Abstract
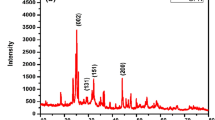
La0.75Sr0.25Cr0.5Mn0.5O3 (LSCM)/graphene oxide (GO)-based composite electrodes are developed for energy storage applications. LSCM was synthesized by solution combustion method and GO by improved Hummers’ method. Synthesized active materials at appropriate ratios (GO and LSCM) were mixed in polyvinylidene fluoride by vacuum centrifuge mixing (VCM) to prepare a composite dispersion of GO and LSCM which was coated on porous Ni foam using the VCM. The effect of GO content on specific capacitance (Csp) of LSCM was studied by varying GO content from 1 to 7 wt%. Both as-synthesized perovskite and GO were characterized by X-ray diffraction, scanning electron microscopy, atomic force microscopy and Fourier transform infrared spectroscopy. Electrochemical characterization of the electrodes was carried out by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) in 3 M KOH. CV measurements revealed that LSCM electrode showed maximum Csp of 751 Fg−1 at 1 mVs−1. By increasing GO content from 0 to 5 wt % in composite electrodes, Csp was increased from 751 to 1223 Fg−1, respectively. Further, evaluation of CV results revealed that predominant charge storage mechanism of electrodes was Faradic in nature which exhibited distinct redox peaks. EIS revealed that LSCM-5%GO electrode showed the lowest solution resistance, charge transfer resistance and highest double layer capacitance among other electrodes. Furthermore, the LSCM-5%GO electrode showed 93% charge retention after 3000 charge–discharge cycles.








Similar content being viewed by others
References
Li, Z.; Zhang, W.; Yuan, C.; Su, Y.: Controlled synthesis of perovskite lanthanum ferrite nanotubes with excellent electrochemical properties. RSC Adv. 7(21), 12931–12937 (2017)
Rai, A.; Sharma, A.; Thakur, A.K.: Evaluation of aluminium doped lanthanum ferrite based electrodes for supercapacitor design. Solid State Ion. 262, 230–233 (2014)
Simon, P.; Gogotsi, Y.: Materials for electrochemical capacitors. Nanosci. Technol. Collect. Rev. Nat. J. 320–329 (2010)
Guo, Y.G.; Hu, J.S.; Wan, L.J.: Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 20(15), 2878–2887 (2008)
Bruce, P.G.; Scrosati, B.; Tarascon, J.M.: Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47(16), 2930–2946 (2008)
Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Van Schalkwijk, W.: Nanostructured materials for advanced energy conversion and storage devices. Mater. Sustain. Energy Collect. Peer-rev. Res. Rev. Artic. Nat. Publ. Group 148–159 (2011)
Liu, F.; Wang, X.; Hao, J.; Han, S.; Lian, J.; Jiang, Q.: High density arrayed Ni/NiO core-shell nanospheres evenly distributed on graphene for ultrahigh performance supercapacitor. Sci. Rep. 7(1), 1–10 (2017)
Winter, M.: What are Batteries, Fuel Cells, and Supercapacitors? ACS Publications, Washington (2004)
Raj, T.V.; Hoskeri, P.A.; Muralidhara, H.; Manjunatha, C.; Kumar, K.Y.; Raghu, M.: Facile synthesis of perovskite lanthanum aluminate and its green reduced graphene oxide composite for high performance supercapacitors. J. Electroanal. Chem. 858, 113830 (2020)
Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H.: Ni (OH) 2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 132(21), 7472–7477 (2010)
Yang, G.-W.; Xu, C.-L.; Li, H.-L.: Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem. Commun. (48), 6537–6539 (2008)
Tian, Y.: Flexible and free-standing 2D titanium carbide film decorated with manganese oxide nanoparticles as a high volumetric capacity electrode for supercapacitor. J. Power Sources 359, 332–339 (2017)
Fan, Z.: Interlaminar shear strength of glass fiber reinforced epoxy composites enhanced with multi-walled carbon nanotubes. Compos. A Appl. Sci. Manuf. 39(3), 540–554 (2008)
Yu, D.: Decorating nanoporous ZIF-67-derived NiCo 2 O 4 shells on a Co 3 O 4 nanowire array core for battery-type electrodes with enhanced energy storage performance. J. Mater. Chem. A 4(28), 10878–10884 (2016)
Porada, S.: Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 58(8), 1388–1442 (2013)
Gao, Z.: Microstructural design of hybrid CoO@ NiO and graphene nano-architectures for flexible high performance supercapacitors. J. Mater. Chem. A 3(28), 14833–14844 (2015)
Liao, Q.: All-solid-state symmetric supercapacitor based on Co3O4 nanoparticles on vertically aligned graphene. ACS Nano 9(5), 5310–5317 (2015)
Zhai, T.: Phosphate ion functionalized Co3O4 ultrathin nanosheets with greatly improved surface reactivity for high performance pseudocapacitors. Adv. Mater. 29(7), 1604167 (2017)
Zhu, Q.: Design of a unique 3D-nanostructure to make MnO2 work as supercapacitor material in acid environment. Chem. Eng. J. 321, 554–563 (2017)
Cheng, S.: Phase evolution of an alpha MnO2-based electrode for pseudo-capacitors probed by in operando Raman spectroscopy. Nano Energy 9, 161–167 (2014)
Xiong, X.: Three-dimensional ultrathin Ni (OH) 2 nanosheets grown on nickel foam for high-performance supercapacitors. Nano Energy 11, 154–161 (2015)
Sahoo, S.; Shim, J.-J.: Facile synthesis of three-dimensional ternary ZnCo2O4/reduced graphene oxide/NiO composite film on nickel foam for next generation supercapacitor electrodes. ACS Sustain. Chem. Eng. 5(1), 241–251 (2017)
Patil, S.B.; Raghu, M.; Kishore, B.; Nagaraju, G.: Enhanced electrochemical performance of few-layered MoS 2–rGO nanocomposite for lithium storage application. J. Mater. Sci. Mater. Electron. 30(1), 316–322 (2019)
Kumar, K.Y.; Saini, H.; Pandiarajan, D.; Prashanth, M.; Parashuram, L.; Raghu, M.: Controllable synthesis of TiO2 chemically bonded graphene for photocatalytic hydrogen evolution and dye degradation. Catal. Today 340, 170–177 (2020)
Wang, X.W.; Wang, X.E.; Zhang, H.C.; Zhu, Q.Q.; Zheng, D.L.; Sun, L.Y.: Preparation and electrochemical properties of LaMnO3 powder as a supercapacitor electrode material. In: Key Engineering Materials, pp. 698–704. Trans Tech Publications Ltd, Switzerland (2017)
Tian, H.; Lang, X.; Nan, H.; An, P.; Zhang, W.; Hu, X.; Zhang, J.: Nanosheet-assembled LaMnO3@ NiCo2O4 nanoarchitecture growth on Ni foam for high power density supercapacitors. Electrochim. Acta 318, 651–659 (2019)
Hu, X.; Nan, H.; Liu, M.; Liu, S.; An, T.; Tian, H.: Battery-like MnCo2O4 electrode materials combined with active carbon for hybrid supercapacitors. Electrochim. Acta 306, 599–609 (2019)
Gómez-Pérez, A.; Azcondo, M.T.; Yuste, M.; Pérez-Flores, J.C.; Bonanos, N.; Porcher, F.; Muñoz-Noval, A.; Hoelzel, M.; García-Alvarado, F.; Amador, U.: The A-cation deficient perovskite series La 2–x CoTiO 6− δ (0≤ x≤ 0.20): new components for potential SOFC composite cathodes. J. Mater. Chem. A 4(9), 3386–3397 (2016)
Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X.: Symmetric/asymmetric supercapacitor based on the perovskite-type lanthanum cobaltate nanofibers with Sr-substitution. Electrochim. Acta 178, 398–406 (2015)
Wang, X.; Zhu, Q.; Wang, X.; Zhang, H.; Zhang, J.; Wang, L.: Structural and electrochemical properties of La0. 85Sr0. 15MnO3 powder as an electrode material for supercapacitor. J. Alloys Compd. 675, 195–200 (2016)
Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X.: Structure, morphology and electrochemical properties of LaxSr1− xCo0. 1Mn0. 9O3− δ perovskite nanofibers prepared by electrospinning method. J. Alloys Compd. 624, 31–39 (2015)
Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J.: Anion charge storage through oxygen intercalation in LaMnO 3 perovskite pseudocapacitor electrodes. Nat. Mater. 13(7), 726–732 (2014)
Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X.: Synthesis, structure and electrochemical properties of lanthanum manganese nanofibers doped with Sr and Cu. J. Alloy. Compd. 638, 204–213 (2015)
Liu, Y.; Dinh, J.; Tade, M.O.; Shao, Z.: Design of perovskite oxides as anion-intercalation-type electrodes for supercapacitors: cation leaching effect. ACS Appl. Mater. Interfaces 8(36), 23774–23783 (2016)
Zhao, W.; Chen, G.: Exfoliation of Graphite toward Graphene from Lab to Industry. Graphite, Graphene, and Their Polymer Nanocomposites, p. 169. Taylor & Francis, USA (2012)
Züttel, A.; Sudan, P.; Mauron, P.; Wenger, P.: Model for the hydrogen adsorption on carbon nanostructures. Appl. Phys. A 78(7), 941–946 (2004)
Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A.: Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 39(4), 507–514 (2001)
Xia, J.; Chen, F.; Li, J.; Tao, N.: Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 4(8), 505 (2009)
Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S.: Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7), 1558–1565 (2007)
Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Katsnelson, M.; Grigorieva, I.; Dubonos, S.; Firsov, A.A.: Two-dimensional gas of massless Dirac fermions in graphene. Nature 438(7065), 197 (2005)
Zhang, X.; Zhang, H.; Li, C.; Wang, K.; Sun, X.; Ma, Y.: Recent advances in porous graphene materials for supercapacitor applications. RSC Adv. 4(86), 45862–45884 (2014)
Mao, X.; Yang, W.; He, X.; Chen, Y.; Zhao, Y.; Zhou, Y.; Yang, Y.; Xu, J.: The preparation and characteristic of poly (3, 4-ethylenedioxythiophene)/reduced graphene oxide nanocomposite and its application for supercapacitor electrode. Mater. Sci. Eng. B 216, 16–22 (2017)
Phiri, J.; Gane, P.; Maloney, T.C.: General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 215, 9–28 (2017)
Lang, X.; Sun, X.; Liu, Z.; Nan, H.; Li, C.; Hu, X.; Tian, H.: Ag nanoparticles decorated perovskite La0. 85Sr0. 15MnO3 as electrode materials for supercapacitors. Mater. Lett. 243, 34–37 (2019)
Elsiddig, Z.A.; Wang, D.; Xu, H.; Zhang, W.; Zhang, T.; Zhang, P.; Tian, W.; Sun, Z.; Chen, J.: Three-dimensional nitrogen-doped graphene wrapped LaMnO 3 nanocomposites as high-performance supercapacitor electrodes. J. Alloys Compd. 740, 148–155 (2018)
Liu, P.; Liu, J.; Cheng, S.; Cai, W.; Yu, F.; Zhang, Y.; Wu, P.; Liu, M.: A high-performance electrode for supercapacitors: Silver nanoparticles grown on a porous perovskite-type material La0. 7Sr0. 3CoO3− δ substrate. Chem. Eng. J. 328, 1–10 (2017)
Galal, A.; Hassan, H.K.; Jacob, T.; Atta, N.F.: Enhancing the specific capacitance of SrRuO 3 and reduced graphene oxide in NaNO 3, H 3 PO 4 and KOH electrolytes. Electrochim. Acta 260, 738–747 (2017)
Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.-W.; Voon, C.: Synthesis of graphene oxide using modified hummers method: solvent influence. Procedia Eng. 184, 469–477 (2017)
Rehman, Z.U.; Raza, M.A.; Tariq, A.; Chishti, U.N.; Maqsood, M.F.; Lee, N.; Awais, M.H.; Mehdi, S.M.Z.; Inam, A.: La0. 75Sr0. 25Cr0. 5Mn0. 5O3 perovskite developed for supercapacitor applications. J. Energy Storage 32, 101951 (2020)
Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M.: Improved synthesis of graphene oxide. ACS Nano 4(8), 4806–4814 (2010)
Cao, A.; Xu, C.; Liang, J.; Wu, D.; Wei, B.: X-ray diffraction characterization on the alignment degree of carbon nanotubes. Chem. Phys. Lett. 344(1–2), 13–17 (2001)
Tao, S.; Irvine, J.T.: Synthesis and characterization of (La0 75Sr0 25) Cr0 5Mn0 5 O 3− δ, a Redox-Stable, efficient perovskite anode for SOFCs. J. Electrochem. Soc. 151(2), A252 (2004)
Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X.: Sr-doped lanthanum nickelate nanofibers for high energy density supercapacitors. Electrochim. Acta 174, 41–50 (2015)
Raza, M.A.; Maqsood, M.F.; Rehman, Z.U.; Westwood, A.; Inam, A.; Sattar, M.M.S.; Ghauri, F.A.; Ilyas, M.T.: Thermally reduced graphene oxide-reinforced acrylonitrile butadiene styrene composites developed by combined solution and melt mixing method. Arab. J. Sci. Eng. 45(11), 9559–9568 (2020)
Wang, T.; Wang, L.-X.; Wu, D.-L.; Xia, W.; Jia, D.-Z.: Interaction between nitrogen and sulfur in co-doped graphene and synergetic effect in supercapacitor. Sci. Rep. 5(1), 1–9 (2015)
Yang, W.; Xu, X.; Tu, Z.; Li, Z.; You, B.; Li, Y.; Raj, S.I.; Yang, F.; Zhang, L.; Chen, S.: Nitrogen plasma modified CVD grown graphene as counter electrodes for bifacial dye-sensitized solar cells. Electrochim. Acta 173, 715–720 (2015)
Yang, W.; Xu, X.; Hou, L.; Ma, X.; Yang, F.; Wang, Y.; Li, Y.: Insight into the topological defects and dopants in metal-free holey graphene for triiodide reduction in dye-sensitized solar cells. J. Mater. Chem. A 5(12), 5952–5960 (2017)
Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y.: Binder-free electrodes and their application for li-ion batteries. Nanoscale Res. Lett. 15, 1–19 (2020)
Nian, Y.-R.; Teng, H.: Nitric acid modification of activated carbon electrodes for improvement of electrochemical capacitance. J. Electrochem. Soc. 149(8), A1008 (2002)
Come, J.; Taberna, P.-L.; Hamelet, S.; Masquelier, C.; Simon, P.: Electrochemical kinetic study of LiFePO4 using cavity microelectrode. J. Electrochem. Soc. 158(10), A1090 (2011)
Iqbal, M.Z.; Haider, S.S.; Siddique, S.; Karim, M.R.A.; Zakar, S.; Tayyab, M.; Faisal, M.M.; Sulman, M.; Khan, A.; Baghayeri, M.: Capacitive and diffusion-controlled mechanism of strontium oxide based symmetric and asymmetric devices. J. Energy Storage 27, 101056 (2020)
Ardizzone, S.; Fregonara, G.; Trasatti, S.: “Inner” and “outer” active surface of RuO2 electrodes. Electrochim. Acta 35(1), 263–267 (1990)
Faraji, S.; Ani, F.N.: Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors–a review. J. Power Sources 263, 338–360 (2014)
Yavarinasab, A.; Janfaza, S.; Tasnim, N.; Tahmooressi, H.; Dalili, A.; Hoorfar, M.: Graphene/poly (methyl methacrylate) electrochemical impedance-transduced chemiresistor for detection of volatile organic compounds in aqueous medium. Anal. Chim. Acta 1109, 27–36 (2020)
Yavarinasab, A.; Janfaza, S.; Tahmooressi, H.; Ghazi, M.; Tasnim, N.; Hoorfar, M.: A selective polypyrrole-based sub-ppm impedimetric sensor for the detection of dissolved hydrogen sulfide and ammonia in a mixture. J. Hazard. Mater. 416, 125892 (2021)
Elsiddig, Z.A.; Wang, D.; Xu, H.; Zhang, W.; Zhang, T.; Zhang, P.; Tian, W.; Sun, Z.; Chen, J.: Three-dimensional nitrogen-doped graphene wrapped LaMnO3 nanocomposites as high-performance supercapacitor electrodes. J. Alloy. Compd. 740, 148–155 (2018)
Acknowledgements
Authors would like to thank higher education commission of Pakistan (HEC) for providing financial support to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ur Rehman, Z., Raza, M.A. La0.75Sr0.25Cr0.5Mn0.5O3/Graphene Oxide-Based Composite Electrodes for Energy Storage Applications. Arab J Sci Eng 47, 6365–6377 (2022). https://doi.org/10.1007/s13369-021-06345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-06345-5