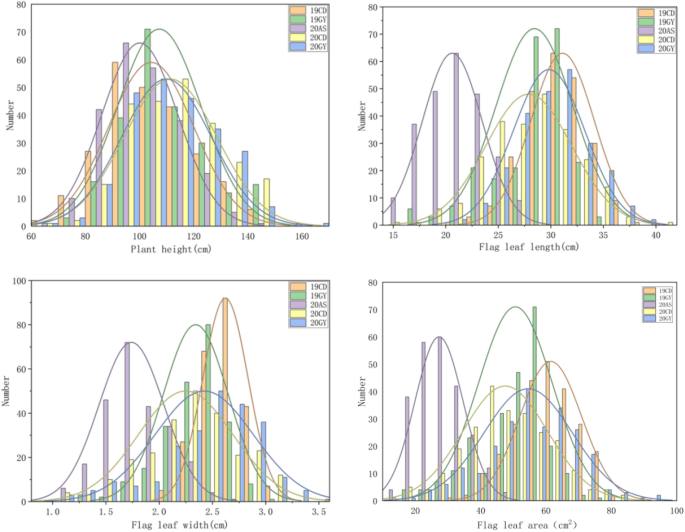
The PH and FL of the 228 RILs did not exhibit significant genetic variation in field trials in five different environments. In each environment, the PH and FL presented continuous normal distribution (Fig. 1, Table 2), indicating that PH and FL were quantitative trait controlled by polygenes and suitable for QTL analysis. Generalized heritability of 0.73 for FLL, 0.64 for FLW, 0.69 for FLA and 0.91 for PH was calculated in the experiments in five environments, the maximum Pearson correlation coefficient between FLL and FLW was 0.75, between FLW and FLA was 0.93, between FLW and FLA was 0.94 (p < 0.01) (Fig. 2).
Frequency distributions of plant height (PH), flag leaf length (FLL), flag leaf width (FLW) and flag leaf area (FLA) in the RIL population derived from a cross between M96-5 and GX3 across five environments. 19CD, 2019 Chengdu; 19GY, 2019 Guiyang; 20AS, 2020 Anshun; 20CD, 2020 Chengdu; 20GY, 2020 Guiyang
QTL mapping
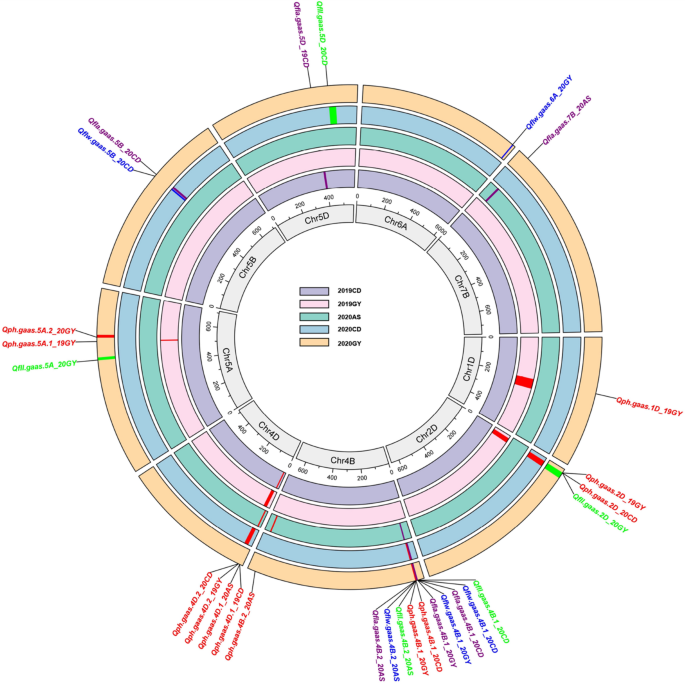
Using ICIM method, a total of 22 QTLs of wheat PH and FL were detected in multiple environments, located on chromosomes 1D (1), 2D (2), 4B (8), 4D (2), 5A (3), 5B (2), 5D (2), 6A (1) and 7B (1), among them, eight, five, four, and five QTLs were associated with PH, FLL, FLW and FLA, respectively.
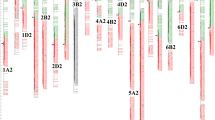
Eight PH QTLs were mapped on chromosomes 1D (1), 2D (1), 4B (2), 4D (2), and 5A (2). Four QTLs were detected in two environments and located on chromosomes 2D, 4B, and 4D. On chromosome 2D, a vital QTL (Qph.gaas.2D) was at a confidence interval (CI) of 20 cM in the 2019GY and 2020CD environments with the highest LOD value of 11.56, explaining the maximum phenotype variation of 15.62%. On chromosome 4B, a QTL (Qph.gaas.4B.2) was at a CI of 1 cM in the 2020CD and 2020GY environments with the highest LOD value of 17.74, explaining the maximum phenotype variation of 22.14%. On chromosome 4D, one QTL (Qph.gaas.4D.1) was detected at a CI of 3 cM in the 2019CD and 2020AS environments with the highest LOD value of 12.21, explaining the maximum phenotype variation of 21.04%. Another QTL (Qph.gaas.4D.2) was located at a CI of AX-89398511 ~ AX-94547815 (7 cM) at the 2019GY and 2020CD sites, with the highest LOD value of 15.39, explaining the maximum phenotype variation of 12.80%. The remaining four QTLs (Qph.gaas.1D, Qph.gaas.4B.1, Qph.gaas.5A.1, and Qph.gaas.5A.2) were only detected in one environment. A QTL (Qph.gaas.4B.1) with the negative additive effect of reduced PH was derived from GX3, whereas the remaining QTLs with the positive additive effect were derived from the parent M96-5 (Fig. 3, Table 3).
Graphical displays of genetic positions of 22 QTLs for plant height and flag leaf relative traits across five environments for the M96-5 × GX3 population. Different colors represent different traits. E.g., red for plant height, green for flag leaf length, deep blue for flag leaf width, and purple for flag leaf area. The different environments were exhibited in order from the inner circle to the outer circle, the innermost being 2019CD, 2019GY, 2020AS, 2020CD, and the outermost 2020GY, respectively
Five QTLs associated with FLL were located on chromosomes 2D (1), 4B (2), 5A (1) and 5D (1). Among them, Qfll.gaas.2D had the largest LOD value (11.26) in the 2020GY environment, and the largest explainable phenotypic variation rate was 18.41%. The additive effects of the three QTLs located on chromosomes 2D and 4B were derived from the parental M96-5, while the two QTLs located on 5A and 5D were derived from the parental GX3.
Four QTLs associated with FLW were located on chromosomes 4B (2), 5B (1) and 6A (1). Among them, an important QTL (Qflw.gaas.4B.1) located on 25.83 ~ 29.20 Mb of 4B was detected in two environments (2020CD and 2020GY) with the highest LOD value of 13.84 and the largest explainable phenotypic variation rate was 22.45%. The additive effects of all QTLs were derived from the parental M96-5.
Five QTLs associated with FLA were located on chromosomes 4B (2), 5B (1), 5D (1) and 7B (1). Consistent with the flag leaf width Qflw.gaas.4B.1, an important QTL (Qfla.gaas.4B.1) located at the same position on chromosome 4B was detected in both 2020CD and 2020GY environments, with the highest LOD value of 15.74 and the largest explainable phenotypic variation rate was 22.90%. The additive effect of three QTLs located on chromosomes 4B and 5B came from M96-5, and the remaining two QTLs came from GX3.
In addition, two important segments located on chromosome 4B were detected in multiple environments and multiple traits. The QTLs associated with FFL (Qfla.gaas.4B.1), FLW (Qflw.gaas.4B.1) and FLA (Qfla.gaas.4B.1) were clustered in chromosome segments 25.83 ~ 29.20 Mb, and QTLs associated with FLL (Qfla.gaas.4B.2), FLW (Qflw.gaas.4B.2), and FLA (Qfla.gaas.4B.2) were clustered in chromosomal segments 32.96–35.63 Mb (Fig. 3, Table 3).
Candidate gene prediction analysis
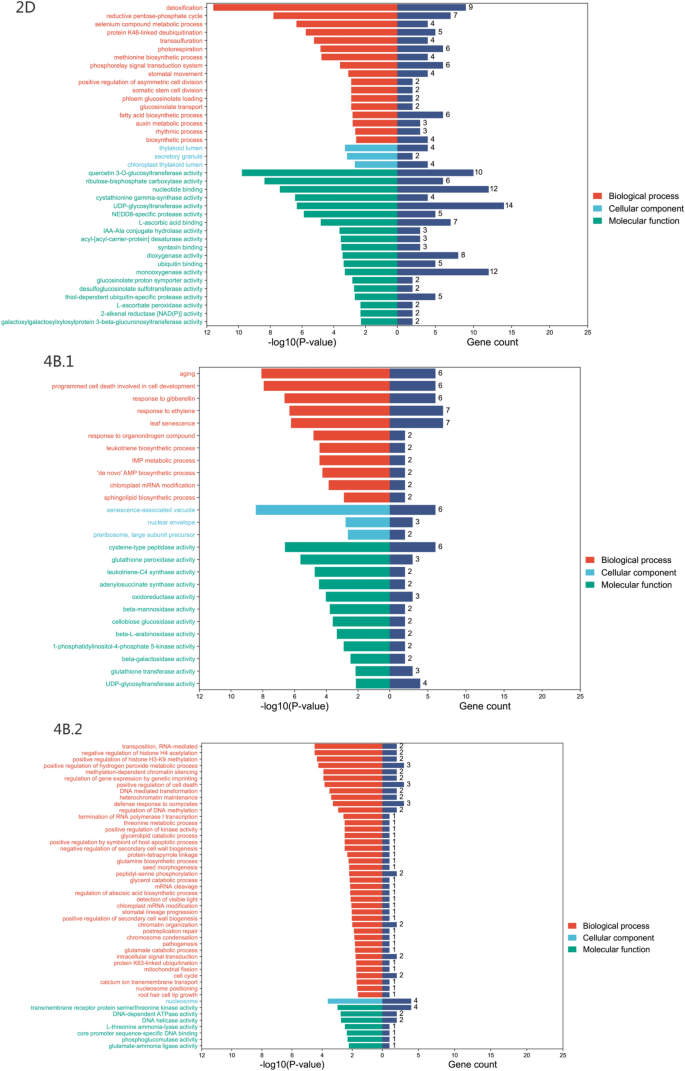
To further investigate the genes underlying important QTL loci, we performed candidate gene prediction. GO analysis was performed on the genes in the physical interval of the 2D QTL (Qfll.gaas.2D/Qph.gaas.2D), and seven genes (TraesCS2D02G065100, TraesCS2D02G065200, TraesCS2D02G065300, TraesCS2D02G065400, TraesCS2D02G065500, TraesCS2D02G065600, TraesCS2D02G073900), the first six genes were all related to the photorespiration stage of plant photosynthesis.
The 25.83 ~ 29.20 Mb segment of chromosome 4B.1 contains six genes (TraesCS4B02G039500, TraesCS4B02G039600, TraesCS4B02G039700, TraesCS4B02G039800, TraesCS4B02G039900, TraesCS4B02G040000), which are not only related to the process of leaf senescence, but also involved in the metabolism of gibberellin and developmental programmed cell death. Two genes (TraesCS4B02G051400 and TraesCS4B02G053600) located in another important segment (32.96–35.63 Mb) of chromosome 4B.2 are related to plant growth and development, and are involved in RNA-mediated transposition, negative regulation of histones, and methylation-dependent metabolic pathways such as chromatin silencing (Fig. 4, Table S1).
Analysis of known Rht genes
Additionally, the two parental lines were scanned using the Rht-linked markers to investigate the relationship between the eight QTLs and known Rht genes. M96-5 contained the Rht-B1a (Rht1 mutant type), Rht-D1b (Rht2 wild type), and Rht8 (210 bp) target fragments, whereas GX3 contained the Rht-D1a (Rht2 mutant type) and Rht8 (210 bp) target fragments (Fig. 5).
Discussion
Phenotype
Wheat PH is affected by several factors with a close relationship of yield and quality, roughly divided into endogenous hormones and the external environment. Endogenous hormones generally refer to substances such as GAs, cytokinins, auxins, and abscisic acid, which participate in the entire plant life cycle and regulate PH (Ford et al. 2018). The present study analyzed the PH phenotype data of wheat grown in different environments and exhibited a higher correlation coefficient of wheat PH in different environments, indicating that PH has a high heritability. FL of wheat is also a typical quantitative trait, controlled by multiple genes, and the QTLs detected in different genetic backgrounds and environments are quite different (Coleman et al. 2001; Tsukaya 2005; Jin et al. 2020). At present, there have been many reports of QTL research on FL-related traits, but there are problems such as a large range of located markers and unclear physical locations, which are difficult to use in breeding practice. In this study, the genetic map was constructed by the wheat 55K SNP chip, the physical location can be clearly known, which is convenient for subsequent marker development and utilization and breeding.
QTL mapping compared with previous Rht genes
Presently, numerous high-quality QTLs related to wheat PH have been mapped (McIntosh et al. 2017). The present study identified eight QTLs for PH, of which four QTLs detected in multiple environments were located on chromosomes 2D, 4B, and 4D. Using a combination of the physical location methods and known markers of genes or QTLs, the major QTLs were compared with those of other studies.
A major QTL (Qph.gaas.2D) was located in the interval of 15.79–37.04 Mb on chromosome 2D, overlapping with the reported Rht8 (TraesCSU02G024900, 24 Mb) locus (Chai et al. 2022). Rht8/RNHL-D1 loss is responsible for the semi-dwarf trait in Rht8-carrying wheat plants. Another study demonstrated that Rht8 encodes a protein containing a zinc finger BED-type motif [a protein domain that was named after the Drosophila BEAF (Boundary Element-associated factor) protein, thought to be involved in chromatin insulation and the Drosophila DREF protein, a transcriptional regulator for S-phase genes] and an RNase H-like domain (RNHL-D1) that regulates PH by influencing bioactive GA biosynthesis (Xiong et al. 2022).
Two major QTLs were identified on chromosome 4B. Qph.gaas.4B.1 was located in the interval of 31.88–32.96 Mb, which was close to Rht-B1 (TraesCS4B02G043100, 30.86–30.86 Mb) (Peng et al. 1999). Its tightly linked marker scan exhibited that M96-5 contained Rht-B1, indicating Qph.gaas.4B.1 is consistent with the Rht-B1 gene. Qph.gaas.4B.2 was located in the interval of 643.50–646.67 Mb, which is inconsistent with the physical map position of two PH genes, TaSRL1-4B (TraesCS4B02G299400, 585.821–585.823 Mb), encoding a novel ethylene-responsive factor (Zhuang et al. 2021) and TaPRR73-4B (TraesCS4B02G198700, 427.490–427.496 Mb), being a paralog of “Green Revolution” gene Ppd1 (Zhang et al. 2016), but Qph.gaas.4B.2 was consistent with the loci Q.Ht_GFS99-4B.1 (643.79–649.47 Mb) and Q.Ht_Pu06-4B (643.65–645.30 Mb) (Arif et al. 2021).
Additionally, two major QTLs were identified in the proximal region of chromosome 4D. Qph.gaas.4D.1 was located in the interval of 11.87–14.43 Mb, whereas Qph.gaas.4D.2 was located in the interval of 14.99–17.08 Mb. The two QTLs were located close to Rht-D1 (TraesCS4D02G040400, 18.781–18.782 Mb) (Peng et al. 1999). Marker scanning results exhibited that they were identical at the Rht-D1 locus.
Similarly, two QTLs [Qph.gaas.5A.2 (523.07–528.26 Mb) and Qph.gaas.5A.1 (515.06–523.07 Mb)] were observed in the proximal region of chromosome 5AL. A dominant GA responsive dwarfing gene Rht12 (TraesCS5A02G543100) was mapped to the interval of 698.888–698.889 Mb, the distal region of chromosome 5AL (Sun et al. 2019), which is quite a physical distance from Qph.gaas.5A.1 and Qph.gaas.5A.2.
Analysis with reported flag leaf QTLs
The Qfll.gaas.2D (15.79 ~ 37.04 Mb) was different from the Qfll-2D (~ 44.73 Mb) detected by Zhao et al. (2015). Ma et al. (2020a) detected a QTL of sicau-2D.3/QFlw.sicau-2D/QFla.sicau-2D (35.02 ~ 38.54 Mb) overlapped the positioning interval of Qfll.gaas.2D, indicating that it may be the same QTL, and further verification and analysis are needed. The GO analysis of the candidate genes at the Qfll.gaas.2D locus found that six genes were involved in important biological pathways such as photorespiration, indicating that the flag leaf of wheat is indeed an important functional leaf during the grain filling period of wheat, reducing the carbon consumption in the photorespiration process. It can indirectly increase the accumulation of assimilates, and the potential yield (Zhou et al. 2020). The Qfll.gaas.5D (457.27–483.12 Mb) had overlapped physical interval with Q.Fll_Pu07-5D (479.88–542.45 Mb) (Arif et al. 2021).
At the same time, two important chromosome segments (25.83–29.20 Mb, 32.96–35.63 Mb) related to the FL (length, width and area) were identified on chromosome 4B, which were detected on chromosome 4B with the physical locations of QFLA-4B.1 (15.76 ~ 75.73 Mb) overlap Liu et al. (2018), and may be the same locus, which needs further analysis and verification. GO analysis of genes in the above-mentioned QTL clustering region showed that there are six genes that may cause programmed cell death in the later stage of wheat growth and development (Locato and Gara 2018), resulting in the withering of wheat leaves, which in turn produces wheat after maturity. These factors may be an important link in the control of wheat yield on the FL as the “source” (Chen and Zhan 2021).
The above studies have shown that there is a certain correlation between the length, width and area of FLs, and the genes that control FL-related traits tend to form gene aggregation regions, and then there is a situation of one cause and multiple effects, which makes FLs in the later stage of wheat growth and development, plays a key role in the grain filling of wheat grains and indirectly affects the yield formation of wheat.
Conclusion
Using a RIL population of M96-5 × GX3 based on a wheat 55K SNP array, eight QTLs were detected for PH. Of these, four QTLs, namely Qph.gaas.2D, Qph.gaas.4B.1, Qph.gaas.4D.1, and Qph.gaas.4D.2, were consistent with known Rht8, Rht-B1, and Rht-D1 loci. A major QTL clustered regions (Qfll.gaas.2D/Qph.gaas.2D, 15.79 ~ 37.04 Mb) was detected on chromosome 2D, where six candidate genes related to the photo-respiration stage of photosynthesis were found. Two important regions on chromosome 4B (25.83 ~ 29.20 Mb and 32.96 ~ 35.63 Mb) were related to the length, width and area of flag leaf at multi-environments, and including genes related to programmed cell death and metabolic regulation in the wheat grain-filling stages. Our results would be useful for molecular marker-assisted breeding and identification of candidate genes for plant height and flag leaf traits in wheat.
References
Agarwal P, Balyan HS, Gupta PK (2020) Identification of modifiers of the plant height in wheat using an induced dwarf mutant controlled by RhtB4c allele. Physiol Mol Biol Plants 26(11):2283–2289
Akram S, Arif MAR, Hameed A (2021) A GBS-based GWAS analysis of adaptability and yield traits in bread wheat (Triticum aestivum L.). J Appl Genet 62(1):27–41
Arif MAR, Shokat S, Plieske J, Ganal M, Lohwasser U, Chesnokov YV, Kocherina NV, Kulwal P, Kumar N, McGuire PE, Sorrells ME, Qualset CO, Börner A (2021) A SNP-based genetic dissection of versatile traits in bread wheat (Triticum aestivum L.). Plant J 108(4):960–976
Arif MAR, Waheed MQ, Lohwasser U, Shokat S, Alqudah AM, Volkmar C, Börner A (2022) Genetic insight into the insect resistance in bread wheat exploiting the untapped natural diversity. Front Genet 13:828905
Bailey-Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI (2019) Genetic strategies for improving crop yields. Nature 575(7781):109–118
Chai L, Xin M, Dong C, Chen Z, Zhai H, Zhuang J, Cheng X, Wang N, Geng J, Wang X, Bian R, Yao Y, Guo W, Hu Z, Peng H, Bai G, Sun Q, Su Z, Liu J, Ni Z (2022) A natural variation in Ribonuclease H-like gene underlies Rht8 to confer “Green Revolution” trait in wheat. Mol Plant. https://doi.org/10.1016/j.molp.2022.01.013
Chen QC, Zhan Y (2021) Regulation of source-to-sink transport of plant photosynthetic products and its response to drought. J Shanxi Agric Sci 49(12):1367–1375 (in Chinese with English abstract)
Chen C, He Z, Lu J, Li J, Ren Y, Ma C, Xia X (2016) Molecular mapping of stripe rust resistance gene YrJ22 in Chinese wheat cultivar Jimai 22. Mol Breed 36:118
Chen Y, Song W, Xie X, Wang Z, Guan P, Peng H, Jiao Y, Ni Z, Sun Q, Guo W (2020) A collinearity-incorporating homology inference strategy for connecting emerging assemblies in the triticeae tribe as a pilot practice in the plant pangenomic era. Mol Plant 13(12):1694–1708
Chen CJ, Wu Y, Xia R (2022) A painless way to customize circos plot: from data preparation to visualization using TBtools. iMeta 1:e35. https://doi.org/10.1002/imt2.35
Coleman RK, Gill GS, Rebetzke GJ (2001) Identification of quantitative trait loci for traits conferring weed competitiveness in wheat (Triticum aestivum L.). Aust J Agric Res 52(12):1235–1246
Curtis T, Halford NG (2014) Food security: the challenge of increasing wheat yield and the importance of not compromising food safety. Ann Appl Biol 164(3):354–372
Ellis H, Spielmeyer W, Gale R, Rebetzke J, Richards A (2002) “Perfect” markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor Appl Genet 105(6–7):1038–1042
Farokhzadeh S, Fakheri BA, Nezhad NM, Tahmasebi S, Mirsoleimani A (2019) Mapping QTLs of flag leaf morphological and physiological traits related to aluminum tolerance in wheat (Triticum aestivum L.). Physiol Mol Biol Plants 25(4):975–990
Ford BA, Foo E, Sharwood R, Karafiatova M, Vrána J, MacMillan C, Nichols DS, Steuernagel B, Uauy C, Doležel J, Chandler PM, Spielmeyer W (2018) Rht18 semi-dwarfism in wheat is due to increased GA2-oxidaseA9 expression and reduced GA content. Plant Physiol 177(1):168–180
Guan P, Lu L, Jia L, Kabir MR, Zhang J, Lan T, Zhao Y, Xin M, Hu Z, Yao Y, Ni Z, Sun Q, Peng H (2018) Global QTL analysis identifies genomic regions on chromosomes 4A and 4B harboring stable loci for yield-related traits across different environments in wheat (Triticum aestivum L.). Front Plant Sci 9:529
Hedden P (2003) The genes of the green revolution. Trends Genet 19(1):5–9
Hu J, Wang X, Zhang G, Jiang P, Chen W, Hao Y, Ma X, Xu S, Jia J, Kong L, Wang H (2020) QTL mapping for yield-related traits in wheat based on four RIL populations. Theor Appl Genet 133(3):917–933
Hussain W, Baenziger PS, Belamkar V, Guttieri MJ, Venegas JP, Easterly A, Sallam A, Poland J (2017) Genotyping-by-sequencing derived high-density linkage map and its application to QTL mapping of flag leaf traits in bread wheat. Sci Rep 7(1):16394
Jin J, Liu D, Qi Y, Ma J, Zhen W (2020) Major QTL for seven yield-related traits in common wheat (Triticum aestivum L.). Front Genet 11:1012
Kroupin PY, Karlov GI, Bespalova LA, Salina EA, Chernook AG, Watanabe N, Bazhenov MS, Panchenko VV, Nazarova LA, Kovtunenko VY, Divashuk MG (2021) Effects of Rht17 in combination with Vrn-B1 and Ppd-D1 alleles on agronomic traits in wheat in black earth and non-black earth regions. BMC Plant Biol 20(Suppl 1):304
Kumari S, Jaiswal V, Mishra VK, Paliwal R, Balyan HS, Gupta PK (2018) QTL mapping for some grain traits in bread wheat (Triticum aestivum L.). Physiol Mol Biol Plants 24(5):909–920
Li C, Ma J, Liu H, Ding PY, Yang CC, Zhang H, Qin NN, Lan XJ (2019) Detection of QTLs for spike length and pant height in wheat based on 55K SNP array. J Triticeae Crops 39(11):1284–1292 (in Chinese with English abstract)
Li C, Tang H, Luo W, Zhang X, Mu Y, Deng M, Liu Y, Jiang Q, Chen G, Wang J, Qi P, Pu Z, Jiang Y, Wei Y, Zheng Y, Lan X, Ma J (2020a) A novel, validated, and plant height-independent QTL for spike extension length is associated with yield-related traits in wheat. Theor Appl Genet 133(12):3381–3393
Li X, Xu X, Liu W, Li X, Yang X, Ru Z, Li L (2020b) Dissection of superior alleles for yield-related traits and their distribution in important cultivars of wheat by association mapping. Front Plant Sci 11:175
Li XM, Wang SY, Ni SL (2021) QTL mapping for traits of flag leaf and seedling in wheat. J Triticeae Crops 41(5):532–537 (in Chinese with English abstract)
Lian JF, Zhang DQ, Wu BJ, Song XP, Ma WJ, Zhou LM, Feng Y, Sun DJ (2016) QTL mapping of flag leaf traits using an integrated high-density 90K genotyping chip. J Triticeae Crops 36(6):689–698 (in Chinese with English abstract)
Liu K, Xu H, Liu G, Guan P, Zhou X, Peng H, Yao Y, Ni Z, Sun Q, Du J (2018) QTL mapping of flag leaf-related traits in wheat (Triticum aestivum L.). Theor Appl Genet 131(4):839–849
Locato V, Gara LD (2018) Programmed cell death in plants: an overview. Methods Mol Biol 1743:1–8
Lu LH, Yang B, Zhang T, Zhang W, Yuan K, Shi XF, Peng HR, Ni ZF, Sun QX (2018) Quantitative trait loci analysis of flag leaf size and grain relative traits in winter wheat. Huabei Nong Xuebao (acta Agriculturae Boreali-Simica) 33(5):1–8 (in Chinese with English abstract)
Lv D, Zhang C, Yv R, Yao J, Wu J, Song X, Jian J, Song P, Zhang Z, Han D, Sun D (2021) Utilization of a Wheat 50K SNP microarray-derived high-density genetic map for QTL mapping of plant height and grain traits in wheat. Plants (basel) 10(6):1167
Ma J, Tu Y, Zhu J, Luo W, Liu H, Li C, Li S, Liu J, Ding P, Habib A, Mu Y, Tang H, Liu Y, Jiang Q, Chen G, Wang J, Li W, Pu Z, Zheng Y, Wei Y, Kang H, Chen G, Lan X (2020a) Flag leaf size and posture of bread wheat: genetic dissection, QTL validation and their relationships with yield-related traits. Theor Appl Genet 133(1):297–315
Ma RQ, Tao ZQ, Wang DM, Wang YJ, Yang YS, Xu ZL, Zhao GC, Chang XH (2020b) Effects of topdressing nitrogen rate on photosynthetic characteristics and yield of flag leaves of wheat in different regions. He Nong Xuebao (j Nucl Agric Sci) 34(6):1281–1293 (in Chinese with English abstract)
Ma S, Wang M, Wu J, Guo W, Chen Y, Li G, Wang Y, Shi W, Xia G, Fu D, Kang Z, Ni F (2021) WheatOmics: A platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol Plant 14(12):1965–1968
McIntosh RA, Dubcovsky J, Rogers WJ, Morris C, Xia XC (2017) Catalogue of gene symbols for wheat: 2017 supplement. Downloaded from http://shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp on 02. Feb. 2017.
Mo Y, Vanzetti LS, Hale I, Spagnolo EJ, Guidobaldi F, Al-Oboudi J, Odle N, Pearce S, Helguera M, Dubcovsky J (2018) Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theor Appl Genet 131(10):2021–2035
Muhammad A, Li J, Hu W, Yu J, Khan SU, Khan MHU, Xie G, Wang J, Wang L (2021) Uncovering genomic regions controlling plant architectural traits in hexaploid wheat using different GWAS models. Sci Rep 11(1):6767
Pearce S, Saville R, Vaughan SP, Chandler PM, Wilhelm EP, Sparks CA, Al-Kaff N, Korolev A, Boulton MI, Phillips AL, Hedden P, Nicholson P, Thomas SG (2011) Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat. Plant Physiol 157(4):1820–1831
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) “Green revolution” genes encode mutant gibberellin response modulators. Nature 400(6741):256–261
Pérez-Pérez JM, Esteve-Bruna D, Micol JL (2010) QTL analysis of leaf architecture. J Plant Res 123(1):15–23
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81(24):8014–8018
Sun L, Yang W, Li Y, Shan Q, Ye X, Wang D, Yu K, Lu W, Xin P, Pei Z, Guo X, Liu D, Sun J, Zhan K, Chu J, Zhang A (2019) A wheat dominant dwarfing line with Rht12, which reduces stem cell length and affects gibberellic acid synthesis, is a 5AL terminal deletion line. Plant J 97(5):887–900
Sun C, Dong Z, Zhao L, Ren Y, Zhang N, Chen F (2020) The Wheat 660K SNP array demonstrates great potential for marker-assisted selection in polyploid wheat. Plant Biotechnol J 18(6):1354–1360
Tian X, Wen W, Xie L, Fu L, Xu D, Fu C, Wang D, Chen X, Xia X, Chen Q, He Z, Cao S (2017) Molecular mapping of reduced plant height gene Rht24 in bread wheat. Front Plant Sci 8:1379
Tian X, Xia X, Xu D, Liu Y, Xie L, Hassan MA, Song J, Li F, Wang D, Zhang Y, Hao Y, Li G, Chu C, He Z, Cao S (2021) Rht24b, an ancient variation of TaGA2ox-A9, reduces plant height without yield penalty in wheat. New Phytol 233(2):738–750
Tsukaya H (2005) Leaf shape: genetic controls and environmental factors. Int J Dev Biol 49(5–6):547–555
Tu Y, Liu H, Liu J, Tang H, Mu Y, Deng M, Jiang Q, Liu Y, Chen G, Wang J, Qi P, Pu Z, Chen G, Peng Y, Jiang Y, Xu Q, Kang H, Lan X, Wei Y, Zheng Y, Ma J (2021) QTL mapping and validation of bread wheat flag leaf morphology across multiple environments in different genetic backgrounds. Theor Appl Genet 134(1):261–278
Wang Y, Zhao L, Dong ZD, Ren Y, Zhang N, Chen F (2019) QTL mapping for plant height and flag leaf traits in common wheat. J Triticeae Crops 39(7):761–767 (in Chinese with English abstract)
Worland AJ, Law CN (1986) Genetic analysis of chromosome 2D of wheat I. The location of genes affecting height, day-length insensitivity, hybrid dwarfism and yellow-rust resistance. J Plant Breed 96:331–345
Worland AJ, Korzun V, Röder MS, Ganal MW, Law CN (1998) Genetic analysis of the dwarfing gene Rht8 in wheat. Part II. The distribution and adaptive significance of allelic variants at the Rht8 locus of wheat as revealed by microsatellite screening. Theor Appl Genet 96:1110–1120
Wu J, Kong X, Wan J, Liu X, Zhang X, Guo X, Zhou R, Zhao G, Jing R, Fu X, Jia J (2011) Dominant and pleiotropic effects of a GAI gene in wheat results from a lack of interaction between DELLA and GID1. Plant Physiol 157(4):2120–2130
Wu QH, Chen YX, Fu L, Zhou SH, Chen JJ, Zhao XJ, Zhang D, Ouyang SH, Wang ZZ, Li D, Wang GX, Zhang DY, Yuan CG, Wang LX, You MS, Han J, Liu ZY (2016) QTL mapping of flag leaf traits in common wheat using an integrated high-density SSR and SNP genetic linkage map. Euphytica 208:337–351
Xiong H, Zhou C, Fu M, Guo H, Xie Y, Zhao L, Gu J, Zhao S, Ding Y, Li Y, Zhang J, Wang K, Li X, Liu L (2022) Cloning and functional characterization of Rht8, a “green revolution” replacement gene in Wheat. Mol Plant. https://doi.org/10.1016/j.molp.2022.01.014
Yan X, Wang S, Yang B, Zhang W, Cao Y, Shi Y, Sun D, Jing R (2020) QTL mapping for flag leaf-related traits and genetic effect of QFLW-6A on flag leaf width using two related introgression line populations in wheat. PLoS ONE 15(3):e0229912
Yang D, Liu Y, Cheng H, Chang L, Chen J, Chai S, Li M (2016) Genetic dissection of flag leaf morphology in wheat (Triticum aestivum L) under diverse water regimes. BMC Genet 17(1):94
Yao JX, Zhang CL, Song XP, Xu XW, Xing YF, Lu DY, Song PB, Yang MY, Sun DJ (2020) QTL analysis of wheat spike length and flag leaf length based on 90K SNP assay. J Triticeae Crops 40(11):1283–1289 (in Chinese with English abstract)
Zeng Q, Wu J, Liu S, Huang S, Wang Q, Mu J, Yu S, Han D, Kang Z (2019) A major QTL co-localized on chromosome 6BL and its epistatic interaction for enhanced wheat stripe rust resistance. Theor Appl Genet 132(5):1409–1424
Zhai H, Feng Z, Li J, Liu X, Xiao S, Ni Z, Sun Q (2016) QTL analysis of spike morphological traits and plant height in winter wheat (Triticum aestivum L.) Using a high-density SNP and SSR-based linkage map. Front Plant Sci 7:1617
Zhang L, Zhao YL, Gao LF, Zhao GY, Zhou RH, Zhang BS, Jia JZ (2012) TaCKX6-D1, the ortholog of rice OsCKX2, is associated with grain weight in hexaploid wheat. New Phytol 195(3):574–584
Zhang W, Zhao G, Gao L, Kong X, Guo Z, Wu B, Jia J (2016) Functional studies of heading date-related gene TaPRR73, a paralog of Ppd1 in common wheat. Front Plant Sci 7:772
Zhao P, Xu F, Jiang WH, Qi P, Li CL, Bai HB, Lv XL, Dong JL, Wang ZH (2015) Quantitative trait loci analysis of flag leaf length, width and chlorophyll content of spring wheat. J Triticeae Crops 35(5):603–608 (in Chinese with English abstract)
Zhou TJ, Ding XH, Wang JH (2020) Strategies for modulation and optimization of the photorespiration pathway in plants. Zhejiangdaxue Xuebao (j Zhejiang Univ) 46(3):271–279 (in Chinese with English abstract)
Zhuang M, Li C, Wang J, Mao X, Li L, Yin J, Du Y, Wang X, Jing R (2021) The wheat SHORT ROOT LENGTH 1 gene TaSRL1 controls root length in an auxin-dependent pathway. J Exp Bot 72(20):6977–6989
Funding
The current work was funded by grants from the Natural Science Foundation of Guizhou Province (QKHJC-ZK[2022]YB 234), Innovation Capacity Building Project of Guizhou Scientific Institutions (QKFQ[2022]007), Guizhou Academy of Agricultural Sciences Project (Guizhou Agricultural Germplasm Resources (2023) 06), the Construction of Genetic Transformation Platform for Dryland Grain Crops in Guizhou Province (QKZYD[2022]4011), the Natural Science Foundation of Guizhou Province (QKHJC-ZK[2023]YB 170), the Youth Science Foundation of Guizhou Academy of Agricultural Sciences ([2023]23), the Post-National Natural Science Foundation Subsidy from Guizhou Academy of Agricultural Sciences [2021]06, and National Wheat Industry Technology System Guiyang Comprehensive Experimental Station (CARS-3).
Author information
Authors and Affiliations
Contributions
BC analyzed data and wrote the manuscript. BC, XG, YD, and NC scanned the molecular markers. YL, TC, and JX performed the phenotype investigation. LZ and ZX conceived, designed the experiments, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Ethical approval
I declare on behalf of my co-authors that the work described is original, previously unpublished research, and not under consideration for publication elsewhere. The experiments in this study comply with the current laws of China.
Additional information
Communicated by Mian Abdur Rehman Arif.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, B., Gao, X., Luo, Y. et al. Utilization of wheat 55K SNP array for QTL mapping of plant height and flag leaf in a RIL population. CEREAL RESEARCH COMMUNICATIONS (2023). https://doi.org/10.1007/s42976-023-00475-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42976-023-00475-2