Abstract
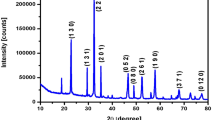
Most recently, graphene-related composite-modified electrode surfaces are been widely employed to improve surface interactions and electron transfer kinetics. Hydrothermally prepared strontium pyro niobate (SPN) and reduced graphene oxide/strontium pyro niobate (RGOSPN) nanostructures reveal excellent morphology. X-ray diffraction analysis of SPN and RGOSPN agree with standard data. Thermogravimetry–differential scanning calorimetry analyses show that RGOSPN has higher thermal stability than SPN. In addition, from the polarization–electric field (P–E) loop measurements, the estimated value of remnant polarization (Pr) and coercive electric field (Ec) of SPN are 0.039 µC cm−2 and − 2.90 kV cm−1 and that of RGOSPN nanocomposite are 0.0139 µC cm−2 and − 2.04 kV cm−1. Cyclic voltammetry measurements show that RGOSPN nanocomposite manifests the possibility of electrochemical reversibility beyond long cycles without change in performance. The redox cycle reveal that RGOSPN can be used as part of a composite electrode for hybrid capacitors dynamic conditions. Moreover, the specific capacitance of SPN and RGOSPN was calculated using galvanostatic charge–discharge (GCD) technique. The observed energy density of 9.1 W h kg−1 in RGOSPN is higher when compared with previous reported values.









Similar content being viewed by others
References
Li R, Wei Z, Gou XL (2015) Nitrogen, phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. ACS Catal 5:4133–4142
Prokhorov AM, Kuzminov YS (1990) Ferroelectric crystals for laser radiation control, vol 81. Adam Hilger, Bristol
Deshpande SB, Potdar HS, Godbole PD, Date SK (1992) Preparation and ferroelectric properties of SBN:50 ceramics. J Am Soc Ceram 75:2581–2585
Yang W, Salim J, Li SH, Sun C, Chen L, Goodenough JB, Kim Y (2012) Perovskite Sr0.95Ce0.05CoO3−δ loaded with copper nanoparticles as a bifunctional catalyst for lithium-air batteries. J Mater Chem 22:18902–18907
Garcia EM, Tarôco HA, Matencio HT, Domingues RZ, Dos Santos JAF (2012) Electrochemical study of La0.6Sr0.4Co0.8Fe0.2O3 during oxygen evolution reaction. Int J Hydrog Energy 37:6400–6406
Jin C, Cao XC, Lu F, Yang Z, Yang R (2013) Electrochemical study of Ba0.5Sr0.5Co0.8Fe0.2O3 perovskite as bifunctional catalyst in alkaline media. J Hydrog Energy 38:10389–10393
Sunarso J, Torriero AAJ, Zhou W, Howlett PC, Forsyth M (2012) Oxygen reduction reaction activity of La-based perovskite oxides in alkaline medium: a thin-film rotating ring-disk electrode study. J Phys Chem C 116:5827–5834
Mendesa RG, Araújob EB, Eirasc JA (2001) Structural characterization and ferroelectric properties of strontium barium niobate (SrxBa1−xNb2O6) thin films. Mater Res 4:113–116
Nanamatsu S, Kimura M, Doi K, Takahashi M (1971) Ferroelectric properties of Sr2Nb2O7 single crystal. J Phys Soc Jpn 30:300–301
Takahashi M, Nanamatsu S, Kimura M (1972) The growth of ferroelectric single crystal Sr2Nb2O7 by means of the floating zone technique. J Cryst Growth 13–14:681–685
Nanamatsu S, Kimura M, Kawamura T (1975) Crystallographic and dielectric properties of ferroelectric A2B2O7 (A = Sr, B = Ta, Nb) crystals and their solid solutions. J Phys Soc Jpn 38:817–824
Shabbir G, Kojima S (2003) Acoustic and thermal properties of strontium pyroniobate single crystals. J Phys D Appl Phys 36:1036–1039
Ishizawa N, Marumo F, Kawamura T, Kimura M (1975) The crystal structure of Sr2Nb2O7, a compound with perovskite-type slabs. Acta Crystallogr B31:1912–1915
Daniels P, Tamazyan R, Kuntscher CA, Dressel AM, Lichtenberg F, Van Smaalen S (2002) The incommensurate modulation of the structure of Sr2Nb2O7. Acta Crystallogr B 58:970–976
Sangiovanni DG, Gueorguiev GK, Kakanakova-Georgieva A (2018) Ab initio molecular dynamics of atomic-scale surface reactions: insights into metal organic chemical vapor deposition of AlN on graphene. Phys Chem Chem Phys 20:17751–17761
Goyenola C, Stafström S, Schmidt S, Hultman L, Gueorguiev GK (2014) Carbon fluoride, CFx: structural diversity as predicted by first principles. J Phys Chem C 118(12):6514–6521
Zhang H, Du X, Ding S, Wang Q, Chang L, Ma X, Hao X, Pen C (2019) DFT calculations of the synergistic effect of λ-MnO2/Graphene composites for electrochemical adsorption of lithium ions. Phys Chem Chem Phys 21:8133–8140
Zaaba NI, Foo KL, Hashim U, Tan SJ, Liu WW, Voon CH (2017) Synthesis of graphene oxide using modified hummers method: solvent influence. Procedia Eng 184:469–477
Kandalkar S, Dhawale D, Kim C, Lokhande C (2010) Chemical synthesis of cobalt oxide thin film electrode for supercapacitor application. Synth Met 160:1299–1302
Jabbar A, Yasin G, Khan WQ, Anwar MY, Korai RM, Nizam MN, Muhyodin G (2017) Electrochemical deposition of nickel graphene composite coatings: effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv 7:31100–31109
Atuchin VV, Grivel JC, Korotkov AS, Zhang Z (2008) Electronic parameters of Sr2Nb2O7 and chemical bonding. J Solid State Chem 181:1285–1291
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev J 56:978–982
Veerappan G, Yoo SY, Zhang K, Ma M, Kang B (2016) High-reversible capacity of perovskite BaSnO3/rGO composite for lithium-ion battery anodes. Electrochem Acta 214:31–37
Gurunathan SJ, Han JW, Park JH, Kim E, Choi YJ, Kwon DN (2015) Reduced graphene oxide–silver nanoparticle nanocomposite: a potential anticancer nanotherapy. Int J Nanomed 10:6257–6276
Cho I-S, Lee S, Noh JH, Kim DW, Jung HS, Kim DW, Hong KS (2010) Facile hydrothermal synthesis of SrNb2O6 nanotubes with rhombic cross sections. Cryst Growth Des 10:2447–2450
Kumar MIS, Kirupavathy SS, Jerusha E, Sureshkumar S, Vinolia M (2018) Synthesis and characterization of novel reduced graphene oxide supported barium niobate (RGOBN) nanocomposite with enhanced ferroelectric properties and thermal stability. J Mater Sci Mater Electron 29:19228–19237
Ke QQ, Wang J (2016) Graphene-based materials for supercapacitor electrodes—a review. J Materiomics 2:37–54
Mathai KC, Vidya S, John A, Solomon S, Thomas JK (2014) Structural, optical, and compactness characteristics of nanocrystalline CaNb2O6 synthesized through an auto igniting combustion method. Adv Condens Matter Phys. https://doi.org/10.1155/2014/735878
Song Z, Liu W, Wei W, Quan C, Sun N, Zhou Q, Liu G, Wen X (2016) Preparation and electrochemical properties of Fe2O3/reduced graphene oxide aerogel (Fe2O3/rGOA) composites for supercapacitors. J Alloy Compd 685:355–363
Alanis J, Rodríguez-Aranda MC, Rodríguez ÁG, Ojeda-Galván HJ, Mendoza ME, Navarro-Contreras HR (2019) Temperature dependence of the Raman dispersion of Sr2Nb2O7: influence of an electric field during the synthesis. J Raman Spectrosc 50:102–114
Monisha M, Priyadarshani N, Durairaj M, Sabari Girisun TC (2020) 2PA induced optical limiting behaviour of metal (Ni, Cu, Zn) niobate decorated reduced graphene oxide. Opt Mater 101:109775
Arunkumar P, Ashish AG, Babu B, Sarang S, Suresh A, Sharma CH, Shaijumon MM (2015) Nb2O5/graphene nanocomposites for electrochemical energy storage. RSC Adv 5(74):59997–60004
Li X, Zhang T, Gu S, Kang SZ, Li G, Mu J (2013) Reduced graphene oxide/potassium niobate composite nanoscrolls with enhanced photocatalytic activity for dye degradation. Sep Purif Technol 108:139–142
Elgrishi N, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, Dempsey JL (2018) A practical beginner’s guide to cyclic voltammetry. J Chem Educ 95:197–206
Kim BK, Sy S, Yu A, Zhang J (2015 ) Electrochemical supercapacitors for energy storage and conversion. Handbook of clean energy systems. Wiley, New York. ISBN:978-1-118-38858-7
Ghosh D, Giri S, Moniruzzaman M, Basu T, Mandala M, Das CK (2014) αMnMoO4/graphene hybrid composite: high energy density supercapacitor electrode material. Dalton Trans 43(28):11067–11076
Yuan L, Lu XH, Zhai T, Dai J, Zhang F, Hu B, Wang X, Gong L, Chen J, Hu C, Tong Y, Zhou J, Wang ZL (2012) Flexible solid-state supercapacitors based on carbon, nanoparticles/MnO2 nanorods hybrid structure. ACS Nano 6:656–661
Wang HW, Hu ZA, Chang YQ, Chen YL, Zhang ZY, Yang YY, Wu HY (2011) Preparation of reduced graphene oxide/cobalt oxide composites and their enhanced capacitive behaviors by homogeneous incorporation of reduced graphene oxide sheets in cobalt oxide matrix. Mater Chem Phys 130:672–679
Liu Z, Wang LD, Ma G, Yuan Y, Jia HN, Fei W (2020) Supercapacitor with ultrahigh volumetric capacitance produced by self-assembly of reduced graphene oxide through phosphoric acid treatment. J Mater Chem A 8:18933–18944
Liu J, Shakir I, Kang DJ (2014) Lithium niobate nanoflakes as electrodes for highly stable electrochemical supercapacitor devices. Mater Lett 119:84–87
Kim CH, Kim B-H, Yang KS (2012) TiO2 nanoparticles loaded on graphene/carbon composite nanofibers by electrospinning for increased photocatalysis. Carbon 50:2472–2481
Ding R, Qia L, Jiac MJ, Wang H (2013) Facile and large-scale chemical synthesis of highly porous secondary submicron/micron-sized NiCo2O4 materials for high-performance aqueous hybrid AC-NiCo2O4 electrochemical capacitors. Electrochim Acta 107:494–502
Azhagan MVK, Vaishampayan MV, Shelke MV (2014) Synthesis and electrochemistry of pseudocapacitive multilayer fullerenes and MnO2 nanocomposites. J Mater Chem A 2:2152
Chen T, Dai L (2014) Flexible supercapacitors based on carbon nanomaterials. J Mater Chem A 2(28):10756
Zhao Y, Liu J, Wang B, Sha J, Li Y, Zheng D, Amjadipour M, MacLeod J, Motta N (2017) Supercapacitor electrodes with remarkable specific capacitance converted from hybrid graphene oxide/NaCl/urea films. ACS Appl Mater Interfaces 9(27):22588–22596
Lei Z, Zhang J, Zhao XS (2012) Ultrathin MnO2 nano fibers grown on graphitic carbon spheres as high-performance asymmetric supercapacitor electrodes. J Mater Chem 22:153–160
Kuang M, Wen ZQ, Guo XL, Zhang SM, Zhang YX (2014) Engineering firecracker-like beta-manganese dioxides@spinel nickel cobaltates nanostructures for high-performance supercapacitors. J Power Sources 270:426–433
Aadil M, Shaheen W, Warsi MF, Shahid M, Khan MA, Ali Z, Haider S, Shakir I (2016) Superior electrochemical activity of α-Fe2O3/rGO nanocomposite for advance energy storage devices. J Alloy Compd 689:648–654
Sumboja A, Foo CY, Wang X, Lee PS (2013) Large areal mass, flexible and free-standing reduced graphene oxide/manganese dioxide paper for asymmetric supercapacitor device. Adv Mater 25:2809–2815
Acknowledgements
We thank Professor R. Sundar, Head, CSRC, Rajalakshmi Engineering College, Chennai for providing the support for lab facility and encouragements. The support received from MSRC, IIT Madras for P–E loop measurements is highly appreciable.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, M.I.S., Kirupavathy, S.S. & Shalini, S. Exploration on reduced graphene oxide/strontium pyro niobate electrode material for electrochemical energy storage applications. Carbon Lett. 31, 619–633 (2021). https://doi.org/10.1007/s42823-020-00203-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-020-00203-4