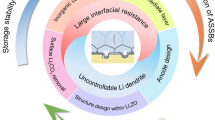
Abstract
Enormous research focusing on solid-state electrolyte promotes the development of solid-state batteries. Compared to lithium-ion batteries using liquid electrolyte, the solid-state batteries feature the high energy density and non-flammability, which accelerates the revolution in portable electronics and transportation. Garnet-type Li7La3Zr2O12 (LLZO) solid-state electrolyte is considered as the promising solid-state electrolyte due to high ionic conductivity, Li transference number and shear modulus. However, surface contaminant and poor contact with lithium inhibit its practical application in lithium metal batteries. The review provides a brief introduction about structure and properties of LLZO. Then, we conclude the modification strategies for increasing ionic conductivity, enhancing interfacial contact and inhibiting lithium dendrite. At last, the challenge and perspectives are discussed for development of LLZO in solid-state batteries.

Reproduced with permission from Ref. [16] Copyright 2012 American Physical Society

Reproduced with permission from Ref. [32] Copyright 2020 Wiley

Reproduced with permission from Ref. [33] Copyright 2020 Springer Nature

Reproduced with permission from Ref. [62]. Copyright 2017 Wiley. b Illustration for synthesis of Li3N-coated LLZTO, and the SEM images of Li2CO3-coated LLZTO and Li3N-coated LLZTO. Reproduced with permission from Ref. [63]. Copyright 2018 ACS. c The mechanism of Li3OCl for better contact between Li metal and LLZTO. Reproduced with permission from Ref. [64]. Copyright 2018 Elsevier. d Schematic illustration for poor contact between LLZTO and Li and the formation of LiySn and Li3N at the interface to realize intimate contact. Right: magnified area. Reproduced with permission from Ref. [65]. Copyright 2020 Wiley

Reproduced with permission from Ref. [66] Copyright 2021 Springer Nature

Reproduced with permission from Ref. [67] Copyright 2020 ACS. b Illustration for poor contact between SSE and Li metal and stable contact between Li–Mg alloy and SSE. Reproduced with permission from Ref. [61] Copyright 2018 Wiley. c Mechanism of the formation of Li3N interlayer by reaction between molten Li and g-C3N4. Reproduced with permission from Ref. [68] Copyright 2019 Wiley

Reproduced with permission from Ref. [70] Copyright 2016 ACS. b Schematic illustrations for microcrack caused within LBO layer during batteries cycling and the SEM images of the microcrack. Reproduced with permission from Ref. [73] Copyright 2019 ACS. c Schematics of the Li2.3C0.7B0.3O3 as solder to realize intact contact between LCO powder and LLZO powder, and between cathode layer and LLZO pellet and cycling performance of Li/LLZO/LCO batteries at 0.05 C at room temperature. Reproduced with permission from Ref. [69] Copyright 2018 Elsevier

a, b Reproduced with permission from Ref. [89] Copyright 2020 Wiley. c Schematics of fabrication procedures of the presented LLZO thin films. d Illustration of the migration barrier and Li vacancy concentration at the conventional grain boundary and the grain boundary with amorphous domains. c, d Reproduced with permission from Ref. [90] Copyright 2020 Springer Nature

a, b Reproduced with permission from Ref. [95] Copyright 2021 Wiley. c Photo showing the flexibility of the hybrid electrolyte, which is associated with the fiber network and conformal structure between ceramic fibers and a polymer electrolyte. Reproduced with permission from Ref. [97] Copyright 2018 Wiley
Similar content being viewed by others
Change history
21 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42864-021-00115-4
References
Famprikis T, Canepa P, Dawson JA, Islam MS, Masquelier C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat Mater. 2019;18:1278.
Ye T, Li LH, Zhang Y. Recent progress in solid electrolytes for energy storage devices. Adv Funct Mater. 2020;30(29):2000077.
Wu C, Huang HF, Lu WY, Wei ZX, Ni XY, Sun F, Qing P, Liu ZJ, Ma JM, Wei WF, Chen LB, Yan CL, Mai LQ. Mg doped Li-LiB alloy with in situ formed lithiophilic LiB skeleton for lithium metal batteries. Adv Sci. 2020;7:1902643.
Duan J, Huang LQ, Wang TR, Huang YM, Fu HY, Wu WY, Luo W, Huang YH. Shaping the contact between Li metal anode and solid-state electrolytes. Adv Funct Mater. 2020;30:1908701.
Banerjee A, Wang XF, Fang CC, Wu EA, Meng YS. Interfaces and interphases in all-solid-state batteries with inorganic solid electrolytes. Chem Rev. 2020;120(14):6878.
Zheng Y, Yao YZ, Ou JH, Li M, Luo D, Dou HZ, Li ZQ, Amine K, Yu AP, Chen ZW. A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures. Chem Soc Rev. 2020;49(23):8790.
Li ZY, Li AJ, Zhang HR, Lin RQ, Jin TW, Cheng Q, Xiao XH, Lee WK, Ge MY, Zhang HJ, Zangiabadi A, Waluyo I, Hunt A, Zhai HW, Borovilas JJ, Wang PY, Yang XQ, Chuan XY, Yang Y. Interfacial engineering for stabilizing polymer electrolytes with 4V cathodes in lithium metal batteries at elevated temperature. Nano Energy. 2020;72:104655.
Wang ZX, Huang BY, Huang H, Chen LQ, Xue RJ, Wang FS. Investigation of the position of Li+ ions in a polyacrylonitrile-based electrolyte by Raman and infared spectroscopy. Electrochim Acta. 1996;41:1443.
Liu WY, Yi C, Li LP, Liu SL, Gui QY, Ba DL, Li YY, Peng DL, Liu JP. Designing polymer-in-salt electrolyte and fully infiltrated 3D electrode for integrated solid-state lithium batteries. Angew Chem Int Ed. 2021;60(23):12931.
Zhang X, Wang S, Xue CJ, Xin CZ, Lin YH, Shen Y, Li LL, Nan CW. Self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly(vinylidene difluoride)-based solid electrolytes. Adv Mater. 2019;31(11):1806082.
Chen R, Li Q, Yu X, Chen L, Li H. Approaching practically accessible solid-state batteries: Stability issues related to solid electrolytes and interfaces. Chem Rev. 2020;120(14):6820.
Manthiram A, Yu X, Wang S. Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater. 2017;2:16103.
Li X, Liang J, Yang X, Adair KR, Wang C, Zhao F, Sun X. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ Sci. 2020;13(5):1429.
Dai J, Chen Q, Glossmann T, Lai W. Comparison of interatomic potential models on the molecular dynamics simulation of fast-ion conductors: a case study of a Li garnet oxide Li7La3Zr2O12. Comput Mater Sci. 2019;162:333.
Meesala Y, Jena A, Chang H, Liu RS. Recent advancements in Li-ion conductors for all-solid-state Li-ion batteries. ACS Energy Lett. 2017;2:2734.
Xu M, Park MS, Lee JM, Kim TY, Park YS, Ma E. Mechanisms of Li+ transport in garnet-type cubic Li3+xLa3M2O12 (M = Te, Nb, Zr). Phys Rev B. 2012;85:052301.
Kasper HM. A new series of rare earth garnets Ln3+3M2Li+3O12 (M = Te, W). Inorg Chem. 1969;8(4):1000.
Thangadurai V, Kaack H, Weppner WJF. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J Am Chem Soc. 2003;86(3):437.
Murugan R, Thangadurai V, Weppner W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed. 2007;46(41):7778.
Murugan R, Thangadurai V, Weppner W. Lattice parameter and sintering temperature dependence of bulk and grain-boundary conduction of garnet-like solid Li-electrolytes. J Electrochem Soc. 2008;155:A90.
Wang C, Fu K, Kammampata SP, Mcowen DW, Samson AJ, Zhang L, Hitz GT, Nolan AM, Wachsman ED, Mo Y, Thangadurai V, Hu L. Garnet-type solid-state electrolytes: materials, interfaces, and batteries. Chem Rev. 2020;120(10):4257.
Posch P, Lunghammer S, Berendts S, Ganschow S, Redhammer GJ, Wilkening A, Lerch M, Gadermaier B, Rettenwander D, Wilkening HMR. Ion dynamics in Al-stabilized Li7La3Zr2O12 single crystals – macroscopic transport and the elementary steps of ion hopping. Energy Storage Mater. 2020;24:220.
Sharafi A, Yu S, Naguib M, Lee M, Ma C, Meyer HM, Nanda J, Chi M, Siegel DJ, Sakamoto J. Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. J Mater Chem A. 2017;5:13475.
Krauskopf T, Mogwitz B, Hartmann H, Singh DK, Zeier WG, Janek J. The fast charge transfer kinetics of the lithium metal anode on the garnet-type solid electrolyte Li6.25Al0.25La3Zr2O12. Adv Energy Mater. 2020;10:2000945.
Han F, Westover AS, Yue J, Fan X, Wang F, Chi M, Leonard DN, Dudney NJ, Wang H, Wang C. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat Energy. 2019;4:187.
Xia W, Xu B, Duan H, Tang X, Guo Y, Kang H, Li H, Liu H. Reaction mechanisms of lithium garnet pellets in ambient air: the effect of humidity and CO2. J Am Ceram Soc. 2017;100:2832.
Cheng L, Wu CH, Jarry A, Chen W, Ye Y, Zhu J, Kostecki R, Persson K, Guo J, Salmeron M, Chen G, Doeff M. Interrelationships among grain size, surface composition, air stability, and interfacial resistance of Al-substituted Li7La3Zr2O12 solid electrolytes. ACS Appl Mater Interfaces. 2015;7:17649.
Galven C, Dittmer J, Suard E, Le Berre F, Crosnier-Lopez MP. Instability of lithium garnets against moisture structural characterization and dynamics of Li7−xHxLa3Sn2O12 and Li5−xHxLa3Nb2O12. Chem Mater. 2012;24:3335.
Truong L, Thangadurai V. First total H+/Li+ ion exchange in garnet-type Li5La3Nb2O12 using organic acids and studies on the effect of Li stuffing. Inorg Chem. 2012;51(3):1222.
Cheng L, Crumlin EJ, Chen W, Qiao R, Hou H, Franz Lux S, Zorba V, Russo R, Kostecki R, Liu Z, Persson K, Yang W, Cabana J, Richardson T, Chen G, Doeff M. The origin of high electrolyte-electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys Chem Chem Phys. 2014;16:18294.
Besli MM, Usubelli C, Metzger M, Pande V, Harry K, Nordlund D, Sainio S, Christensen J, Doeff MM, Kuppan S. Effect of liquid electrolyte soaking on the interfacial resistance of Li7La3Zr2O12 for all-solid-state lithium batteries. ACS Appl Mater Interfaces. 2020;12:20605.
Duan H, Chen WP, Fan M, Wang WP, Yu L, Tan SJ, Chen X, Zhang Q, Xin S, Wan LJ, Guo YG. Building an air stable and lithium deposition regulable garnet interface from moderate-temperature conversion chemistry. Angew Chem Int Ed. 2020;59:12069.
Yang YN, Li YX, Li YQ, Zhang T. On-surface lithium donor reaction enables decarbonated lithium garnets and compatible interfaces within cathodes. Nat Commun. 2020;11:5519.
Yu S, Schmidt RD, Garcia-Mendez R, Herbert E, Dudney NJ, Wolfenstine JB, Sakamoto J, Siegel DJ. Elastic properties of the solid electrolyte Li7La3Zr2O12 (LLZO). Chem Mater. 2016;28:197.
Rangasamy E, Wolfenstine J, Sakamoto J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ion. 2012;206:28.
Im C, Park D, Kim H, Lee J. Al-incorporation into Li7La3Zr2O12 solid electrolyte keeping stabilized cubic phase for all-solid-state Li batteries. J Energy Chem. 2018;27:1501.
Yang SH, Kim MY, Kim DH, Jung HY, Ryu HM, Han JH, Lee MS, Kim HS. Ionic conductivity of Ga-doped LLZO prepared using Couette-Taylor reactor for all-solid lithium batteries. J Ind Eng Chem. 2017;56:422.
Chen C, Sun Y, He L, Kotobuki M, Hanc E, Chen Y, Zeng K, Lu L. Microstructural and electrochemical properties of Al- and Ga-doped Li7La3Zr2O12 garnet solid electrolytes. ACS Appl Energy Mater. 2020;3:4708.
Rangasamy E, Wolfenstine J, Allen J, Sakamoto J. The effect of 24c-site (A) cation substitution on the tetragonal–cubic phase transition in Li7−xLa3−xAxZr2O12 garnet-based ceramic electrolyte. J Power Sources. 2013;230:261.
Dumon A, Huang M, Shen Y, Nan CW. High Li ion conductivity in strontium doped Li7La3Zr2O12 garnet. Solid State Ion. 2013;243:36.
Chen X, Cao T, Xue M, Lv H, Li B, Zhang C. Improved room temperature ionic conductivity of Ta and Ca doped Li7La3Zr2O12 via a modified solution method. Solid State Ion. 2018;314:92.
Li Y, Wang Z, Cao Y, Du F, Chen C, Cui Z, Guo X. W-doped Li7La3Zr2O12 ceramic electrolytes for solid state Li-ion batteries. Electrochim Acta. 2015;180:37.
Gao J, Guo X, Li Y, Ma Z, Guo X, Li H, Zhu Y, Zhou W. The Ab initio calculations on the areal specific resistance of Li-metal/Li7La3Zr2O12 interphase. Adv Theory Simul. 2019;2:1900028.
Ma C, Cheng Y, Yin K, Luo J, Sharafi A, Sakamoto J, Li J, More KL, Dudney NJ, Chi M. Interfacial stability of Li metal-solid electrolyte elucidated via in situ electron microscopy. Nano Lett. 2016;16:7030.
Gao J, Zhu J, Li X, Li J, Guo X, Li H, Zhou W. Rational design of mixed electronic-ionic conducting Ti-doping Li7La3Zr2O12 for lithium dendrites suppression. Adv Funct Mater. 2020;31:2001918.
Yeandel SR, Chapman BJ, Slater PR, Goddard P. Structure and lithium-ion dynamics in Fluoride-doped cubic Li7La3Zr2O12 (LLZO) garnet for Li solid-state battery applications. J Phys Chem C. 2018;122:27811.
Liu C, Wen ZY, Rui K. High ion conductivity in garnet-type F-doped Li7La3Zr2O12. J Inorg Mater. 2015;30(9):995.
Yang T, Li Y, Wu W, Cao Z, He W, Gao Y, Liu J, Li G. The synergistic effect of dual substitution of Al and Sb on structure and ionic conductivity of Li7La3Zr2O12 ceramic. Ceram Int. 2018;44:1538.
Shen L, Wang L, Wang Z, Jin C, Peng L, Pan X, Sun J, Yang R. Preparation and characterization of Ga and Sr co-doped Li7La3Zr2O12 garnet-type solid electrolyte. Solid State Ion. 2019;339:114992.
Wang C, Lin PP, Gong Y, Liu ZG, Lin TS, He P. Co-doping effects of Ba2+ and Ta5+ on the microstructure and ionic conductivity of garnet-type solid state electrolytes. J Alloys Compd. 2021;854:157143.
Liu X, Gao M, Liu Y, Xiong L, Chen J. Improving the room temperature ionic conductivity of Al-Li7La3Zr2O12 ceramics by Ba and Y or Ba and W co-doping. Ceram Int. 2019;45:13488.
Amardeep A, Kobi S, Mukhopadhyay A. Mg-doping towards enhancing the composition-phase-structural stability of Li-La-zirconate based cubic garnet upon exposure to air. Scr Mater. 2019;162:214.
Murugan R, Ramakumar S, Janani N. High conductive yttrium doped Li7La3Zr2O12 cubic lithium garnet. Electrochem Commum. 2011;13:1373.
Song S, Chen B, Ruan Y, Sun J, Yu L, Wang Y, Thokchom J. Gd-doped Li7La3Zr2O12 garnet-type solid electrolytes for all-solid-state Li-ion batteries. Electrochim Acta. 2018;270:501.
Liu X, Li Y, Yang T, Cao Z, He W, Gao Y, Liu J, Li G, Li Z. High lithium ionic conductivity in the garnet-type oxide Li7−2x La3Zr2−xMox O12 (x = 0–0.3) ceramics by sol-gel method. J Am Ceram Soc. 2017;100:1527.
Xiang X, Chen F, Yang W, Yang J, Ma X, Chen D, Su K, Shen Q, Zhang L. Dual regulation of Li+ migration of Li64La3Zr14M0.6O12 (M = Sb, Ta, Nb) by bottleneck size and bond length of M−O. J Am Ceram Soc. 2019;103:2483.
Zeier WG. Structural limitations for optimizing garnet-type solid electrolytes: a perspective. Dalton Trans. 2014;43:16133.
Jalem R, Rushton MJD, Manalastas W, Nakayama M, Kasuga T, Kilner JA, Grimes RW. Effects of gallium doping in garnet-type Li7La3Zr2O12 solid electrolytes. Chem Mater. 2015;27:2821.
Wang C, Xie H, Zhang L, Gong Y, Pastel G, Dai J, Liu B, Wachsman ED, Hu L. Universal soldering of lithium and sodium alloys on various substrates for batteries. Adv Energy Mater. 2018;8:1701963.
Zhou C, Samson AJ, Hofstetter K, Thangadurai V. A surfactant-assisted strategy to tailor Li-ion charge transfer interfacial resistance for scalable all-solid-state Li batteries. Sustain Energy Fuels. 2018;2:2165.
Yang C, Xie H, Ping W, Fu K, Liu B, Rao J, Dai J, Wang C, Pastel G, Hu L. An electron/ion dual-conductive alloy framework for high-rate and high-capacity solid-state lithium-metal batteries. Adv Mater. 2019;31:e1804815.
Luo W, Gong Y, Zhu Y, Li Y, Yao Y, Zhang Y, Fu KK, Pastel G, Lin CF, Mo Y, Wachsman ED, Hu L. Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer. Adv Mater. 2017;29:1606042.
Xu H, Li Y, Zhou A, Wu N, Xin S, Li Z, Goodenough JB. Li3N-modified garnet electrolyte for all-solid-state lithium metal batteries operated at 40 °C. Nano Lett. 2018;18:7414.
Tian Y, Ding F, Zhong H, Liu C, He YB, Liu J, Liu X, Xu Q. Li6.75La3Zr1.75Ta0.25O12@amorphous Li3OCl composite electrolyte for solid state lithium-metal batteries. Energy Storage Mater. 2018;14:49.
Shi K, Wan Z, Yang L, Zhang Y, Huang Y, Su S, Xia H, Jiang K, Shen L, Hu Y, Zhang S, Yu J, Ren F, He YB, Kang F. In situ construction of an ultra-stable conductive composite interface for high-voltage all-solid-state lithium metal batteries. Angew Chem Int Ed Engl. 2020;59:11784.
Huo H, Gao J, Zhao N, Zhang D, Holmes NG, Li X, Sun Y, Fu J, Li R, Guo X, Sun X. A flexible electron-blocking interfacial shield for dendrite-free solid lithium metal batteries. Nat Commun. 2021;12:176.
Zhang Y, Meng J, Chen K, Wu H, Hu J, Li C. Garnet-based solid-state lithium fluoride conversion batteries benefiting from eutectic interlayer of superior wettability. ACS Energy Lett. 2020;5:1167.
Huang Y, Chen B, Duan J, Yang F, Wang T, Wang Z, Yang W, Hu C, Luo W, Huang Y. Graphitic carbon nitride (g-C3N4): an interface enabler for solid-state lithium metal batteries. Angew Chem Int Ed. 2020;59:3699.
Han F, Yue J, Chen C, Zhao N, Fan X, Ma Z, Gao T, Wang F, Guo X, Wang C. Interphase engineering enabled all-ceramic lithium battery. Joule. 2018;2:497.
Park K, Yu BC, Jung JW, Li Y, Zhou W, Gao H, Son S, Goodenough JB. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: Interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem Mater. 2016;28:8051.
Zhou W, Wang S, Li Y, Xin S, Manthiram A, Goodenough JB. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J Am Chem Soc. 2016;138:9385.
Liu B, Gong Y, Fu K, Han X, Yao Y, Pastel G, Yang C, Xie H, Wachsman ED, Hu L. Garnet solid electrolyte protected Li-metal batteries. ACS Appl Mater Interfaces. 2017;9(22):18809.
Wang D, Sun Q, Luo J, Liang J, Sun Y, Li R, Adair K, Zhang L, Yang R, Lu S, Huang H, Sun X. Mitigating the interfacial degradation in cathodes for high-performance oxide-based solid-state lithium batteries. ACS Appl Mater Interfaces. 2019;11:4954.
Fu K, Gong Y, Hitz GT, Mcowen DW, Li Y, Xu S, Wen Y, Zhang L, Wang C, Pastel G, Dai J, Liu B, Xie H, Yao Y, Wachsman ED, Hu L. Three-dimensional bilayer garnet solid electrolyte based high energy density lithium metal–sulfur batteries. Energy Environ Sci. 2017;10:1568.
Zhong G, Wang C, Wang R, Ping W, Xu S, Qiao H, Cui M, Wang X, Zhou Y, Kline DJ, Zachariah MR, Hu L. Rapid, high-temperature microwave soldering toward a high-performance cathode/electrolyte interface. Energy Storage Mater. 2020;30:385.
Bai L, Xue W, Qin H, Li Y, Li Y, Sun J. A novel dense LiCoO2 microcrystalline buffer layer on a cathode-electrolyte interface for all-solid-state lithium batteries prepared by the magnetron sputtering method. Electrochim Acta. 2019;295:677.
Cheng L, Chen W, Kunz M, Persson K, Tamura N, Chen G, Doeff M. Effect of surface microstructure on electrochemical performance of garnet solid electrolytes. ACS Appl Mater Interfaces. 2015;7(3):2073.
Mo F, Ruan J, Sun S, Lian Z, Yang S, Yue X, Song Y, Zhou YN, Fang F, Sun G, Peng S, Sun D. Inside or outside: origin of lithium dendrite formation of all solid-state electrolytes. Adv Energy Mater. 2019;9:1902123.
Zhao CZ, Chen PY, Zhang R, Chen X, Li BQ, Zhang XQ, Cheng XB, Zhang Q. An ion redistributor for dendrite-free lithium metal anodes. Sci Adv. 2018;4:eaat3446.
Kim JS, Kim H, Badding M, Song Z, Kim K, Kim Y, Yun DJ, Lee D, Chang J, Kim S, Im D, Park S, Kim SH, Heo S. Origin of intergranular Li metal propagation in garnet-based solid electrolyte by direct electronic structure analysis and performance improvement by bandgap engineering. J Mater Chem A. 2020;8(33):16892.
Xie H, Li C, Kan WH, Avdeev M, Zhu C, Zhao Z, Chu X, Mu D, Wu F. Consolidating the grain boundary of the garnet electrolyte LLZTO with Li3BO3 for high-performance LiNi0.8Co0.1Mn0.1O2/LiFePO4 hybrid solid batteries. J Mater Chem A. 2019;7(36):20633.
Huang X, Lu Y, Song Z, Xiu T, Badding ME, Wen Z. Preparation of dense Ta-LLZO/MgO composite Li-ion solid electrolyte: Sintering, microstructure, performance and the role of MgO. J Energy Chem. 2019;39:8.
Li HY, Huang B, Huang Z, Wang CA. Enhanced mechanical strength and ionic conductivity of LLZO solid electrolytes by oscillatory pressure sintering. Ceram Int. 2019;45(14):18115.
Shen F, Guo W, Zeng D, Sun Z, Gao J, Li J, Zhao B, He B, Han X. A simple and highly efficient method toward high-density garnet-type LLZTO solid-state electrolyte. ACS Appl Mater Interface. 2020;12:30313.
Zhang H, An X, Lu Z, Liu L, Cao H, Xu Q, Liu H, Ni Y. A three dimensional interconnected Li7La3Zr2O12 framework composite solid electrolyte utilizing lignosulfonate/ cellulose nanofiber bio-template for high performance lithium ion batteries. J Power Sources. 2020;477:228752.
Ding J, Xu R, Yan C, Xiao Y, Liang Y, Yuan H, Huang J. Integrated lithium metal anode protected by composite solid electrolyte film enables stable quasi-solid-state lithium metal batteries. Chin Chem Lett. 2020;31(9):2339.
Xu R, Xiao Y, Zhang R, Cheng XB, Zhao CZ, Zhang XQ, Yan C, Zhang Q, Huang JQ. Dual-phase single-ion pathway interfaces for robust lithium metal in working batteries. Adv Mater. 2019;31(19):e1808392.
Zhao Y, Yan J, Cai W, Lai Y, Song J, Yu J, Ding B. Elastic and well-aligned ceramic LLZO nanofiber based electrolytes for solid-state lithium batteries. Energy Storage Mater. 2019;23:306.
Zhu J, Li X, Wu C, Gao J, Xu H, Li Y, Guo X, Li H, Zhou W. A multilayer ceramic electrolyte for all-solid-state Li batteries. Angew Chem Int Ed Engl. 2021;60(7):3781.
Zhu Y, Wu S, Pan Y, Zhang X, Yan Z, Xiang Y. Reduced energy barrier for Li+ transport across grain boundaries with amorphous domains in LLZO thin films. Nanoscale Res Lett. 2020;15:153.
Wan Z, Lei D, Yang W, Liu C, Shi K, Hao X, Shen L, Lv W, Li B, Yang QH, Kang F, He YB. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder. Adv Funct Mater. 2019;29(1):1805301.
Li Y, Zhang W, Dou Q, Wong KW, Ng KM. Li7La3Zr2O12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. J Mater Chem A. 2019;7(8):3391.
Li Z, Xie HX, Zhang XY, Guo X. In situ thermally polymerized solid composite electrolytes with a broad electrochemical window for all-solid-state lithium metal batteries. J Mater Chem A. 2020;8(7):3892.
Zhao CZ, Zhang XQ, Cheng XB, Zhang R, Xu R, Chen PY, Peng HJ, Huang JQ, Zhang Q. An anion-immobilizd composite electrolyte for dendrite-free lithium metal anodes. Proc Natl Acad Sci USA. 2017;114:11069.
He K, Cheng SH, Hu J, Zhang Y, Yang H, Liu Y, Liao W, Chen D, Liao C, Cheng X, Lu Z, He J, Tang J, Li RKY, Liu C. In-situ intermolecular interaction in composite polymer electrolyte for ultralong life quasi-solid-state lithium metal batteries. Angew Chem Int Ed. 2021;60(21):12116.
Jiang T, He P, Wang G, Shen Y, Nan CW, Fan LZ. Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solid-state lithium batteries. Adv Energy Mater. 2020;10:1903376.
Xie H, Yang C, Fu KK, Yao Y, Jiang F, Hitz E, Liu B, Wang S, Hu L. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose. Adv Energy Mater. 2018;8(18):1703474.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51672156), Local Innovative Research Teams Project of Guangdong Pearl River Talents Program (Grant No. 2017BT01N111), Shenzhen Technical Plan Project (Grant Nos. JCYJ20170412170706047, JCYJ20170307153806471 and GJHS20170314165324888).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, JS., Guo, SK. & He, YB. Modification strategies of Li7La3Zr2O12 ceramic electrolyte for high-performance solid-state batteries. Tungsten 3, 260–278 (2021). https://doi.org/10.1007/s42864-021-00102-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42864-021-00102-9