Abstract
Crop residue-based biochar (CRB) has shown great potential for removing trace elements (TEs) from aquatic matrices. Despite the increasing interest in this area, no review has focused specifically on the efficacy of CRB for TEs removal in aquatic environments. This comprehensive review examines the global TEs water contamination status with an emphasis on their sources, compositional metrics for crop residue feedstock (proximate, ultimate, and lignocellulosic properties), and the potential use of CRB for TEs removal in aquatic media. It also evaluates the factors that affect the ability of CRB to remove TEs, such as feedstock type, production conditions, water pH, background electrolytes, water temperature, CRB/water ratio, and underlying pollutant sorption mechanisms. This review also discusses the practical applications of CRB in real water samples and engineering considerations for designing CRB with improved physicochemical properties, treatment efficiencies, and regeneration abilities. Additionally, the cost–benefit and economic assessment of CRB, challenges, and future research directions related to CRB are highlighted to promote research on this sustainable source of biochar. By elucidating the prospects of CRB as an adsorbent, this review emphasizes the need for continued research on its practical implications for environmentally relevant pollutant concentrations.
Graphical Abstract

Highlights
-
Crop residue-based biochar (CRB) emerged as a top-notch adsorbent for TEs in aquatic systems.
-
The CRB adsorption potential depended on the experimental conditions.
-
The application of CRB to real water samples highlights its potential on a larger scale.
-
Engineer-designed CRB exhibits high treatment efficiencies and regeneration abilities.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Introduction
The rapid development of multiple industries has resulted in the significant discharge of trace elements (TEs) into aquatic ecosystems, posing a direct threat to the safety of water resources, agricultural productivity, and human health (Haris et al. 2021; Nabi et al. 2011; Wang et al. 2022). For example, chromium (Cr) pollution in potable water is prime for the emergence of “cancer villages” in some regions of Liaoning Province, China (Xia et al. 2019). Numerous water treatment approaches have been used to remove TEs, including electrocoagulation, precipitation, ion exchange, ozonation, membrane separation, capacitive deionization, biosorption, and adsorption etc. (Haris et al. 2023a). Nevertheless, multiple drawbacks limit their practical applications, predominantly at the community level, where they are most required. For example, membrane capacitive deionization (MCDI), capacitive deionization, and electrocoagulation approaches are expensive (He et al. 2023) and require professional assistance for installation and operation. Therefore, it is imperative to develop innovative, facile, and cost-effective technologies for the treatment of TEs in aquatic matrices. Among these, adsorption has been documented as a promising technique because of its facile operation, high efficiency, and low cost (Rosales et al. 2017; Neolaka et al 2023a, b; Haris et al. 2022b). However, some drawbacks limit its practical application, such as low removal capacity, narrow pH range, and the use of expensive adsorbents (Jabar et al. 2022). Therefore, it is important to select appropriate adsorbents to mitigate and offset these limitations.
Biochar is considered an effective and eco-friendly material for TEs treatment because of its micro- and/or mesoporous framework, high surface area, various surface functionalities, and the presence of inorganic mineral species (Zama et al. 2017). In addition, the use of waste materials as feedstock for developing biochar synchronizes with the principles of the circular bioeconomy and sustainable development (Darmokoesoemo et al. 2016; Khera et al. 2020; Ambika et al. 2022), with a significant positive impact on environmental health (Hizal et al. 2013; Putranto et al. 2016; Nidheesh et al. 2021). To date, various biomasses such as agricultural wastes, forest residues, algae wastes, wood, and bagasse have been applied to produce biochar for the removal of toxic pollutants from aquatic matrices (Kuncoro et al 2018a, b; Kusuma et al. 2024; Naat et al. 2021). Among biomasses, crop residues are the most available source for the low-cost production of biochar, which has gained significant attention as an adsorbent for environmental applications (Muhammad et al. 2021). Moreover, crop residue-based biochar (CRB) has more advantages, including greater surface area and inferior ash content, than biochar produced from other feedstocks, which can be attributed to the presence of high amounts of lignocellulose in the crop residue feedstock (Haris et al. 2022a, b).
Recent reviews have focused on specific feedstocks and their applications in wastewater treatment of organic and inorganic pollutants. For example, Foong et al. reviewed rice straw-derived biochar adsorbents and their applications in wastewater treatment (Foong et al. 2022). Yasir et al. (2022) focused on engineered biochar developed from different feedstocks for TEs removal from wastewater. These reviews have made significant contributions by summarizing and characterizing biochar production and its diverse applications in contaminated environments, thereby promoting further research. To the best of our knowledge, a comprehensive review that specifically covers the potential use of CRB to remove TEs from contaminated aquatic matrices has not been published to date despite the considerable amount of available research data. The intent of this review is to compile and analyze publications pertaining to biochar production from different crop residue feedstocks, with a specific focus on their application in treating TEs in contaminated aquatic systems. Considering the immense amount of data on the application of CRB for remediation of water matrices, we are convinced that this review will be of significant interest for researchers involved in the “waste-to-wealth” approach for addressing TEs in contaminated aquatic matrices.
This review advances our understanding of the potential applications of engineered/designed CRBs for the removal of TEs from aquatic media and their associated challenges. We provide a critical summary of several key aspects: (i) sources of TEs contaminated aquatic matrices; (ii) composition of crop residue feedstock; (iii) factors affecting the removal efficiency of CRB under different conditions including the feedstock type and production condition, water pH, background electrolytes, water temperature and CRB/water ratio; (iv) the removal mechanisms by which CRBs remove TEs; (v) the applications of CRBs in real water samples; and (vi) engineering consideration for designing CRBs with improved properties, treatment efficiency and regeneration ability. Furthermore, this review presents a cost–benefit and economic assessment of CRB, highlights the underlying challenges, and provides prospective strategies and possibilities for future research in this field.
2 Sources of TE polluted water system
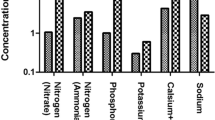
The TE contamination in drinking water is an unavoidable environmental concern, attributed to both geogenic and anthropogenic activities (Fig. 1). The geogenic sources of TEs are weathering and leaching of metals from parent rocks and are predominately lower/or-within the range of permissible values recommended by the WHO, USEPA, and local environmental protection agencies (WHO 2011). However, Arslan and Ayyildiz Turan (2015) carried out a trial in the Northern Develi Closed Basin, Kayseri, Turkey, and reported that the As (0.0634 mg L–1) and Fe (0.9978 mg L–1) concentrations surpassed the Turkish drinking water standard values (0.010 and 0.300 mg L–1, respectively) in groundwater, resulting from the attraction of extremely transformed volcanic and pyroclastic rocks to water. In addition, the concentration of uranium (U) in groundwater is increasing globally owing to geogenic sources, and is responsible for cancer in humans (Burow et al. 2017). Naturally, U originates from silicates (Swamboite and Uraninite), phosphates (Torbernite and Autunite), carbonates (Andersonite and Bayleyite), and oxides (Uraninite and Metaschoepite). In natural environments, uraninite (U-enriched silicate) is immobile and instantly oxidized to soluble uranyl ions (U(VI)O22+) on rock-water attractions. During anoxic states, the Fe and S species can reduce immobile U(VI) to highly mobile forms [U3O8/U4O9/UO2] (explained in the following equations) and account for the increasing U concentration in groundwater (Hua et al. 2018).
Anthropogenic sources that contribute to TE contamination in water resources are divided into two groups: (I) point sources (industries and mining activities); and (II) non-point sources (fertilizers). Worldwide, growing industries (such as textiles, pharmaceuticals, electronics, paint, and electroplating) release untreated wastewater that accumulates on soil surfaces and poses a risk to groundwater quality. For instance, pervasive Cr application in wood treatment via the depletion of copper–chromium–arsenate, leather tanning, pigments, and electronic and electroplating industries could increase the Cr content in wastewater (Gürkan et al. 2022; Gürkan et al. 2023b). Wastewater discharged from chemical and electronic factories into groundwater accounts for an increase in Hg(II) concentration. Similarly, Noreen et al. (2017) reported that the textile industry is predominantly responsible for the Ni(II), Pb(II), and Cd(II) contamination of groundwater in Faisalabad, Pakistan. Moreover, Shaheen et al. (2013) reported that dyeing and textile industry effluents are the main sources of Cu(II) and Zn(II) contamination in groundwater. Sewage wastewater contains a high concentration of TEs, such as Cr, Ni, Pb, and Cd, resulting in the contamination of groundwater. For example, Leung and Jiao (2006) noticed that the concentrations of Se and B substantially increased near the urban area of Hong Kong, China, owing to the corrosion/leakage of stormwater drains.
Mining is recognized as one of the dominant sources of TE pollution in both surface and groundwater around the globe (Haris et al. 2023b). Mines engender sulfide-enriched wastes, such as tailings and overstrained rocks, through mining and mineral processing accountable for producing acid-mine drainage (AMD), which comprises copious amounts of TEs via contact with water and oxygen, and ultimately contaminates the environment (Ahmadi et al. 2016). Runoff produced from AMD and mine waste piles at active and unrestrained mining sites is attributed to the TE contamination of surface water streams. Fertilizers are considered nonpoint sources of groundwater contamination by TEs. Fertilizers produced from phosphorite have high contents of TEs (i.e., As, Cd, and U) and carbonate-derived fertilizers are enriched with rare earth elements (REE) (i.e., Th, Sr, and Ba) (Otero et al. 2005). Wastewater from phosphate fertilizer factories is extremely polluted with Cd and leads to contamination of groundwater (Huang et al. 2018). Therefore, the removal of TEs from aquatic systems has received considerable attention. The development of high-potential, low-cost materials is important for the effective removal of TE-polluted water matrices. Preferably, these materials should be attractive from both economic and industrial perspectives (i.e., readily scalable and cost-effective) and synthesized from renewable and naturally abundant resources.
3 Crop residues composition
The composition of feedstocks plays an indispensable role in the production of biochar and governs the quality and properties of the final product (Tomczyk et al. 2020). Crop residues contain lower ash content, fewer voids, and greater calorific value than organic waste (such as compost, manure, and sewage sludge) and woody biomass (Ji et al. 2022). Recently, various crop residues have been used as feedstock for biochar production (Table 1). The proximate, ultimate, and lignocellulosic components are the major compositional metrics of crop residues. The proximate biomass components include moisture content (MC), fixed carbon (FC), ash, and volatile matter (VM). The MC, FC, ash, and VM contents of the crop residues were within the ranges of 0–10%, 3–26%, 1–15%, and 65–90%, respectively (Table 1). The VM content and the yield of biochar were more sensitive to the pyrolysis environment (i.e., temperature and residence time), whereas the feedstock type predominantly affected the ash and FC contents of biochar. Biochar containing a high ash content has great potential as a catalyst for thermal conversion approaches; however, it is unsuitable for adsorption-associated applications (i.e., TEs adsorption from aquatic media) because it can limit the availability of binding sites on the biochar surface, and biochar with a high ash content typically reduces the microporous surface area. Predominantly, crop residues contain a lower ash content than organic wastes, which leads to a higher surface area and porosity of crop residue-based biochar (CRB) (Haris et al. 2021; Leng et al. 2021). Recent research attempts (i.e., Wang et al. 2018; Singh et al. 2021) have been made to use crop residues as a feedstock source to produce CRB and have used them as adsorbents in TE-contaminated water matrices owing to their lower ash content in comparison to other feedstock biomass and organic wastes. The ultimate composition is another significant compositional feature that includes oxygen (O), carbon (C), nitrogen (N), sulfur (S) and hydrogen (H). Among these elements, C exhibited a prodigious percentage in various biomasses, followed by O and H, accounting for 40–65%, 25–50%, and 5–10%, respectively. The C content of biochar depends on the feedstock type, and CRB has a higher C content than organic waste (e.g., sewage sludge and manure) (Ji et al. 2022). It has been reported that greater O and C contents in feedstocks might result in higher yields and net calorific value of biochar (Leng and Huang 2018). The N content of biochar is considered an essential aspect of fertilizer application. The presence of high levels of proteins and macromolecular amino acids in the feedstock resulted in an increased N content in the biochar. Among feedstocks, the N content of crop residues is generally higher than that of woody biomass and lower than that of organic waste (Pariyar et al. 2020). The structural composition of crop residues is determined by their hemicellulose, cellulose, and lignin (H–C–L) contents, which strongly control the biochar characteristics and yield. For agricultural biomass, the H–C–L content was within the ranges of 11–39%, 28–47%, and 9–27%, respectively (Haris et al. 2021; Muhammad et al. 2021) (Table 1). These characteristics make crop residues the most appropriate feedstock for the production of biochar, which can be used as adsorbents for the removal of TEs from aquatic systems.
4 Removal of TEs from water with CRB
Recently, various crop feedstocks, such as wheat straw (Bandara et al. 2020), sugarcane straw (Singh et al. 2021), corn straw (Zhao et al. 2019), and rice straw (Pan et al. 2015) have been used to synthesize CRB to remove TEs from aqueous systems. The synthesis techniques, physiochemical properties, characterization approaches, and morphology of the synthesized crop residue biochar were critically reviewed in our previous article (Haris et al. 2021). The removal efficiency of CRB for TEs is given in Table 2 and indicates that removal efficiency of CRB towards TEs is dependent on multiple factors including physicochemical properties of CRB, solution pH, feedstock type, background electrolyte in binary solution and water temperature. The higher removal efficiency of TEs by CRB can be attributed to the characteristics of CRB, such as its higher surface area, low H/C ratio, pH buffering capacity, rational pore structure, inferior ash content, and dosage rate.
4.1 Feedstock type and production conditions of CRB
The source of crop feedstock and pyrolysis temperature are the dominant factors governing the potential of CRB to remove TEs (Jindo et al. 2014). Kwak et al. (2019) investigated the effect of pyrolysis temperature and feedstock type (wheat, canola straw, and manure pellets) on the removal of Pb(II) from water matrices. They found that increasing the pyrolysis temperature (300 and 700 °C) significantly improved the removal capacity of Pb(II) for all feedstock-produced biochars (wheat straw biochar, 55 and 109 mg g–1; canola straw biochar, 84 and 108 mg g–1 and manure pellets, 49 and 68 mg g–1). CRB showed a higher adsorption capacity for Pb(II) than the manure pellets, which was attributed to its lower ash content and higher surface area (Table 2). In another study, biochars were synthesized from different crop feedstocks (rice husk, cotton stem, wheat straw and corn stem) at slow pyrolysis temperatures (350 and 550 °C) and used to remove TEs including Zn(II), Cu(II), Cd(II) and Pb(II) in aqueous media. They concluded that cotton stem biochar showed a higher removal efficiency for Cu(II) (0.34‒12.83%) and Pb(II) (1.90‒30.42%) in multi-metal systems than other feedstock biochars (Wang et al. 2018). The variations between the biochars produced from different feedstocks could provide a comprehensive understanding of the selection of the most appropriate CRB to remediate TE-polluted water matrices.
Zama et al. evaluated the influence of pyrolysis temperature and feedstock type on the sorption performance of biochar derived from buckwheat husk (Bw), peanut shells (Ps), and corncobs (Cc) towards TEs (As(III), Pb(II), and Cd(II)). They found that different feedstocks showed varying sorption percentages of the target pollutants with changes in pyrolysis temperature. The Bw biochar exhibited an increase in sorption percentage of Pb(II) (90.2%, 99.6% and 99.8%) and Cd(II) (10.9%, 50.9% and 60.8%) with increasing pyrolysis temperature (350, 450, 650 °C, respectively), while a decrease in sorption percentage was observed for As(III) (39.5%, 37.2% and 33.8%). In contrast, the Ps biochar showed a decrease in the sorption percentage of Cd(II) (99.2%, 96.7%, and 93.1%) and As(III) (28.9%, 27.1%, and 26.4%) with increasing pyrolysis temperature, whereas the Pb(II) sorption percentage remained relatively stable. Cc biochar exhibited the highest sorption percentage of Cd(II) (80.3%), Pb(II) (98.9%), and As(III) (33.4%) at a pyrolysis temperature of 450 °C (Zama et al. 2017). Pyrolysis temperature had an antagonistic influence on the CRB removal potential of CRB, reliant on the feedstock source. In some scenarios, the removal efficiency increased [wheat and canola straw biochar; Kwak et al. (2019)], likely due to increased surface area and the existence of more oxygen functionalities on the surface of biochar, which facilitate the adsorption of metal ions, whereas in other cases [peanut shells and corncobs; Zama et al. (2017)], it was noticed that the removal efficiency of CRB decreased with increasing pyrolysis temperature for TEs in water solutions, which is attributed to the development of more stable aromatic structure and lower surface area at higher pyrolysis temperatures, resulting in decreasing the active sites available for adsorption of metal ions (Haris et al. 2023a). These findings could be useful for selecting a suitable temperature for the pyrolysis of various crop feedstocks to obtain greater removal efficiency of TEs in aquatic matrices based on the source of crop residue feedstocks and the type of pollutant.
4.2 pH of the aqueous solution
Functional groups on the CRB surface influence the adsorption of TEs because of their capacity to ionize water (Mukherjee et al. 2021; Gürkan et al. 2022; 2023a). The generation of charged ions and electrons on the CRB surface was primarily dependent on the pH of the aqueous medium. For pH values > pKa values of the functional groups on the CRB surface, these groups are predominately disassociated and might commute H+ with metal cations (e.g., Cd(II) and Pb(II)) in solution (Kang et al. 2022). However, the pH < pKa values result in a complexation process, predominantly for the COOH groups (Tong et al. 2011; Shakoor et al. 2016). Various studies have explored the adsorption of TEs onto CRB as a multifaceted function of pH, owing to fluctuations in the removal potential of biochar and the speciation of metal ions with pH (Fan et al. 2020; Kwak et al. 2019). The studies listed in Table 2 show that the sorption of TEs is pH dependent. For instance, some studies (e.g. [Muhammad et al. (2021); Mukherjee et al. (2021)] noticed that acidic environments (pH = 2.0–3.0) resulted in lower TEs adsorption with CRB, unveiling that the existence of positively charged groups on CRB surface could be responsible for lower adsorption of aforementioned pH values.
Similarly, Wang et al. (2018) examined Cd(II) adsorption onto corncob-derived biochar and observed that the maximum Cd(II) removal capacity was attained within the pH range of 2.0‒5.0. At pH < 2.0, the biochar surface was protonated and accounted for a higher electrostatic repulsion with the positively charged Cd(II) ions, resulting in a decrease in the removal efficiency of the biochar. Chen et al. (2011) found that the adsorption capacity of Zn(II) and Cu(II) was enhanced with increasing pH by cornstraw biochar and maximum removal capacity (10.76 and 8.20 mg g−1 respectively) was achieved at pH 5.0. Similar annotations regarding TEs adsorption on CRB surfaces have been reported. For instance, Bandara et al. found that canola shoot, lucerne shoot and wheat straw derived biochar attained their maximum adsorption capacities at pH 5.0‒5.5 for Cu(II) and Cd(II) (Bandara et al. 2020). However, Cr(VI) exhibited different behavior, and its maximum removal capacity was observed under highly acidic conditions (pH 1.0). This phenomenon could be attributed to the significant protonation occurring on the biochar surface at lower pH, facilitating the development of an ion-pair attraction mechanism among positively charged groups and chromate (CrO42−) ions (Sinha et al. 2022).
The influence of solution pH on As(III) and As(V) removal capacity was investigated using rice husk, empty fruit bunch biochar, and their engineered biochars. They observed that at pH 8.0‒9.0, the highest As (III) removal capacity was achieved for all biochars, with maximum As(V) adsorption at pH < 6.0. However, the sorption affinity of As(V) significantly declined in the pH range of 7.0‒9.0, which could be associated with the highly negatively charged biochar surface at pH > 7.0 (Samsuri et al. 2013). Similarly, Pan et al. (2015) observed the effect of pH on As (V) removal capacity using peanut and rice straw biochars. They found that As(V) sorption decreased with increasing pH, and maximum adsorption was observed at pH 3.7 (~ 27 and 18 g kg–1, respectively). The solution pH affects the As speciation in distinct forms (anionic and neutral form, i.e., AsO43−, HAsO42−, H2AsO4−, H3AsO4, etc.). At acidic pH (pH = 3.0‒6.0), As predominantly exists as H2AsO4−, whereby under alkaline conditions (pH > 8.0), AsO43− and HAsO42− are considered as foremost species (Vithanage et al. 2017). Furthermore, AsO33−, H2AsO3− and AsO3−3 are recognized as more stable form of As at pH 12.0‒13.0, 9.0‒12.0 and 13.0‒14.0, respectively. Therefore, the sorption mechanisms of various forms of As on certain types of biochar are complex because of the distribution of As(V) and As(III) species in aquatic media as a function of pH (Alkurdi et al. 2019).
Surface charge is another significant factor that controls the adsorption of TEs on CRB and is highly dependent on solution pH following its application in aquatic systems. The point of zero charge (pHPZC) of CRB signifies the pH of the solution where its surface manifests a zero net charge. For example, the CRB surface is highly negatively charged when the pH of the solution is greater than that of pHPZC and readily binds cationic metals such as Cd(II), Hg(II), and Pb(II). Whereas, the biochar surface shows higher affinity for anionic metals species (HCrO4− and HAsO42–) when pH < pHPZC due to its significant positive charge (Li et al. 2017a, b). Yuan et al. evaluated the influence of zeta potential of biochars derived from crop residues (peanut, soyabean, corn and canola straws) at various pyrolysis temperatures (300, 500 and 700 °C). They stated that biochars produced from crop residues within solution pH of 3.0–7.0 exhibited negatively charged; however, the synthesized biochar at higher pyrolysis temperature of 700 °C manifests inferior negative charged in relation to those produced at 300 and 500 °C. The amount of negatively charged functional groups, such as –OH, –COOH, and –COO– substantially decreased at higher pyrolysis temperatures and accounted for lower negatively charged surfaces and increased pHPZC (Yuan et al. 2011). The aforementioned discussion indicates that pH is a significant factor that influences the ability of CRB to remove TEs from water matrices. This information is significant because it highlights the importance of manipulating water pH to achieve the maximum adsorption of TEs by CRB.
4.3 Background electrolyte and temperature
The existence of background electrolytes, either in individual (monometal) or competitive (binary) forms in solution, can significantly influence the sorption affinity of CRB for specific metals (Tehreem et al. 2022; Gürkan et al. 2022, 2023b). In general, the sorption affinity for TEs is affected more in binary solutions than in mono-metal removal systems (Fan et al. 2020). Mobile metals (such as Zn(II), Ni(II), and Cd(II)) are typically more affected than sharply adsorbed metals, such as Cu(II) and Pb(II) (Alkurdi et al. 2019). For example, Fan et al. (2020) investigated the sorption affinity of rice straw and its engineered modified biochar for TEs in single and binary metal sorption systems. They reported that biochar showed a higher adsorption capacity for Pb(II) and Cd(II) in monometal sorption solutions than in binary-metal systems. Moreover, the adsorption capacity of Cd (II) decreased more (52%) than that of Pb(II) (6%). The distribution coefficient (Kd) is recognized as a significant metric for evaluating the adsorption capacity of adsorbents for specific metals in multiple metal systems and exhibits more prominent selectivity towards Cd(II) over Pb(II).
Kang et al. (2022) examined the potential of pristine biochar (derived from corn residue) and engineered biochar for single and multiple metal adsorption systems. They noted that Cd(II) and Zn(II) were adsorbed more efficiently from single-metal solutions than from competing solutions. Similarly, Haris et al. (2022b) investigated the adsorption of Tl(I) using wheat straw biochar in the presence of competing ions including Mg(II), Ca(II), Zn(II), and Cu(II). They reported that, at a concentration of 1.0 mmol L–1, the proficiency of biochar in removing Tl(I) was reduced to 28% and 24%, respectively, owing to the presence of Cu(II) and Zn(II) in the metal solution. This could be associated with the greater electronegativity values of Cu(II) and Zn(II) (1.90 and 1.65, respectively) than that of Tl(I) (1.62). Higher electronegativity increases competition at the binding sites with Tl(I), leading to decreased Tl(I) sorption on the biochar surface. These findings suggest that the removal potential of TEs by CRB in monometal polluted water could be greater than that in multiple-metal polluted water. Therefore, CRB could exhibit a lower efficiency in removing TEs in saline water than in fresh water.
The removal potential of CRB is also affected by water temperature, as reported in various studies [i.e., Chen et al. (2011); Kang et al. (2022); Liu et al. (2016)], where higher temperatures favor TEs adsorption. For example, the Hg(II) removal potential of different crop residues biochar (corn stover, corncob, wheat shaft and cotton seed husk) produced at various temperatures (300, 600 and 700 °C) was investigated by Liu et al. (2016). They stated that the concentration of Hg(II) in adsorption trial was decreased > 90% with biochar produced at 600 and 700 °C, whereas 40‒90% decrease was observed in biochar produced at 300 °C. Hg(II) adsorption showed a positive correlation with increasing temperature, indicating that higher water temperatures deliver sufficient energy for metal ions to overwhelm the diffuse double layer and sequester into the interior structure of the CRB. Furthermore, Zhang et al. performed an experiment to determine the influence of water temperature on rice straw biochar for Cd(II) adsorption and suggested that Cd(II) adsorption onto biochar surface is spontaneous endothermic reaction and accounted for increase in Cd(II) adsorption with increasing water temperature (Zhang et al. 2019a, b). These findings increase our knowledge of the influence of water temperature on TE removal efficiency by CRB, and it could be possible to increase the removal potential of TEs by increasing water temperature.
4.4 CRB/water ratio
The CRB dosage added to aquatic media is recognized as a significant factor affecting TEs removal efficiency (Cao et al. 2019) (Table 2). For instance, Singh et al. (2021) performed a sorption experiment to determine the influence of dosage (0.1 to 0.5 g) on TEs adsorption, while reserved other parameters (i.e., temperature, contact time and pH) constant. They observed that the removal efficiencies of Cr(VI), Ni(II), Cd(II), Pb(II), and Cu(II) increased from 87.62 to 99.36%, 11.56 to 43.31%, 11.76 to 99.24%, 59.0 to 95.52% and 36.43 to 91.59% as the dosage increased from 0.1 to 0.5 g L–1. This could be associated with an increase in the surface area with increasing biochar dosage, providing more binding sites for metal adsorption on the biochar surface.
In contrast, a higher biochar dosage also decreased the uptake of TEs on the biochar surface owing to the aggregation of binding sites. Mukherjee et al. studied the impact of rice straw biochar dosage (0.5, 2, and 5 g L–1) on As(V) removal. They concluded that at 2 g L–1, biochar exhibited > 45% (14.8 in comparison to 9.03 and 2.75 µg g–1) higher adsorption capacity, which was approximately 3 times greater compared to 0.5 and 5 g L–1. The adsorption efficiency at a biochar dosage of 5 g L–1 increased to 64%, owing to the availability of more binding sites and a mass-transfer concentration gradient, resulting in faster diffusion of As(V) to the exterior structure of the biochar. The removal percentage of As(V) increased from ~ 31 to 64% at biochar dosages of 0.5 and 5 g L–1 but led to 5 times decrease in the equilibrium adsorption capacity (qe). This indicated that the decrease in the dispersion capacity of biochar in the presence of the As(V) solution was a consequence of the aggregation of binding sites caused by collisions between biochar particles (Mukherjee et al. 2021).
In addition, Cao et al. stated that with increasing adsorbent dosage (0.2‒1.0 g L–1), adsorption capacity of wheat straw, its derived biochar and ball-milled biochar decreased from 46.33, 119.55, and 134.68 mg g–1 to 27.44, 99.45 and 100.0 mg g–1, respectively, which was linked to the unsaturation of adsorption sites, and the overlapping or aggregation of adsorption sites. However, the removal rates (Re) of all adsorbents increased significantly with increasing adsorbent dosage because of the increased availability of binding sites (Cao et al. 2019). The above discussion indicates that the biochar solution dosage in the metal solution is a significant factor controlling the adsorption capacity and removal percentage of CRB for TEs in contaminated water matrices. This dosage is dependent on the type of biochar and metal, as well as on its concentration.
5 Real water samples treatment with CRB
Although numerous studies have explored the effectiveness of CRB in removing TEs from laboratory prepared aqueous solutions, research on their application in real-world scenarios is still lacking. Studies investigating the application of CRB in the removal of TEs from real water samples are limited. Therefore, the existing research on the application of CRB for TEs removal in real water samples is summarized.
For example, Han et al. (2020) investigated the Cr(VI) removal from wastewater (25 °C) and ice (– 20 °C) by using rice husk biochar. This study revealed that dissolved organic matter (DOM) plays a more significant role in enhancing Cr(VI) removal from ice than from wastewater. It was concluded that DOM acted as an electron shuttle, thereby facilitating efficient Cr(VI) removal under freezing conditions. The successful removal of Cr(VI) using rice husk biochar was demonstrated in real-world Cr(VI)-contaminated wastewater under freezing conditions, highlighting the environmental significance of freezing-assisted Cr(VI) removal in cold regions. Similarly, Ahmad et al. used biochar (derived from dew melon peel) to test its potential for Cr(VI) removal from electroplating and tannery industrial wastewater. They found that biochar removed 99.9% and 100% of the Cr(VI) from electroplating and tannery wastewater, respectively, within 40 min. This highlights the potential of biochar as a cost-effective solution for industrial wastewater treatment (Ahmadi et al. 2016).
The TEs (e.g., Cd(II) and Cu(II)) removal efficiency of different CRB (such as canola shoots, lucerne shoots, and wheat straw) in mining areas (collected from New South Wales, Australia) containing 0.58 and 2.28 mg L–1 concentrations, respectively, which are above the standard values in drinking water (0.003 and 2.0 mg L–1, respectively) was tested. The results indicated that the adsorption efficiency of Cu(II) in mine water followed the order of lucerne shoots (99.8%) > canola shoots (95.0%) > wheat straw (0.55%), whereas a similar trend of removal efficacy for Cd(II) was also observed, i.e. lucerne shoots (82%) > canola shoots (20%) > wheat straw (6.5%). Notably, biochar derived from lucerne and canola shoots were effective in removing Cu(II) from mine water to levels below the WHO standard for drinking water (2 mg L–1), whereas biochar derived from wheat straw exhibited limited effectiveness in removing Cu(II) from mine water (Bandara et al. 2020). River water has a complex composition characterized by the presence of multiple coexisting ions that can compete for binding sites on the biochar surface. Moreover, the presence of organic substances in this natural system limits the mobility of TEs and complicates their uptake by adsorbent surfaces. Haris et al. used river water to evaluate the potential of exfoliated biochar nanosheets (derived from wheat straw). The results showed that the removal potential (89.66%) of the exfoliated biochar was influenced in the river water samples compared with that in the aqueous solution (92.2%). This could be imputed to higher value of COD (≈ 33.8 mg L–1) observed in the tested river water samples, manifesting the presence of organic materials in significant amounts (Haris et al. 2022b). CRB exhibited greater removal potential for TEs in both potable water and aqueous solutions. These findings demonstrate the potential application of CRB as an adsorbent to remediate TE-polluted water matrices. However, limited research has been conducted on TEs removal from actual water matrices. Therefore, future studies should prioritize the development of CRB and its application in the treatment of TE-polluted real water matrices.
6 Removal mechanisms of TEs by CRB
The TEs adsorption efficiency of CRB in aquatic media can be attributed to the pyrolysis conditions (temperature and residence time) and the intrinsic characteristics of the biomass (Ambika et al. 2022). For instance, the presence of functional groups (e.g., carboxylic and hydroxyl groups) accounts for the increasing number of negatively charged surface groups on the CRB. These sites ultimately bind to cationic TEs such as Pb(II), Cd(II), Cu(II), and Zn(II) in aquatic media (Zhang et al. 2018; Gao et al. 2019; Qian et al. 2016; Zheng et al. 2021). TEs adsorption on the surface of CRB can be explained by different mechanisms (Fig. 2), such as surface complexation, electrostatic interaction, ion exchange, and coprecipitation (Liu et al. 2022).
Various characterization strategies (FTIR, XPS, XRD, etc.) have been applied to demystify the adsorption of TEs on CRB by changing the spectral peaks (Tripathi et al. 2016). For instance, the FTIR analysis of peanut shell biochar after Pb(II) adsorption was performed by Shan et al. (2020), who concluded that hydroxyl peaks at 3200–3500 cm–1 decreased after Pb(II) adsorption, indicating that the oxygen-containing functional groups are responsible for complexes with metals on the biochar surface through surface complexation and electrostatic interaction processes. The crystalline minerals involving quartz (SiO2), calcite (CaCO3), gonnardite [(Na, Ca)2(Si, Al)5O10·3H2O] etc., may exist as inorganic mineral species in CRB (Tripathi et al. 2016). These minerals release anions (SO42– and CO32–) and bond with cationic TEs via precipitation and ion exchange processes on CRB. Several studies [i.e., Rizwan et al. (2020); Xu et al. (2013)] investigated the precipitation of Cd3(PO4)2, Pb3(CO3)2(OH), PbCO3 etc., which should be considered as a significant mechanism for Pb(II) and Cd(II) removal by rice husk biochar.
In addition, Haris et al. used different characterization approaches (e.g., FTIR and XPS) to ratify the adsorption mechanism of Tl(I) on an exfoliated biochar surface derived from wheat straw. FTIR analysis indicated three main differences in the spectrum pattern of the exfoliated biochar surface after the adsorption of Tl(I), as presented in Fig. 3a. The broader peak at 3433 cm–1 attributed to stretching vibration of –OH was decreased and slightly shifted to 3428 cm–1, manifesting that surface complexation is accountable for Tl(I) adsorption on exfoliated biochar surface. The peak at 1692 cm–1 was assigned to C = O functional groups and diminished after reacting with Tl(I). Furthermore, the peak at 1620 cm–1 represented the stretching vibrations of carbonyl groups in form of quinone and shifted towards lower value (1608 cm–1) after adsorption, indicating that oxygen-containing functional groups is responsible for adsorption of Tl(I) on biochar surface. Moreover, to further understand the adsorption mechanism, XPS was used to observe Tl(I) adsorption, and the obtained survey scan signified the existence of Tl(I) species with the inset of the narrow spectrum Tl 4f (Fig. 3b). An obvious doublet peak was observed at approximately 119 and 123 eV, which were assigned to Tl 4f7/2 and Tl 4f5/2, respectively (Fig. 3c). The high-resolution O 1 s spectrum was further obtained before and after the adsorption of Tl(I), and significant differences after the adsorption of Tl(I) were evidenced by the respective deconvoluted peaks of the O 1 s spectrum. The peaks at 530.73, 533.43 and 534.8 eV (C–O/O–H/C–O–C) were altered towards 531.30, 533.20 and 534.09 eV after Tl(I) adsorption, which suggested that oxygen-containing functional groups (hydroxyl) is accountable for Tl(I) complexation. The hydroxyl group content decreased from 63.21 to 46.66%, which further corroborated that the –OH groups accounted for the adsorption of Tl(I) (Fig. 3d). Based on these results, a potential mechanism governing Tl(I) removal using exfoliated biochar is proposed, suggesting that negatively charged biochar surfaces (–OH groups) play a substantial role in Tl(I) adsorption through surface complexation and electrostatic attraction processes (Fig. 3e) (Haris et al. 2022b).
FTIR spectrum of the exfoliated biochar before and after adsorption (a), XPS survey after Tl(I) adsorption (b), high-resolution Tl 4f (c), high-resolution O 1 s spectrum before and after Tl(I) adsorption (d), and anticipated reaction mechanism of Tl(I) adsorption on exfoliated biochar surface (e); reproduced with the permission of the publisher (Haris et al. 2022b)
Carboxylic and phenolic groups are considered the most substantial binding sites for TEs adsorption (Kamali et al. 2021; Xie et al. 2022). Xiong et al. (2013) reported that complexation of Pb (II) by carboxylic and phenolic functional groups in the structure of humic substances induces the formation of inner-sphere complexes with oxygen and carbon atoms. In comparison to Pb(II) and Cd(II), there were negligible or insignificant variations in the bond energy by XPS for As(III), indicating that complexation and electrostatic attraction are substantial mechanisms for As(III) adsorption, whereas ion exchange and precipitation were considered the dominant mechanisms for Pb(II) and Cd(II) removal by peanut shell biochar (Zama et al. 2017). However, Fan et al. (2020) observed that surface complexation and electrostatic attraction mechanisms accounted for the removal of Cd(II) and Pb(II) from aquatic media using rice straw biochar. These findings suggest that precipitation, ion exchange, complexation, and electrostatic attraction are the dominant mechanisms of Cd(II) and Pb(II) sorption by CRB. However, their sorption is dictated by the biochar properties, which are affected by the feedstock source, pyrolysis conditions, and adsorption parameters (temperature, pH, etc.).
The adsorption of cationic chromite [Cr(III)] onto CRB can be explained by three mechanisms: (i) electrostatic interactions, (ii) cation exchange, and (iii) complexation with oxygen-containing functional groups (Liu et al. 2019; Li et al. 2017a, b). For example, Pan et al. evaluated the influence of various CRB on Cr(III) adsorption in water matrices and stated that adsorption capacity increased in the following order peanut > soybean > canola > rice biochar (0.48, 0.33, 0.28 and 0.27 mmol kg–1), owing to the increase in the number of oxygen-containing functional groups (1.34, 1.13, 0.80 and 0.63 mmol g–1). These findings indicate that complexation of Cr(III) with oxygen-containing functional groups is important for CRB adsorption (Pan et al. 2013a, b). However, two dominant mechanisms have been suggested for the adsorption of Cr(VI): (i) electrostatic interaction; and (ii) reduction of Cr(VI) to Cr(III), primarily facilitated by ‒COOH, ‒OH, and the consequent complexation of Cr(III) with functional groups present on the CRB surface (Zhou et al. 2016; Xu et al. 2016).
Mukherjee et al. (2021) observed that the binding mechanisms of As(V) to oxygen-containing functionalized mesoporous rice husk biochar were electrostatic attraction/repulsion, intraparticle diffusion, and surface complexation (Fig. 4). Ion exchange between the TEs and protons on ‒COOH and ‒OH groups is another primary attraction mechanism between the TEs and CRB. The efficiency of the ion-exchange approach on the biochar surface is primarily determined by the size of the metal ions and the chemistry of the surface functionalities of the CRB (Agrafioti et al. 2014; Xiao et al. 2018). CEC is considered to be a dominant factor in TEs sequestration during ion exchange. Ion exchange generally occurs between ‒COOH and C = O groups, divalent metal ions (M2+), and hydrogen ions (H+). These processes are outlined as follows.
Schematic description of As(V) adsorption on rice straw biochar via various physicochemical interaction/electronic bonding mechanisms (reproduced from Mukherjee et al. (2021), with permission from the publisher)
Moreover, surface functional moieties (i.e., 9CH2/–CH3, –COOH, and C–H) can also participate in the sequestration of TEs in aquatic media (Nkoh et al. 2022). The presence of sulfur in CRB may also facilitate the formation of metalsulfide-like species, sequestering TEs on the biochar surface. Similarly, ion exchange between PO43– and SO42– with oxyanions (e.g., As(V)) can be a plausible alternative process for As removal by CRB in aquatic matrices (Luo et al. 2021). Nevertheless, further research is required to thoroughly examine the significant aspects of TEs removal using CRB to precisely define the attraction between metals and sulfur species present in biochar.
Spectroscopic analyses provide useful insights into CRB efficiency and removal mechanisms of TEs, which are crucial for correctly assessing the role of CRBs in removing TEs from various polluted environments (such as surface water, groundwater, sediments, and soils). EXAFS spectroscopy and μ-XRF maps are authoritative approaches for bestowing spatial localization of a specific metal ion, its relationship with further related metals, and local structural information entailing binding elements, coordination numbers and bond distances. The integration of such techniques could improve our understanding of the binding of TEs on the biochar surface and the constituents that might coexist in CRB to possibly influence the alteration of TEs in field-scale applications. For instance, Liu et al. (2016) applied XAS to determine the Hg(II) distribution and local coordination geometry of Hg(II) bound to CRB. Their μ-XRF mapping findings showed that the Hg(II) was distributed heterogeneously around CRB (Fig. 5). The principal mechanisms for Hg (II) removal are based on the chemical bonds between Hg(II) and the diverse functionalities of CRB. Future efforts that combine various spectroscopic approaches including XAFS, μ-XRF, XANES, etc., are required to improve our understanding of the fate and mechanism of TEs adsorbed from aquatic matrices by CRB.
The µ-XRF findings specify Hg is distributed on the edges, inside the pores, and even inside the pore walls of the particles in all thin sections of biochar ((reproduced from Liu et al. (2016), with permission from the publisher)
7 Engineering consideration for designing CRB adsorbents
CRB has significant potential for adsorbing TEs from water matrices. However, several efficient approaches have been employed to synthesize engineered CRBs, resulting in improved physicochemical properties, enhanced adsorption capacities, and higher stabilities (Fig. 6). Methods, such as physical activation (steam activation and gas purging) and chemical activation (acid and alkaline treatment, metal impregnation, etc.), have been reviewed in detail by Silva-Medeiros et al. (2022) and Wang et al. (2019). The following is a brief description of the strategies that improve the efficiency of CRBs for higher adsorption of TEs (Table 3).
7.1 Improving physicochemical properties and treatment efficiency of CRB using various engineering techniques
Engineered CRB is synthesized through modification of the biochar structure or direct pyrolysis of pre-treated biomass (pre- or post-pyrolysis) (Lima et al. 2010; Xue et al. 2012; Zhang et al. 2013; Song et al. 2014; Fang et al. 2016; Li et al. 2017a, b). These methods are considered to be low-cost and appropriate for the economical production of biochar for practical large-scale applications. Furthermore, these methods produce programmable engineered CRB for the efficient removal of TEs from aquatic media. For example, Haris et al. synthesized programmable exfoliated biochar via an innovative technique (Fig. 7) that involved the pretreatment of biomass (wheat straw) and thermal chemical flash heat treatment. This exfoliated biochar was used as an effective Tl(I) adsorbent in aquatic matrices. The authors concluded that the pore volume and surface area of exfoliated biochar considerably increased to 0.267838 cm3 g–1 and 421.24 m2 g–1 in comparison to pristine wheat straw biochar 0.00475 cm3 g−–1 and 3.812 m2 g–1, respectively. The O and N contents substantially improved to 32.53% and 6.25%, respectively, compared to those of the pristine biochar (18.21% and 2.77%, respectively). Furthermore, the exfoliated biochar exhibited superior adsorption capacity for Tl(I) (382.38 mg g–1), which was over nine times greater than that of pristine biochar (Haris et al. 2022b). Alkaline modification can also improve the physicochemical properties of CRB and its potential to adsorb TEs from aquatic matrices. Liu et al. (2022) synthesized KOH modified rice straw biochar for Hg(II) removal in water matrices and stated that the pore volume and surface area of engineered biochar increased to 1.20 cm3 g−– and 2371.52 m2 g–1 compared to 0.24 cm3 g–1 and 273.26 m2 g–1 of pristine rice straw biochar, respectively. The engineered biochar exhibited removal capacity of 209.75 mg g–1, which was much greater than that of the pristine biochar.
The schematic of the synthetic approach for designing exfoliated biochar nanosheets (reproduced from Haris et al. (2022b), with permission from the publisher)
Engineered CRB can also be synthesized by a surface reduction technique that increases the content of functional groups on the CRB surface, predominantly nitrogen functionalities (e.g., primary, secondary, and tertiary amines; quaternary ammonium; and imidazole). CRB has been frequently modified by reductants including NH3.H2O, Na2S2O3, FeSO4, and aniline (Ma et al. 2019; Pan et al. 2013a, b). For example, Zhang et al. (2019a, b) used Na2S2O3 reductant to modify rice husk and remove Cd(II) from aqueous media. This treatment significantly improved the nitrogen-containing functional groups in the engineered biochar, which boosted Cd(II) removal by up to 72.1% compared to pristine biochar.
Magnetization of CRB has also been proposed for the development of magnetic designer biochar (Xiao et al. 2023). In this context, Li et al. (2022a, b) designed a novel amine-functionalized magnetic biochar (MgFe2O4–NH2@sRHB) derived from rice husks and used it as an adsorbent to remediate Cd(II)- and Pb(II)-polluted environments. They concluded that the surface area and pore volume of the engineered biochar considerably improved to 51.30 m2 g–1 and 0.146 cm3 g–1, respectively, compared with 18.77 m2 g–1 and 0.038 cm3 g–1 of pristine biochar. The engineered-designed biochar (MgFe2O4-NH2@sRHB) had strong magnetic nature and exhibited removal ability for Cd(II) and Pb(II) (194.85 and 199.08 mg g–1) in comparison to HNO3-modified biochar (sRHB) (38.22 and 31.23 mg g–1) and pristine rice husk biochar (RHB) (14.88 and 9.98 mg g–1) (Fig. 8). Zhang et al. (2013) developed a magnetic biochar derived from cottonwood residues for As(V) removal. The resulted engineered-biochar manifested top-notch ferromagnetic property having a saturation magnetization of 69.2 emu g–1, and manifested superior adsorption capacity of 3000 mg kg–1 for As(V) in aqueous solution, but resulted in reduced surface area of modified biochar (Table 4).
Recently, an innovative technique involving the production of CRB composites with nano-metal oxide/hydroxide and CRB coated with functional materials was developed to improve the physicochemical characteristics of biochar and its potential to remove contaminants from the water environment (Ramanayaka et al. 2017). For example, nanoscale zero-valent iron (nZVI)-designed biochar has gained substantial research attention owing to its strong adsorption ability, affinity for TEs, and reducibility. Dong et al. (2017) applied nZVI to pretreated HCl biochar derived from corn stalks and showed that the nZVI@HCl-biochar composite increased the Cr(VI) removal capacity owing to its greater surface area (34.8952 m2 g–1) than that of pristine biochar (5.3792 m2 g–1). Nevertheless, recent reports have shown that metal oxide modifications decrease the surface area of CRB, which is attributed to the pore blockage caused by the formation of metal oxide precipitates (Table 4). Yang et al. (2018) developed a hydrophilic corn stalk biochar supported by an nZVI (nZVI-HCS) composite to remove TEs from aquatic media. The specific surface area, total pore volume and average pore diameter of nZVI-HCS were determined as 603.4 m2 g–1, 0.474 cm3 g–1 and 3.14 nm respectively, whereas, the observed specific surface area of cornstalk and hydrophilic cornstalk biochar were 1236 and 1216 m2 g–1, respectively. They reported that loading iron particles onto the biochar surface clogged the micropores, resulting in a significant decrease in the surface area (Yang et al. 2018).
The nanostructure, composite formation, and doping-synthesized CRB have a profound influence on the remediation of numerous toxic pollutants in water media (Dong et al. 2017). The synthesis of CRB-based composite adsorbents is considered an emerging approach for developing engineered CRB (Yang et al. 2018). These composites have a high potential for adsorbing multiple pollutants from water matrices. Among these composites, manganese oxide-modified biochar and zero-valent iron (ZVI) nanobiochar have attracted significant attention in recent years (Ramanayanka et al. 2017; Tan et al. 2016).
Various studies have been conducted to produce binary-oxide CRB composites with improved magnetic properties and TE removal efficiency. For instance, Zhou et al. (2018a, b) developed a ferromanganese binary/oxide-biochar composite (FMBC) from corn straw using an impregnation sintering strategy and determined its potential for removing Cu(II) and Cd(II) in aquatic media. The FMBC exhibited maximum Cu(II) and Cd(II) removal capacities of 64.9 and 101.0 mg g–1, respectively, compared to pristine biochar (21.8 and 28.0 mg g–1, respectively). To date, Qu et al. have designed Fe/Mn binary metal oxide-biochar nanocomposite (Fe/Mn-BMBC) adsorbents from cotton straw, rice husk, and maize straw to evaluate their potential in a Cd(II)-contaminated water environment. They extrapolated that the removal efficiencies of engineered biochar were 4.8–6.1 times greater than those of their pristine biochars, respectively, due to higher surface area and the existence of more negatively charged functional groups (Qu et al. 2022).
Layered double hydroxides (LDHs) have been extensively used to develop engineered biochars that improve the TE removal capacity. In this respect, Liang et al. used corn straw to synthesize Ca-Fe/LDH biochar and applied it to a TE-contaminated environment. They indicated that the removal efficiency of designed biochar for Cu(II), Pb(II), Cd(II) and Zn(II) substantially increased to 39.35, 240.96, 24.58 and 57.54 mg g–1,, compared to that of pristine biochar (Liang et al. 2023). Huang et al. (2019) developed a nano-adsorbent using bamboo biochar coupled with ethylenediaminetetraacetic acid (EDTA) and intercalated with Mg–Al/LDH (BC@EDTA-LDH). They concluded that the adsorption capacity of Cr(VI) was significantly improved to 55.19 mg g–1 by BC@EDTA-LDH compared to that of pristine biochar (5.08 mg g–1). Research efforts have been made to discover innovative approaches for nano-biochar fabrication and its application in TE-contaminated water environments. The ball-milled nano-CRB showed excellent removal potential for TEs in TE-contaminated environments. However, further studies need to be conducted by introducing direct innovative strategies to produce nano-CRB, which shows high potential for TEs in TE-polluted water matrices.
7.2 Regeneration of CRB-adsorbed TEs
The ability of an adsorbent to regenerate is of great practical significance because it involves the efficient utilization of available resources, technical feasibility, and economic viability (Jia et al. 2019; Yang et al. 2021). These factors are crucial for an overall evaluation of the performance and suitability of an adsorbent for particular applications. CRB is recognized as a promising adsorbent for removing TEs from different water matrices owing to its greater surface area, porosity, and superior binding ability to form stable complexes with TEs (Huang et al. 2019). However, their limited recyclability is a significant challenge for practical applications (Yang et al. 2021). To address this drawback, several approaches have been investigated, including chemical, physical (i.e., acid treatment, ion exchange, and metal oxide integration), and hybridization with other materials (such as clay and activated carbon) to increase its mechanical strength and stability under harsh climates. These strategies can substantially improve the recyclability of CRB (Table 3), thereby improving its overall efficiency in removing TEs from aquatic matrices (Zong et al. 2021). Tan et al. (2022) designed an iron-manganese rice straw biochar (Fe–Mn@BC) to evaluate its removal potential in Cd(II)-polluted water. They found that Fe–Mn@BC maintained a removal potential of approximately 50% after 3 adsorption–desorption cycles.
The regeneration of CRB-based adsorbents (i.e., efficient separation and recycling) remains a significant challenge for their development. Moreover, the utilization of CRB in large-scale wastewater treatment plants is difficult because of its small particle size, which leads to sluggish flow rates and pressure drops in the columns. Although filtration can address this drawback, it is a slow method that increases the operating costs associated with replacing filters or membranes (Jia et al. 2019). Introducing a magnetically designed CRB could potentially increase the removal capacity of pollutants and mitigate regeneration issues as it facilitates the recovery of small particle sizes from batch trials (Yang et al. 2021; Zong et al. 2021). Although these techniques have great potential for regenerating CRB, their scalability is limited owing to a lack of knowledge on optimizing the regeneration parameters and concerns about the disposal of spent CRB after multiple cycles. Therefore, the regeneration of CRB is a significant aspect of the development of effective and sustainable adsorbents for the treatment of contaminated water matrices.
8 Cost–benefit and economic assessment of CRB
The cost–benefit analysis of CRB from several precursors has rarely been discussed in the published literature (Table 5). Biochar cost depends on numerous factors, such as availability, collection, and transportation of feedstock, manufacturing unit, synthesis strategies, pyrolysis temperature, marketing, and handling. However, the lack of data related to the entire synthesis process makes it difficult to calculate the economic cost of biochar. The biochar cost varies in various countries, e.g., the calculated break-even price range of biochar was reported $246 ton−1 in USA by Kurniawan et al. (2006) and McCarl et al. (2009). In addition, Shackley et al. (2011) recommend the price of biochar in United Kingdom (UK) ranges from $50 to 682.54 ton−1. Kulyk, (2012) indorses a price range of $300–500 ton−1 of biochar. Nevertheless, in California, the price value for per ton high-quality biochar was estimated to be $2000.
Among the numerous biochars, the range of $50–500 ton−1 is stated for CRB reliant on production environment and storage facility (Galinato et al. 2011). CRB is more cost-effective in comparison to activated carbon derived from crop residues. For instance, Ahmad et al. (2012) investigated that the anticipated price range for CRB is around 1/6 of that of activated carbon produced from crop residues (US $1500 ton−1). Therefore, transforming crop residues into biochar could be an economically viable choice compared to synthesizing activated carbon (Moreira et al. 2017). Furthermore, Hina et al. (2015) reported that the price range of zeolite-based adsorbents is $500 to $600 ton−1, which is comparatively higher compared to the cost of CRB. In addition, the recent market scenario specifies that CRB/or other feedstock biochar applications are economically inviable and expensive compared to their multiple significant environmental advantages (Ifa et al. 2020), which is responsible for the lower incentives provided by government officials to attain carbon negativity and inferior capital costs of pyrolysis units (Li et al. 2022a). Technoeconomic assessment (TEA) is commonly used to determine the distinct what-if circumstances by improved economics. For instance, Haeldermans et al.. performed TEA to compare CRB production through conventional pyrolysis (CP) and microwave pyrolysis (MWP). They stated that the minimum price ranges for MWP- and CP-produced biochar were US$1020/ton and US$588/ton, respectively. They also suggested that MWP-produced biochar has better quality and technical feasibility than CP-produced biochar, whereas CP is considered a more facile and well-developed technique (Haeldermans et al. 2020). Furthermore, the price of biochar per ton is an imperative assessment standard for biochar production plants and relies heavily on the government carbon tax. Economic analysis of the biochar production process was performed using feedstock type, pyrolysis approach, carbon sequestration subsidies, and carbon credits, reflecting the social value of greenhouse gas emission reduction. Compared with other feedstocks, CRB is the most accessible and cost-effective source of feedstock for producing biochar and has the potential to remove toxic metals from wastewater more efficiently than commercial activated carbon (usually produced from coal and petroleum residues).
9 Conclusion, recommendation and future challenges
The use of crop residue feedstocks to develop biochar for TEs treatment from polluted aquatic matrices may be a promising technique that presents a feasible alternative to conventional, high-cost, non-applicable, and less effective materials for emerging cost-effective, easily applicable, and highly effective materials. Nevertheless, it is imperative to overcome the substantial challenge of translating previous bench-scale results into real field scenarios. The production of efficient and economical adsorbents for water treatment is a promising research field. Therefore, it is extremely important to synthesize effective, stable, eco-friendly, and recyclable engineered CRB adsorbents. Magnetic nanomaterial-synthesized CRBs have great potential; however, their practical viability, stability, and environmental fate remain questionable. Therefore, future consideration should be given to the development of engineered/designed CRB using various innovative techniques entailing nano-biochar composites, exfoliation of crop-residue biomass to produce an ultrathin framework for highly accessible reactants and/or ions, integrating ultrasonic exfoliation with sub-micron-size abrasives to utilize the shear and frictional force between the abrasive and nanomaterials to synthesize high lateral size and yield nano-biochar, and testing their potential for TEs removal from aquatic matrices using advanced analytical techniques.
Another critical concern in the field of water treatment using CRB is the reliance on laboratory-scale research. Numerous studies have primarily utilized spiked-polluted aqueous solutions with target contaminants at higher initial concentrations than the environmentally relevant conditions. In a real scenario, natural organic matter is present at considerably higher concentrations than the target contaminants, resulting in a decrease in the removal capacity owing to the non-target consumption of adsorbents. Consequently, the performance of CRBs in complex aquatic environments may be compromised. Further research using representative samples of real water and wastewater is required to identify suitable feedstocks to produce biochar with greater potential for field-level applications. Spectroscopic examination offers valuable insights into the efficiency and underlying mechanisms of TEs adsorption onto CRB, which are crucial for evaluating the potential of CRB to stabilize TEs in aquatic matrices. Thereby, future research should focus on investigating the integration of multiple spectroscopic approaches (i.e., XAFS and μ-XRF) to comprehensively understand the stability and mechanisms involved in TEs adsorbed on CRB in aquatic media.
Availability of data and materials
The datasets used or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Abdelhafez AA, Li J (2016) Removal of Pb(II) from aqueous solution by using biochars derived from sugarcane bagasse and orange peel. J Taiwan Inst Chem Eng 61:367–375
Agrafioti E, Kalderis D, Diamadopoulos E (2014) Ca and Fe-modified biochars as adsorbents of As and Cr in aqueous solutions. J Environ Manage 146:444–450. https://doi.org/10.1016/j.jenvman.2014.07.029
Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang JE (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544. https://doi.org/10.1016/j.biortech.2012.05.042
Ahmadi M, Kouhgardi E, Ramavandi B (2016) Physicochemical study of dew melon peel biochar for chromium attenuation from simulated and actual wastewater. Kor J Chem Eng 33:2589–2601. https://doi.org/10.1007/s11814-016-0135-1
Ahmed MJ, Hameed BH (2018) Adsorption behavior of salicylic acid on biochar as derived from the thermal pyrolysis of barley straw. J Clean Prod 195:1162–1169. https://doi.org/10.1016/j.jclepro.2018.05.257
Alkurdi SSA, Herath I, Bundschuh J, Al-Juboori RA, Vithanage M, Mohan D (2019) Biochar versus bone char for sustainable inorganic arsenic mitigation in water: what needs to be done in future research? Environ Int 127:52–69. https://doi.org/10.1016/j.envint.2019.03.012
Ambika S, Kumar M, Pisharody L, Malhotra M, Kumar G, Sreedharan V (2022) Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: mechanisms, methods, and prospects. Chem Eng J. https://doi.org/10.1016/j.cej.2022.135716
Amin M, Chetpattananondh P (2019) Biochar from extracted marine Chlorella sp. residue for high-efficiency adsorption with ultrasonication to remove Cr(VI), Zn (II), and Ni(II). Bioresour Technol 289:121578. https://doi.org/10.1016/j.biortech.2019.121578
Arslan H, Ayyildiz Turan N (2015) Estimation of spatial distribution of heavy metals in groundwater using interpolation methods and multivariate statistical techniques: its suitability for drinking and irrigation purposes in the Middle Black Sea Region of Turkey. Environ Monit Assess. https://doi.org/10.1007/s10661-015-4725-x
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54(7):951–967
Bandara T, Xu J, Potter ID et al (2020) Mechanisms for the removal of Cd(II) and Cu(II) from aqueous solution and mine water by biochars derived from agricultural waste. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126745
Bao S, Yang W, Wang Y, Yu Y, Sun Y (2020) One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solutions. J Hazard Mater 381:120914. https://doi.org/10.1016/j.jhazmat.2019.120914
Brown P, Jefcoat IA, Parrish D, Gill S, Graham S (2000) Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Adv Environ Res 4:19–29
Burow KR, Belitz K, Dubrovsky NM, Jurgens BC (2017) Large decadal-scale changes in uranium and bicarbonate in groundwater of the irrigated western U.S. Sci Total Environ 586:87–95. https://doi.org/10.1016/j.scitotenv.2017.01.220
Cao Y, Xiao W, Shen G, Ji G, Zhang Y, Gao C (2019) Carbonization and ball milling on the enhancement of Pb(II) adsorption by wheat straw: competitive effects of ion exchange and precipitation. Bioresour Technol 273:70–76. https://doi.org/10.1016/j.biortech.2018.10.065
Chen X, Chen G, Chen L, Chen Y, Lehmann J, McBride MB (2011) Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour Technol 102:8877–8884. https://doi.org/10.1016/j.biortech.2011.06.078
Chen Y, Dong L, Miao J, Wang J et al (2019) Hydrothermal liquefaction of corn straw with mixed catalysts for the production of bio-oil and aromatic compounds. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.122148
Cheng X, Huang Z, Wang Z, Ma C, Chen S (2019) A novel on-site wheat straw pretreatment method: enclosed torrefaction. Bioresour Technol 281:48–55. https://doi.org/10.1016/j.biortech.2019.02.075
Darmokoesoemo H, Setianingsih FR, Putranto TW, Kusuma HS (2016) Horn snail (Telescopium sp.), and mud crab (Scylla sp.) shells were used as low-cost adsorbents for the removal of Cu2+ from synthetic wastewater. Rasayan J Chem 9:550–555
de Muhammad H, Wei T, Cao G, Yu SH, Ren XH, Jia HL (2021) Study of soil microorganisms modified wheat straw and biochar for reducing Cd leaching potential and bioavailability. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.129644
de Silva Medeiros DC, Nzediegwu C, Benally C, Messele SA, Kwak C, Naeth C (2022) Pristine and engineered biochar for the removal of contaminants co-existing in several types of industrial wastewaters: a critical review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151120
Dong H, Deng J, Xie Y, Zhang C, Jiang Z, Cheng Y (2017) Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J Hazard Mater 332:79–86. https://doi.org/10.1016/j.jhazmat.2017.03.002
dos Reis Ferreira RA, da Silva Meireles C, Assunção RM, Barrozo MA, Soares RR (2020) Optimization of the oxidative fast pyrolysis process of sugarcane straw by TGA and DSC analyses. Biomass Bioenergy. https://doi.org/10.1016/j.biombioe.2019.105456
Du Y, Lian F, Zhu L (2011) Biosorption of divalent Pb, Cd, and Zn onto aragonite and calcite mollusk shells. Environ Pollut 159(7):1763–1768
Fan J, Cai C, Chi H, Reid BJ, Coulon F, Zhang Y (2020) Remediation of Cd- and lead-polluted soil using thiol-modified biochar. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.122037
Fang J, Gao B, Zimmerman AR, Ro KS, Chen J (2016) Physically (CO2) activated hydrochars from hickory and peanut hulls: preparation, characterization, and sorption of methylene blue, lead, copper, and cadmium. RSC Adv 6:24906–24911. https://doi.org/10.1039/c6ra01644h
Foong SY, Chan YH, Chin BLF, Lock SSM, Yee CY, Yiin CL (2022) Production of biochar from rice straw and its application for wastewater remediation. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.127588
Galinato SP, Yoder JK, Granatstein D (2011) The economic value of biochar in crop production and carbon sequestration. Energy Policy 39:6344–6350. https://doi.org/10.1016/j.enpol.2011.07.035
Gao LY, Deng JH, Huang GF, Li K, Cai KZ, Liu Y (2019) Relative distribution of Cd2+ adsorption mechanisms on biochar derived from rice straw and sewage sludge. Chem Bioresour Technol 272:114–122. https://doi.org/10.1016/j.biortech.2018.09.138
Gürkan EH, Berkay I, Yusuf T (2022) Adsorption of Cu(II) ve Zn(II) ions by alginate-based composites: full factorial design approach. Fuller Nanotub Carbon Nanostruct 30(8):787–800. https://doi.org/10.1080/1536383X.2021.2021891
Gürkan EH, Berkay I, Yusuf T (2023a) Adsorption performance of heavy metal ions from aqueous solutions by a waste biomass based hydrogel: comparison of isotherm and kinetic models. Int J of Environ Analyt Chem 103(6):1343–1360. https://doi.org/10.1080/03067319.2021.1873314
Gürkan EH, Rasim B, Akyol SÇ (2023b) Kinetic, isotherm modeling analyses of the adsorption of phenol on activated carbon/alginate composites. Int J Phytorem 25(7):832–839. https://doi.org/10.1080/15226514.2022.2112936
Haeldermans T, Campion L, Kuppens T, Vanreppelen K, Cuypers A, Schreurs S (2020) Comparative technoeconomic assessment of biochar production from different residue streams using conventional and microwave pyrolysis. Bioresour Technol 318:124083. https://doi.org/10.1016/j.biortech.2020.124083
Hamid Y, Liu L, Usman M, Naidu R, Haris M, Lin Q (2022) Functionalized biochars: synthesis, characterization, and applications for removing trace elements from water. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2022.129337
Han TU, Kim J, Kim K (2020) Freezing-accelerated removal of chromate by biochar synthesized from waste rice husk. Sep Puri Technol. https://doi.org/10.1016/j.seppur.2020.117233
Haris M, Hamid Y, Usman M, Wang L, Saleem A, Su F (2021) Crop-residue-derived biochar: synthesis, properties, characterization, and application for the removal of trace elements in soils. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.126212
Haris M, Hamid Y, Wang L, Wang M, Yashir N, Su F (2022a) Cd diminution through microbial-mediated degraded lignocellulose maize straw: batch adsorption and bioavailability trials. J Environ Manage. https://doi.org/10.1016/j.jenvman.2021.114042
Haris M, Usman M, Su F, Lei W et al (2022b) Programmable synthesis of exfoliated biochar nanosheets for selective and highly efficient adsorption of thallium. Chem Eng J. https://doi.org/10.1016/j.cej.2022.134842
Haris M, Usman M, Saleem A (2023a) Synthesis of conch-like layered carbon nanosheets by ball-milling-assisted ultrasonic exfoliation for highly selective removal of Cd(II) from multiple water matrices. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2023.124756
Haris M, Hamid Y, Saleem A, Guo J (2023b) Pristine- and engineered wood-derived biochar for abating toxic metal contamination in the soil environment. In: Li P, Elumalai V (eds) Recent advances in environmental sustainability. Springer International Publishing, Cham, pp 271–301. https://doi.org/10.1007/978-3-031-34783-2
Harisankar S, Vishnu Mohan R, Choudhary V, Vinu R (2022) Effect of water quality on the yield and quality of the products from hydrothermal liquefaction and carbonization of rice straw. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.127031
He Z, Li Y, Wang Y (2023) Insufficient desorption of ions in constant-current membrane capacitive deionization (MCDI): problems and solutions. Water Res. https://doi.org/10.1016/j.watres.2023.120273
Hina K, Hedley M, Camps-Arbestain M, Hanly J (2015) Comparison of pine bark, biochar and zeolite as sorbents for NH4+-N removal from water. Clean (weinh) 43:86–91. https://doi.org/10.1002/clen.201300682
Hizal J, Tutem E, Guclu K, Hugul M, Ayhan S, Apak R (2013) Heavy metal removal from water by red mud and coal fly ash: an integrated adsorption-solidification/stabilization process. Desalin Water Treat 51:7181–7193. https://doi.org/10.1080/19443994.2013.771289
Hong Z, Zhong F, Niu W, Zhang K, Su J, Liu J (2020) Effects of temperature and particle size on the composition, energy conversion, and structural characteristics of pyrolysis products from different crop residues. Energy. https://doi.org/10.1016/j.energy.2019.116413
Huang G, Zhang M, Liu C, Li L, Chen Z (2018) Heavy metal(loid)s and organic contaminants in groundwater in the Pearl River Delta that has undergone three decades of urbanization and industrialization: distribution, sources, and driving forces. Sci Total Environ 635:913–925. https://doi.org/10.1016/j.scitotenv.2018.04.210
Huang D, Liu C, Zhang C, Deng R, Wang R, Xue W (2019) Cr(VI) removal from aqueous solution using biochar modified with Mg/Al layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour Technol 276:127–132. https://doi.org/10.1016/j.biortech.2018.12.114
Ifa L, Yani S, Nurjannah N (2020) Techno-economic analysis of bio-briquettes from cashew nutshell waste. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e05009
Intani K, Latif S, Kabir AKMR, Müller J (2016) Effect of self-purging pyrolysis on yield of biochar from maize cobs, husks and leaves. Bioresour Technol 218:541–551. https://doi.org/10.1016/j.biortech.2016.06.114
Jabar JM, Odusote YA, Ayinde YT, Yılmaz M (2022) African almond (Terminalia catappa L.) leaves biochar prepared through pyrolysis using H3PO4 as chemical activator for sequestration of methylene blue dye. Results Eng. https://doi.org/10.1016/j.rineng.2022.100385
Ji M, Wang X, Usman M, Liu F et al (2022) Effects of different feedstock-based biochar on soil remediation: a review. Environ Pollut. https://doi.org/10.1016/j.envpol.2021.118655
Jia Y, Zhang Y, Fu J, Yuan L, Li Z, Liu C (2019) A Novel magnetic biochar/MgFe-layered double hydroxide composite removing Pb2+ from aqueous solution: isotherms, kinetics, and thermodynamics. Colloid Surf A Physicochem Eng Asp 567:278–287. https://doi.org/10.1016/j.colsurfa.2019.01.064
Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11:6613–6621. https://doi.org/10.5194/bg-11-6613-2014
Kamali M, Appels L, Kwon EE, Aminabhavi TM, Dewil R (2021) Biochar in water and wastewater treatment-a sustainability assessment. Chem Eng J. https://doi.org/10.1016/j.cej.2021.129946
Kang X, Geng N, Li Y, Li X, Yu J, Gao S (2022) Treatment of cadmium and zinc-contaminated water systems using modified biochar: contaminant uptake, adsorption ability, and mechanism. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.127817
Khera RA, Iqbal M, Ahmad A (2020) Kinetics and equilibrium studies of copper, zinc, and nickel ion adsorptive removal on Archontophoenix alexandrae: Conditions optimization by RSM. Desalin Water Treat 201:289–300. https://doi.org/10.5004/dwt.2020.25937
Krauklis A, Ozola R, Burlakovs J, Rugele K, Kirillov K, Trubaca-Boginska A, Rubenis K, Stepanova V, Klavinsa M (2017) FeOOH and Mn8O10Cl3modified zeolites for As(V) removal from aqueous media. J Chem Technol Biotechnol 92:1948–1960
Kulyk N (2012) Cost-benefit analysis of biochar application in U.S. cereal crop cultivation. University of Massachusetts, The School of Public Policy, Amherst
Kuncoro EP, Isnadina DRM, Darmokoesoemo H (2018a) Characterization, kinetics, and isotherm data for adsorption of Pb2+ from aqueous solution by adsorbent from a mixture of bagasse and bentonite. Data Brief 16:622–629. https://doi.org/10.1016/j.dib.2017.11.098
Kuncoro EP, Soedarti T, Putranto TWC (2018b) Characterization of a mixture of algal waste-bentonite used as an adsorbent for the removal of Pb2+ from aqueous solution. Data Brief 16:908–913. https://doi.org/10.1016/j.dib.2017.12.030
Kurniawan TA, Babel S (2003) A research study on Cr(VI) removal from contaminated wastewater using low-cost adsorbents and commercial activated carbon. In: Kurniawan TA (ed) Proceedings of the 2nd International conference on energy technology towards a clean environment (RCETE). RCETE, Phuket, pp 1110–1117
Kurniawan TA, Chan GYS, Lo W, Hung BS, Babel S (2006) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366:409–426. https://doi.org/10.1016/j.scitotenv.2005.10.001
Kusuma HS, Illiyanasafa N, Jaya DEC (2024) Utilization of the microalga Chlorella vulgaris for mercury bioremediation from wastewater and biomass production. Sustain Chem Pharm. 37:101346
Kwak JH, Islam MS, Wang S, Messele SA et al (2019) Biochar properties and lead(II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation. Chemosphere 231:393–404. https://doi.org/10.1016/j.chemosphere.2019.05.128
Leng L, Huang H (2018) An overview of the effects of pyrolysis process parameters on biochar stability. Bioresour Technol 270:627–642. https://doi.org/10.1016/j.biortech.2018.09.030
Leng L, Xiong Q, Yang L, Li H, Zhou Y, Zhang W (2021) An overview of engineering of the surface area and porosity of biochar. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.144204
Leung CM, Jiao JJ (2006) Heavy metal and trace element distributions in groundwater in natural slopes and highly urbanized spaces in Mid-Levels area, Hong Kong. Water Res 40:753–767. https://doi.org/10.1016/j.watres.2005.12.016
Li B, Yang L, Wang C, Quan ZQ, Pei LQ, Cheng LY (2017a) Adsorption of Cd(II) from aqueous solutions by rapeseed straw biochar derived from different modification processes. Chemosphere 175:332–340. https://doi.org/10.1016/j.chemosphere.2017.02.061
Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ (2017b) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072
Li X, Zhang D, Sheng F, Qing H (2018a) Adsorption characteristics of copper(II), zinc(II), and mercury(II) by four kinds of immobilized fungal residues. Ecotoxicol Environ Saf 147:357–366
Li R, Liang W, Wang JJ, Gaston LA, Huang D, Huang H (2018b) Facilitative capture of As(V), Pb(II) and methylene blue from aqueous solutions with MgO hybrid sponge-like carbonaceous composite derived from sugarcane leafy trash. J Environ Manage 212:77–87. https://doi.org/10.1016/j.jenvman.2017.12.034
Li G, Zhang J, Li Y, Liu J, Yan Z (2021) Adsorption characteristics of Pb(II), Cd(II), and Cu(II) on carbon nanotube-hydroxyapatite. Technol 42(10):1560–1581. https://doi.org/10.1080/09593330.2019.1674385
Li A, Ge W, Liu L, Zhang Y, Qiu G (2022a) Synthesis and application of amine-functionalized MgFe2O4-biochar for the adsorption and immobilization of Cd(II) and Pb(II). Chem Eng J. https://doi.org/10.1016/j.cej.2022.135785
Li X, Jiang J, Shao S, Lv Z, Ge S, Cai Y (2022b) Catalytic conversion of rape straw into biofuels by direct nonthermal plasma-modified HZSM-5. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.126787
Liang X, Su Y, Wang X, Liang C, Tang C, Wei J (2023) Insights into the heavy metal adsorption and immobilization mechanisms of CaFe-layered double hydroxide corn straw biochar: synthesis and application in a combined heavy metal-contaminated environment. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.137467
Lima IM, Boateng AA, Klasson KT (2010) Physicochemical and adsorptive properties of fast-pyrolysis biochar and their steam-activated counterparts. J Chem Technol Biotechnol 85:1515–1521. https://doi.org/10.1002/jctb.2461
Liu P, Ptacek CJ, Blowes DW, Landis RC (2016) Mechanisms of mercury removal by biochars produced from different feedstocks determined using X-ray absorption spectroscopy. J Hazard Mater 308:233–242. https://doi.org/10.1016/j.jhazmat.2016.01.007
Liu WJ, Jiang H, Yu HQ (2019) Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ Sci 12:1751–1779. https://doi.org/10.1039/c9ee00206e
Liu Z, Zhen F, Zhang Q, Qian X, Li W, Sun Y (2022) Nanoporous biochar with high specific surface area based on rice straw digestion residue for efficient adsorption of mercury ions from water. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.127471
Lu X, Liu X, Zhang W, Wang X, Wang S, Xia T (2020) The residue from the acidic concentrated lithium bromide-treated crop residue as biochar to remove Cr(VI). Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.122348
Luo J, Yu D, Hristovski KD, Fu K, Shen Y, Westerhoff P (2021) Critical review of advances in engineering nanomaterial adsorbents for metal removal and recovery from water: mechanism identification and engineering design. Environ Sci Technol 55:4287–4304. https://doi.org/10.1021/acs.est.0c07936
Lyu H, Gao B, He F, Zimmerman AR, Ding C, Huang H, Tang J (2018) Effects of ball milling on physicochemical and sorptive properties of biochar: experimental observations and governing mechanisms. Environ Pollut 233:54–63
Ma J, Li T, Liu Y, Cai T, Wei Y, Dong W (2019) Rice husk-derived double-network hydrogel as an efficient adsorbent for Pb(II), Cu(II), and Cd(II) removal in individual and multicomponent systems. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.121793
McCarl BA, Peacocke C, Chrisman R (2009) Economics of biochar production, utilization, and greenhouse gas offsets. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 341–358
Mohammed R (2012) Removal of heavy metals from wastewater using black tea waste. Arab J Sci Eng 37:1505–1520
Moreira MT, Noya I, Feijoo G (2017) The prospective use of biochar as adsorption matrix: a review from a lifecycle perspective. Bioresour Technol 246:135–141. https://doi.org/10.1016/j.biortech.2017.08.041
Mukherjee S, Thakur AK, Goswami R, Mazumder P et al (2021) Efficacy of agricultural waste-derived biochar for arsenic removal: tackling water quality in the Indo-Gangetic plain. J Environ Manage. https://doi.org/10.1016/j.jenvman.2020.111814
Naat JN, Neolaka YAB, Lapailaka T (2021) Adsorption of Cu(II) and Pb(II) using silica@mercapto (hs@m) hybrid adsorbent synthesized from silica of Takari sand: optimization of parameters and kinetics. Rasayan J Chem 14:550–560. https://doi.org/10.31788/RJC.2021.1415803
Nabi SA, Shahadat M, Bushra R, Shalla AH (2011) metal separation from industrial effluent, natural water, as well as from synthetic mixture using synthesized novel composite adsorbent. Chem Eng J 175(1):8–16. https://doi.org/10.1016/j.cej.2011.01.022
Nawaz A, Kumar P (2021) Pyrolysis of mustard straw: evaluation of optimum process parameters, kinetic and thermodynamic study. Bioresour Technol. https://doi.org/10.1016/j.biortech.2021.125722
Neolaka YA, Lawa Y, Jl N (2023a) Adsorption of methyl red from aqueous solution using Bali cow bone (Bos javanicus domesticus) hydrochar powder. The Results of Engineering. https://doi.org/10.1016/j.rineng.2022.100824
Neolaka YA, Riwu AAP, Aigbe UO (2023b) Potential of activated carbon from various sources as a low-cost adsorbent for removing heavy metals and synthetic dyes. Results Chem. https://doi.org/10.1016/j.rechem.2022.100711
Nham NT, Tahtamouni TMA, Nguyen TD, Huong PT, Jitae K, Viet NM, Van-Noi N, Phuong NTH (2019) Synthesis of iron modified rice straw biochar toward arsenic from groundwater. Mater Res Express 6:115528. https://doi.org/10.1088/2053-1591/ab4b98
Nidheesh PV, Gopinath A, Ranjith N, Praveen Akre A, Sreedharan V, Suresh Kumar M (2021) Potential role of biochar in advanced oxidation processes: a sustainable approach. Chem Eng J. https://doi.org/10.1016/j.cej.2020.126582
Nkoh JN, Ajibade FO, Atakpa EO, Baquy MA-A, Mia S, Odii EC (2022) Reduction of heavy metal uptake from polluted soils and associated health risks through biochar amendment: a critical synthesis. J Hazard Mat Adv 6:100086. https://doi.org/10.1016/j.hazadv.2022.100086
Noreen M, Shahid M, Iqbal M, Nisar J, Abbas M (2017) Measurement of cytotoxicity and heavy metal load in drain water receiving textile effluents and drinking water in the vicinity of drains. Measurement (length) 109:88–99. https://doi.org/10.1016/j.measurement.2017.05.030
Otero N, Vitòria L, Soler A, Canals A (2005) Fertilizer characterization: major, trace and rare earth elements. Appl Geochem 20:1473–1488. https://doi.org/10.1016/j.apgeochem.2005.04.002
Pan J, Jiang J, Xu R (2013a) Removal of Cr(VI) from aqueous solutions by Na2SO3/FeSO4 combined with peanut straw biochar. Chemosphere 101:71–76. https://doi.org/10.1016/j.chemosphere.2013.12.026
Pan J, Jiang J, Xu R (2013b) Adsorption of Cr(III) from acidic solutions by crop straw-derived biochars. J Environ Sci 25:1957–1965. https://doi.org/10.1016/S1001-0742(12)60305-2
Pan JJ, Jiang J, Qian W, Xu RK (2015) Arsenate adsorption from aqueous solution onto Fe(III)-modified crop straw biochars. Environ Eng Sci 32:922–929. https://doi.org/10.1089/ees.2014.0540
Pariyar P, Kumari K et al (2020) Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperatures for environmental and agricultural applications. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.136433
Putranto T, Darmokoesoemo H, Putranto T, Kusuma H (2016) Telescope snail (Telescopium spp.) and mangrove crabs (Scylla spp.) as adsorbents for the removal of Pb2+ from aqueous solutions. Rasayan J Chem 9:680
Qian L, Zhang W, Yan J, Han L, Gao W, Liu R (2016) Effective removal of heavy metals by biochar colloids at different pyrolysis temperatures. Bioresour Technol 206:217–224. https://doi.org/10.1016/j.biortech.2016.01.065
Qu J, Wang Y, Tian X, Jiang Z, Deng F, Tao Y (2021) KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: affecting factors, mechanisms, and reusability exploration. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.123292
Qu J, Che N, Niu G, Liu L, Li C, Liu Y (2022) Iron/Manganese binary metal oxide-biochar nanocomposites with high adsorption capacities of Cd2+: Preparation and adsorption mechanisms. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2022.103332
Ramanayaka S, Vithanage M, Alessi DS, Liu WJ, Jayasundera ACA, Ok YS (2017) Nanobiochar: production, properties, and multifunctional applications. Environ Sci Nano 7:3279–3302. https://doi.org/10.1039/d0en00486c
Rizwan M, Lin Q, Chen X, Li Y, Li G, Zhao X (2020) Synthesis, characterization, and application of magnetic and acid-modified biochars following alkaline pretreatment of rice and cotton straw. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.136532
Rosales E, Meijide J, Pazos M, Sanromán MA (2017) Challenges and recent advances in biochar as a low-cost biosorbent: from batch assays to continuous-flow systems. Bioresour Technol 246:176–192. https://doi.org/10.1016/j.biortech.2017.06.084
Samsuri AW, Sadegh-Zadeh F, Seh-Bardan BJ (2013) Adsorption of As(III) and As(V) by Fe-coated biochars and biochars produced from empty fruit bunches and rice husks. J Environ Chem Eng 1:981–988. https://doi.org/10.1016/j.jece.2013.08.009
Shackley S, Hammond J, Gaunt J, Ibarrola R (2011) Feasibility and costs of biochar application in the UK. Carbon Manag 2:335–356. https://doi.org/10.4155/cmt.11.22
Shaheen SM, Eissa FI, Ghanem KM, Gamal El-Din HM, Al Anany FS (2013) Heavy metals removal from aqueous solutions and wastewaters by using various by products. J Environ Manage 128:514–521. https://doi.org/10.1016/j.jenvman.2013.05.06
Shakoor MB, Niazi NK, Bibi I, Murtaza G, Kunhikrishnan A, Seshadri B (2016) Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit Rev Environ Sci Technol 46:467–499. https://doi.org/10.1080/10643389.2015.1109910
Shan R, Shi Y, Gu J, Wang Y, Yuan H (2020) Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin J Chem Eng 28:1375–1383. https://doi.org/10.1016/j.cjche.2020.02.012
Singh E, Kumar A, Mishra R, You S, Singh L, Kumar S (2021) Pyrolysis of waste biomass and plastics for production of biochar and its use for removal of heavy metals from aqueous solutions. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.124278
Singha B, Das SK (2013) Adsorptive removal of Cu(II) from aqueous solution and industrial effluent using natural and agricultural waste. Colloid Surfaces B Biointerfaces 107:97–106
Sinha R, Kumar R, Sharma P, Kant N, Shang J, Aminabhavi TM (2022) Removal of hexavalent chromium via biochar-based adsorbents: state-of-the-art, challenges, and future perspectives. J Environ Manage. https://doi.org/10.1016/j.jenvman.2022.115356
Song Z, Lian F, Yu Z, Zhu L, Xing B, Qiu W (2014) Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem Eng J 242:36–42. https://doi.org/10.1016/j.cej.2013.12.061
Tan XF, Liu YG, Gu YL, Xu Y, Zeng GM, Hu XJ, Liu SB, Wang X, Liu SM, Li J (2016) Biochar-based nanocomposites for the decontamination of wastewater: a review. Bioresour Technol 212:318–333. https://doi.org/10.1016/j.biortech.2016.04.093
Tan WT, Zhou H, Tang SF, Zeng P et al (2022) Enhancing Cd(II) adsorption on rice straw biochar by modification of iron and manganese oxides. Environ Pollut. https://doi.org/10.1016/j.envpol.2022.118899
Tehreem S, Yousra M, Alamer KH, Alsudays IM, Sarwar S, Kamal A (2022) Analysis of the role of various biochar in the remediation of heavy metals in contaminated water and its kinetics study. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2022.101518
Thirumavalavan M, Lai Y, Lin L, Lee J (2010) Cellulose-based native and surface-modified fruit peels for the adsorption of heavy metal ions from aqueous solution. J Chem Eng Data 55:1186–1192
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock type effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Tong XJ, Li JY, Yuan JH, Xu RK (2011) Adsorption of Cu(II) by biochars generated from three crop straws. Chem Eng J 172:828–834. https://doi.org/10.1016/j.cej.2011.06.069
Torab-Mostaedi M, Hemmati A, Khosravi A (2013) Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grape fruit peel. J Taiwan Inst Chem Eng 44:295–302
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis. Renew Sustain Ener Rev 55:467–481. https://doi.org/10.1016/j.rser.2015.10.122
Vithanage M, Herath I, Joseph S, Bundschuh J, Bolan N, Ok YS (2017) Interaction of arsenic with biochar in soil and water: a critical review. Carbon 113:219–230. https://doi.org/10.1016/j.carbon.2016.11.032
Wang RZ, Huang DL, Liu YG, Zhang C, Lai C, Zeng GM (2018) Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour Technol 261:265–271. https://doi.org/10.1016/j.biortech.2018.04.032
Wang L, Wang Y, Ma F, Tankpa V, Bai S, Guo X (2019) Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater. Sci of Total Environ 668:1298–1309. https://doi.org/10.1016/j.scitotenv.2019.03.011
Wang H, Wang H, Zhao H, Yan Q (2020) Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu Blue algae. Eng J 379:122372. https://doi.org/10.1016/j.cej.2019.122372
Wang Z, Luo P, Zha X, Xu C, Kang S, Zhou M, Nover D, Wang Y (2022) Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and soil. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.134043
Wei Y, Wei S, Liu C, Chen T, Tang Y, Ma J, Yin K, Luo S (2019) Efficient removal of as from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high fe utilization and fast adsorption kinetics. Water Res 167:115107. https://doi.org/10.1016/j.watres.2019.115107
World Health Organization (WHO) (2011) Guidelines for drinking water quality. 4 recommendations, 3rd edn. World Health Organization, Geneva
Wu J, Huang D, Liu X, Meng J, Tang C, Xu J (2018) Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J Hazard Mater 348:10–19. https://doi.org/10.1016/j.jhazmat.2018.01.011
Xia S, Song Z, Jeyakumar P, Shaheen SM, Rinklebe J, Ok YS, Bolan N, Wang H (2019) A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit Rev Environ Sci Technol 49(12):1027–1078. https://doi.org/10.1080/10643389.2018.1564526
Xiao X, Chen B, Chen Z, Zhu L, Schnoor JL (2018) Insight into multiple and multilevel structures of biochars and their potential environmental applications: a critical review. Environ Sci Technol 52:5027–5047. https://doi.org/10.1021/acs.est.7b06487
Xiao Z, Zhang L, Wu L, Chen D (2019) Adsorptive removal of Cu(II) from aqueous solutions using a novel macroporous bead adsorbent based on poly(vinyl alcohol)/sodium alginate/KMnO4 modified biochar. J Taiwan Inst Chem Eng 102(2019):110–117
Xiao B, Jia J, Wang W, Zhang B, Ming H, Ma S (2023) A review on magnetic biochar for the removal of heavy metals from contaminated soils: preparation, application, and microbial response. J Hazard Mat Adv 10:100254. https://doi.org/10.1016/j.hazadv.2023.100254
Xiaorui L, Longji Y, Xudong Y (2021) Evolution of chemical functional groups during torrefaction of rice straw. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.124328
Xie Y, Wang L, Li H, Westholm LJ et al (2022) A critical review on production, modification, and utilization of biochar. J Anal Appl Pyrolysis. https://doi.org/10.1016/j.jaap.2021.105405
Xiong J, Koopal LK, Tan W, Fang L, Wang M, Zhao W (2013) Lead binding to soil fulvic and humic acids: NICA-Donnan modeling and XAFS spectroscopy. Environ Sci Technol 47:11634–11642. https://doi.org/10.1021/es402123v
Xu X, Cao X, Zhao L (2013) Comparison of rice husk and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:955–961. https://doi.org/10.1016/j.chemosphere.2013.03.009
Xu X, Schierz A, Xu N, Cao X (2016) Comparison of the characteristics and mechanisms of Hg(II) sorption by biochar and activated carbon. J Colloid Interface Sci 463:55–60. https://doi.org/10.1016/j.jcis.2015.10.003
Xue Y, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman AR (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J 200–202:673–680. https://doi.org/10.1016/j.cej.2012.06.116
Yang S, Wu Y, Aierken A, Zhang M, Fang P, Fan Y, Ming Z (2016) Mono-competitive adsorption of As (III) and Ni (II) using modified green tea waste. J Taiwan Inst Chem Eng 60:213–221
Yang F, Zhang S, Li H, Li S, Cheng K, Li JS (2018) Corn straw-derived biochar impregnated with Α-FeOOH nanorods for highly effective copper removal. Chem Eng J 348:191–201. https://doi.org/10.1016/j.cej.2018.04.161
Yang L, He L, Xue J, Wu L, Ma Y, Li H, Peng P, Li M, Zhang Z (2019) Highly efficient nickel (II) removal by sewage sludge biochar supported α-Fe2O3 and α-FeOOH: sorption characteristics and mechanisms. PLoS ONE 14:1–16. https://doi.org/10.1371/journal.pone.0218114
Yang D, Wang L, Li Z, Tang X, He M, Yang S, Liu X, Xu J (2020) Simultaneous adsorption of Cd(II) and As(III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems. Total Environ 708:134823. https://doi.org/10.1016/j.scitotenv.2019.134823
Yang L, Hu W, Chang Z, Liu T, Fang D, Shao P (2021) Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: recent advances and future trends. Environ Int. https://doi.org/10.1016/j.envint.2021.106512
Yi Y, Tu G, Zhao D, Tsang PE, Fang Z (2019) Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstock and steel pickling waste liquor. Chem Eng J 360:212–220. https://doi.org/10.1016/j.cej.2018.11.205
Yuan JH, Xu RK, Zhang H (2011) The forms of alkali in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018
Zama EF, Zhu YG, Reid BJ, Sun GX (2017) The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II), and As(III) in aqueous solutions. J Clean Prod 148:127–136. https://doi.org/10.1016/j.jclepro.2017.01.125
Zhang M (2011) Adsorption study of Pb(II), Cu(II), and Zn(II) from simulated acid mine drainage using dairy manure compost. Chem Eng J 172:361–368
Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, Inyang M (2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol 130:457–462. https://doi.org/10.1016/j.biortech.2012.11.132
Zhang X, Fu W, Yin Y, Chen Z, Qiu R, Simonnot MO (2018) Adsorption-reduction removal of Cr(VI) by tobacco petiole pyrolytic biochar: batch experiment, kinetic and mechanism studies. Bioresour Technol 268:149–157. https://doi.org/10.1016/j.biortech.2018.07.125
Zhang Y, Yue X, Xu W (2019a) Amino modification of rice straw-derived biochar to enhance its Cd (II) ion adsorption from water. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.120783
Zhang L, Tang S, He F, Liu Y, Mao W, Guan Y (2019b) Highly efficient and selective capture of heavy metals by poly(acrylic acid)–grafted chitosan and biochar composite for wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122215
Zhao X, Song Z, Liu H, Li Z, Li L, Ma C (2010) Microwave pyrolysis of cornstalk bale: a promising method for direct utilization of large-sized biomass and syngas production. J Anal Appl Pyrolysis 89:87–94
Zhao JJ, Shen XJ, Domene X, Alcañiz JM, Palet C (2019) Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple metal solutions. Sci Rep. https://doi.org/10.1038/s41598-019-46234-4
Zheng C, Yang Z, Si M, Zhu F, Yang W, Zhao F (2021) Application of biochars in the remediation of chromium contamination: fabrication, mechanisms, and interfering species. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124376
Zhou L, Liu Y, Liu S, Yin Y, Zeng G, Tan X (2016) Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars at different pyrolytic temperatures. Bioresour Technol 218:351–359. https://doi.org/10.1016/j.biortech.2016.06.102
Zhou Q, Liao B, Lin L, Qiu W, Song Z (2018a) Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci Total Environ 615:115–122. https://doi.org/10.1016/j.scitotenv.2017.09.220
Zhou X, Zhou J, Liu Y, Guo J, Ren J, Zhou F (2018b) Preparation of IA-modified magnetic biochar by carbonization magnetization and functional modification for Cd(II)removal from water. Fuel 233:469–479
Zhou W, Wu P, Zhang L, Zhu D, Zhao X, Cai Y (2022) Heavy metal ions and particulate pollutants can be effectively removed by a gravity-driven ceramic foam filter optimized by carbon nanotube implantation. J Hazard Mater 421:126721. https://doi.org/10.1016/j.jhazmat.2021.126721
Zong Y, Xiao Q, Lu S (2021) Biochar derived from cadmium-contaminated rice straw at various pyrolysis temperatures: cadmium immobilization mechanisms and environmental implications. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.124459
Acknowledgements
Not applicable.
Funding
Funding for this study was supported by the National Natural Science Foundation of China (41,977,274,42,130,710), Shaanxi Province Science and Technology Innovation Team (2022TD-09), and the Key Industrial Chain Project of Shaanxi Province (2019ZDLNY01-05–02; 2022ZDLNY02-02).
Author information
Authors and Affiliations
Contributions
Muhammad Haris: Conceptualization, writing, original draft. Zainab Amjad and Muhammad Usman: Review and editing. Atif Saleem, Ainur Dyussenova, and Zarak Mahmood: Graphic design and formatting; Kukybayeva Dina: Language editing; Junkang Guo and Wenke Wang: Supervision, review and editing, and funding acquisition. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing financial interests to declare.
Additional information
Handling editor: Wenfu Chen
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haris, M., Amjad, Z., Usman, M. et al. A review of crop residue-based biochar as an efficient adsorbent to remove trace elements from aquatic systems. Biochar 6, 47 (2024). https://doi.org/10.1007/s42773-024-00341-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-024-00341-2