Abstract
Bamboo biochar was modified by lignin impregnation and microwave irradiation to enhance its performance for CO2 capture. The pore structure of lignin-impregnated biochar was significantly affected by the impregnation ratio. The maximum specific surface area of 377.32 m2 g−1 and micropore volume of 0.163 cm3 g−1 were observed on the biochar with an impregnation ratio of 1:20 (mass ratio of lignin to biochar). Lignin impregnation increased the CO2 adsorption capacity of biochar up to 134.46 mg g−1. Correlation analysis confirmed the crucial role of biochar’s pore structure in adsorption. The Avrami model fitted the CO2 capture curves well. The calculation of adsorption activation energy suggested that the adsorption process was dominated by physical mechanism assisted with partial chemical mechanism. Meanwhile, Langmuir isotherm analysis indicated that lignin impregnation transformed the larger pores of biochar into more uniform micropores, thereby making the adsorption process closer to monolayer adsorption. Both the high reusability (89.79–99.06%) after 10 successive cycles and the excellent CO2 selectivity in competitive adsorption confirmed that lignin-impregnated biochar is an outstanding adsorbent for CO2 capture.
Graphical Abstract

Highlights
-
Microwave energy absorbed by biochar carbonized the impregnated lignin to increase micropore structure.
-
CO2 uptake on lignin-impregnated biochar reached 134.46 mg g−1 and was associated with micropore, alkalinity, and temperature.
-
CO2 adsorption on lignin-impregnated biochar was governed by physisorption with slight chemisorption.
-
Lignin-impregnated biochar demonstrated excellent reusability and selectivity in CO2 capture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The continuous emission of greenhouse gas leads to the rising of global temperature. As the main greenhouse gas, CO2 is posing a huge threat to the environment (Karimi et al. 2022). Therefore, finding a high-efficiency and low-energy method to control the CO2 emission is significant to the whole world. Various methods, such as adsorption, absorption, chemical looping combustion, cryogenic distillation, and membrane separations have been employed for CO2 capture (Sreedhar et al. 2017). Adsorption is one of the most promising strategies in CO2 capture for its superiorities of high efficiency, cost-effectiveness, stability, and easy operation (Hong 2022). Many adsorbents, such as activated carbon (Zhang et al. 2021), carbon nano-tubes (Baghery et al. 2019), MOFs (Ma et al. 2022), zeolitic imidazolate frameworks (ZIFs) (Chang et al. 2020) and metal oxides (Liao et al. 2022) have already been employed for CO2 adsorption. As a newly emerged carbon material, biochar has been widely used in environmental management and ecological restoration, which is also a promising candidate for CO2 capture (Jellali et al. 2022; Zhang et al. 2022b).
Adsorption capacity of biochar is affected by many factors, such as porosity (Guo et al. 2022), alkalinity (Kumar et al. 2015), surface functional groups (Torrisi et al. 2010), mineral content (Li et al. 2017), hydrophilicity and hydrophobicity (Dissanayake et al. 2020).Nevertheless, in terms of CO2 adsorption, micropores and alkalinity play more crucial role (Goel et al. 2021; Petrovic et al. 2021). Biochar has well-developed pore structure. When it comes to CO2 adsorption, micropores play a more significant role than macropores and mesopores. This is because micropores enable stronger van der Waals force between CO2 molecules and biochar (Zhang et al. 2022d). Igalavithana et al. (2020a) found that KOH-activated biochar showed excellent CO2 adsorption capacity due to its abundant micropores developed after activation. Beyond the micropores, CO2 adsorption is also influenced by alkalinity of biochar for the acidic nature of CO2. Cao et al. (2022) prepared various straw and wood biochars, and found that the biochar with the highest alkalinity had the highest CO2 uptake of 79.99 mg g−1 at 0 °C. As reported, CO2 can react with alkaline functional groups like amine through several different pathways. For example, the primary amines (–NH2) and secondary amines (–NH) can directly react with CO2 to form carbamates (Cao et al. 2022). Thus, improving the microporosity and alkalinity of biochar is conducive to enhancing its CO2 capture ability.
Various modification methods have been developed to improve the microporosity and alkalinity of biochar, such as CO2 activation (Dissanayake et al. 2020), ultrasonic treatment (Shafawi et al. 2021), impregnation (Zhang et al. 2022b), grafting (Zhang et al. 2022a), solid–solid mixing (Rashidi and Yusup 2016), ligand functionalization (Mankar et al. 2021), etc. Among them, impregnation has the advantage of high efficiency and low cost, making it more competitive for modifying the biochar. Impregnation is a mild and economical modification process, by contrast, other methods like KOH activation and steam activation are usually carried out under strict conditions requiring high temperature and high pressure (Panwar and Pawar 2022). Also, the selection of impregnation precursor may bring different changes to the properties of biochar. For example, metal and metal oxide impregnation improves the basicity of biochar (Jung et al. 2019; Zubbri et al. 2020), while glucose and chitosan impregnation strengthens the pore structure of biochar (Zhang et al. 2022b). Therefore, the properties of target biochar should be considered in the selection of impregnation precursor. In addition, cheap and readily available precursors can decrease the modification cost and have a better application prospect. Thus, it is also necessary to consider the economics of impregnation precursors. Based on the above, lignin with huge yield and high carbon content is an excellent impregnation precursor (Xiang et al. 2022b).
Lignin is an aromatic polymer compound in plants and its natural reserves are second only to cellulose. Global paper industry extracts about 140 million tons of natural fiber from various plants every year, and produces about 50–70 million tons of natural wood by-products annually (Cao et al. 2023), which contributes to most of the lignin production. Nevertheless, more than 95% of lignin was burned after concentration or discharged directly into rivers. This results in low lignin utilization efficiency and even causes serious environmental pollution (Chio et al. 2019). How to improve lignin utilization efficiency has become an important problem faced by the whole world. The application of lignin in biochar modification could be a potential way to improve its utilization.
Microwave pyrolysis is an effective method for biochar preparation. It transfers electromagnetic energy into heat energy and accumulate it inside the material via frequency oscillation of alternating electric field, making polar molecule rub against each other to generate heat (Foong et al. 2020). Compared with traditional pyrolysis methods, microwave irradiation has more advantages, such as low energy consumption, fast start-stop, good controllability, selective heating, large penetration depth (Xiang et al. 2022a). Therefore, biochar prepared by microwave irradiation has demonstrated more uniform micropores, superior specific surface area (SSA) and surface functional groups (Li et al. 2016; Shirvanimoghaddam et al. 2022). Chu et al. (2017) found biochar prepared by microwave irradiation had better pore structure compared with that prepared by muffle furnace. Similar observations have been reported by Mašek et al. (2013). Zhang et al. (2022c) prepared porous biochar via microwave irradiation using activated carbon as a catalyst. They found the obtained biochar had high specific surface area (312.62 m2·g−1) and superior VOC adsorption capacity (75.80 mg g−1). Although microwave irradiation has obvious advantages, its application in biochar preparation is still hindered by certain factors, such as the poor microwave absorption ability of biomass.
Biomass usually has poor microwave absorption capacity because of its low loss tangent value (Kostas et al. 2017; Salema et al. 2017). However, this changes completely once the biomass is pyrolyzed into biochar. Biochar with plentiful pores can absorb an abundance of microwave and converted it into heat energy (Lin et al. 2022). Many studies have proven that biochar has higher loss tangent value than raw biomass (Mushtaq et al. 2014; Salema et al. 2017; Siddique et al. 2022). Thus biochar derived from biomass is an excellent microwave adsorbent. An ingenious solution can be designed based on the facts that (i) precursor lignin requires heat to be carbonized and create more micropores; (ii) carrier biochar can absorb microwave to generate heat. Therefore, precursor lignin could be effectively pyrolyzed by the heat energy from microwave, and much more micropores would be created on the lignin-impregnated biochar.
In this work, biochar derived from bamboo was modified by lignin impregnation and microwave irradiation. After modification, the physiochemical properties of biochar were improved to enhance its CO2 capture ability. The major objectives of this work were to: (1) develop a pore-size modulation method for biochar via lignin impregnation and microwave irradiation, (2) evaluate the CO2 capture ability of lignin-impregnated biochar, and (3) illustrate the adsorption mechanism.
2 Materials and methods
2.1 Biochar preparation
Bamboo was collected from Wuxi, China. Lignin (BSF190725) was purchased from Hefei BASF Biotechnology Co., LTD, China. High purity CO2 cylinder gas (99.999%) was purchased from Xuzhou special gas factory, China.
Bamboo was cut into strips (~5 cm) to fit the quartz crucible and then pyrolyzed at 600 °C for 5 h. After pyrolysis, the obtained bamboo biochar (BB) was ground in an agate mortar and then sieved through 40 to 100 mesh sieves. To modulate biochar’s aperture, lignin was introduced into the biochar skeleton using the wet impregnation method. Lignin-impregnated biochar with different proportion (lignin to BB mass ratio of 1:3, 1:5, 1:10, 1:15, 1:20, respectively) was prepared. For this, a calculated amount of lignin was dissolved, and then BB was added to the solution. The mixture was stirred at 25 °C for 12 h, then a thermostatic water bath was used to evaporate the water. After drying, each sample was heated via microwave irradiation at 600 W for 20 min to carbonize the impregnated lignin. Finally, the obtained samples were marked as LBB3, LBB5, LBB10, LBB15 and LBB20 according to the impregnation mass ratio.
2.2 Characterization
Pore structure parameters of biochar were measured on a specific surface area analyzer (Kubo X1000, Bibuilder, China) by N2 adsorption and desorption isotherms at −196 °C. The elemental analyzer (Elementar Vario MACRO cube, Germany) was employed to analyze the C, H, N, and S element contents of biochar. The thermal gravimetric analyzer (TGA, Mettler-Toledo TGA/DSC 3 +, Switzerland) was employed to test ash content and thermostability. Raman spectra were determined by a Raman microscope (DXR2, Thermo Fisher Scientific, USA). Boehm titration method was employed to determine the total basicity (Tsechansky and Graber 2014). Surface functional groups were characterized by a Fourier transform infrared spectrometer (FTIR, IS10, Thermo Fisher Scientific, USA).
2.3 Adsorption experiment
According to gravimetric method reported previously (Creamer et al. 2014), CO2 capture experiment was carried out on the TGA. Each sample was placed in an alumina crucible with 10 mg biochar, then the sample was heated to 200 °C to get rid of the impurities under N2 atmosphere. When the temperature reduced to the preset adsorption temperature (25, 35, 45, 55, and 65 °C), high purity CO2 of 50 mL min−1 was injected under atmospheric pressure. The weight gain of biochar sample was the CO2 adsorbed. Adsorption isotherm experiment was conducted on the Kubo X 1000 at 0 °C. Adsorption/desorption cycle experiment was used to test the reusability of biochar. Before all the cycles started, the biochar was heated to 200 °C to remove the moisture, then CO2 was adsorbed at 25 °C. After adsorption equilibrium, the biochar was desorbed by increasing the temperature from 25 °C to 200 °C. The competitive adsorption experiment was carried out on a multi-component adsorption breakthrough curve analyzer (BSD-MAB, BSD Instrument, China). The standard gas of SO2 (1 vol%) and NO (1 vol%) was chosen as the competing gas, and high purity N2 was used as the carrier gas. Specific procedure can be found in a previous study (Cao et al. 2023). Inlet and outlet concentrations of gas were measured by mass spectrum. All tests were repeated three times.
2.4 Data analysis
N2 adsorption/desorption data were analyzed by the Brunauer-Emmett-Teller equation. Pore size distribution was calculated by density function theory method. Aiming to investigate the adsorption mechanisms, various models (Pseudo-first order, pseudo-second order, Avrami kinetic models, Langmuir and Freundlich models) were employed to fit the experimental results. Adsorption activation energy was calculated by the Arrhenius equation. The models and equations are described in Additional file 1.
3 Results and discussion
3.1 Characterization of pristine and lignin-impregnated biochar
The SSA of lignin-impregnated biochar was 146.66–377.32 m2 g−1, which increased with decreasing impregnation ratio from 1:3 to 1:20 (Table 1). Notably, the SSA of BB was higher than that of LBB3, LBB5, and LBB10, but lower than that of LBB15 and LBB20. This result suggest that, while lignin impregnation generally increased SSA of biochar, excessive lignin could cover and block the pores of biochar to decrease its SSA. Only large pores of biochar would be filled with lignin because it has high molecular weight and large molecular size (Sun et al. 2021). Therefore, biochar’s macropores and mesopores could be converted into micropores by lignin filling, while the micropores remain as it because the lignin cannot enter them. During microwave irradiation, the impregnated lignin was carbonized and fixed in the biochar with the assistance of heat converted from microwave energy. Therefore, much more micropores were created on the lignin-impregnated biochar, which was verified in the following total pore volume (PV) and micropore volume (MV) analysis. To sum, excessive lignin impregnation would lead to lower SSA than pristine biochar, while suitable dosages of lignin impregnation would increase biochar’s SSA.
The PV of biochar ranged from 0.112 cm3 g−1 to 0.235 cm3 g−1. It increased as the impregnation ratio decreased from 1:3 to 1:20 (Table 1). Additionally, the PV of LBB3 was lower than that of BB. This could also be attributed to the excessive impregnation of lignin, which covered and blocked the intrinsic pores of biochar. Furthermore, MV and pore distribution of biochar (Table 1 and Fig. 1a) confirmed that lignin mainly entered macropores and mesopores. The MV of lignin-impregnated biochar ranged from 0.065 cm3 g−1 to 0.163 cm3 g−1, and it increased with the decrease of impregnation ratio. The MV of LBB3 and LBB5 was lower than that of precursor biochar, because the excessive lignin impregnation blocked the original micropores. However, with the impregnation ratio decreased, lignin was less agglomerated, and most of lignin would enter the large apertures that might create more micropores. Besides, impregnated lignin would also be carbonized and generated plenty of new micropores with the assistance of microwave irradiation.
The total basicity of biochar ranged from 0.248 mmol g−1 to 0.743 mmol g−1, which decreased as the impregnation ratio reduced from 1:3 to 1:20 (Table 1). Compared with that of a previous study (Cao et al. 2022), basicity of lignin-impregnated biochar was higher than that of most the straw-based biochar (0.11–0.27 mmol g−1), suggesting that lignin impregnation could significantly enhance the alkalinity of biochar. This is because lignin is an alkaline substance containing numerous alkaline metals (Zhang et al. 2022b), which are mainly derived from additives used in the extraction of lignin (Santos et al. 2020). So, the ash content of lignin (48.71%) was much higher than that of BB (1.09%). As a result, lignin-impregnated biochar also exhibited higher ash content (1.74–15.81%) than BB.
The carbon content of biochar was 70.78–81.40% (Table 1), among which BB had the highest carbon content of 81.40%. The higher carbon content of BB contributed to its excellent microwave absorption performance. Li et al. (2019) prepared sawdust and oil sludge biochar, and found that biochar with higher carbon content exhibited higher heating rate and pyrolysis efficiency under microwave irradiation. It was attributed to the fact that carbon materials have excellent dielectric properties. Therefore, lignin could be well carbonized and create new micropores on the biochar with higher carbon content. Nitrogen and oxygen contents of lignin-impregnated biochar were 0.61–0.74% and 17.16–24.07%, respectively, which were higher than those of BB. However, the sulfur and hydrogen contents of lignin-impregnated biochar showed opposite trends, lower than those of BB. This can be ascribed to the volatilization of organic sulfur content (Zhang et al. 2015), and some of the hydrogen bonds were broken during the dehydration process during microwave irradiation (Devi and Saroha 2015a).
Raman spectra are exhibited in Fig. 1b. Two major bands ranging at 1601–1605 cm−1 and 1344–1350 cm−1 were dedicated to graphite band and disorder-induced band, which are also known as G and D band. The intensities of G and D bands were assessed by the integral of peak area value and the ratio of D to G band (AD/AG) to reflect the graphitization of biochar. High AD/AG means more structural defects, while low ratio indicates more ordered carbon (Dissanayake et al. 2020). Notably, the AD/AG of LBB3, LBB5, LBB10 were very close to that of BB, which could be attributed to that part of the overlarge impregnated lignin may not be completely carbonized. As a porous carbon material, biochar can adsorb microwave energy and convert it into heat, while the biomass of lignin cannot. Therefore, the carbonization of impregnated lignin mainly depends on the heat transmitted from biochar. High impregnation ratio made lignin pile up on the surface of biochar, so the lignin close to biochar was easily carbonized and had higher degree of graphitization. However, the lignin far away from biochar could not get enough heat energy to be completely carbonized. The AD/AG values of LBB15 and LBB20 were lower than that of BB. Moreover, the AD/AG of LBB20 was even lower than that of biochar activated by KOH and CO2 (Dissanayake et al. 2020), indicating that biochar with less lignin impregnation had a higher degree of graphitization. At low impregnation ratio, lignin was uniformly distributed in the aperture of biochar and was fully carbonized. Meanwhile, after lignin carbonization, excess energy would be absorbed by precursor biochar and promote further pyrolysis of biochar itself, which would enhance its graphitization degree. Excessive microwave irradiation may also have side effects on biochar, such as the collapse of pore tunnel at high temperature (Atinafu et al. 2021). Therefore, impregnated lignin not only modulates the pore structure but also absorbs excessive heat energy and supports pore tunnel during microwave irradiation. To sum, low lignin impregnation assisted with appropriate microwave irradiation was beneficial to improving the physiochemical properties of biochar.
The thermostability of biochar is demonstrated in Fig. 1c. The weight loss observed on the curve from room temperature to 150 °C was mainly caused by the removal of moisture. Elevating temperature resulted in much more weight loss, including the organic components decomposition and inorganic material degradation (Dai et al. 2022). Notably, the TGA curves of LBB3 and LBB5 were similar to those of the original lignin, which showed obvious decrease during 150–650 °C and 650–830 °C. Nevertheless, the TGA curves of LBB10, LBB15 and LBB20 showed only a slight decrease and were similar to those of BB. The weight loss could be ascribed to the decomposition of aromatic rings. Kim et al. (2020) prepared sawdust biochar and found the absorption band of aromatic rings and aromatic C–H bands disappeared when the pyrolysis temperature was elevated to 700 °C. Therefore, the difference between thermostability curves could be ascribed to the decomposition of lignin components that were not sufficiently carbonized. This reconfirmed that biochar with lower impregnation ratio had higher carbonization degree, and vice versa.
Prominent FTIR peaks at 3441 cm−1 and 1634 cm−1 were observed on all the biochar samples, which could be dedicated to the bending vibration of C–OH and C=O, respectively (Fig. 1d). Also, there was a distinct FTIR vibration peak at 1416 cm−1, which could be attributed to amide vibration. The presence of amide was related with lignin impregnation, and increasing the lignin impregnation ratio made amide vibration stronger, and vice versa. Amide is an alkaline group, so the increase of amide further indicated that the alkalinity of biochar was enhanced after lignin impregnation.
3.2 CO2 adsorption
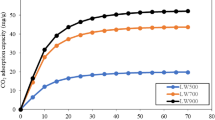
CO2 adsorption amount on lignin-impregnated biochar at 0 °C was 105.85–134.46 mg g−1 (Fig. 2a), which was higher than that of BB. Moreover, it was also larger than the results of most previous studies including Cao et al. (2022), who reported the CO2 adsorption amount ranged 51.99–94.08 mg g−1 at 0 °C and 100 kPa on straw and woody based biochar. Besides, Zhang et al. (2022b) prepared fine-tuned micropore biochar and compared its CO2 capture ability with other biochar. They found the CO2 uptake on pristine biochar usually less than 45.85 mg g−1; while it could increase to 106.92 mg g−1 on the modified biochar (e.g., KOH activated, N-enriched, metal-incorporation, etc.). The variation of CO2 uptake on biochar was suspected to be influenced by two factors. Firstly, the aperture, especially micropores, of precursor biochar was improved by lignin impregnation assisted with microwave irradiation, making it more suitable for CO2 capture. Secondly, the basicity of biochar decreased with reducing the lignin impregnation ratio, which had adverse effect on CO2 capture. Therefore, combining the influences of the two opposite effects, the CO2 uptake increased as the impregnation ratio decreased from 1:3 to 1:15, and then decreased.
Linear correlation analysis was conducted to determine the key factors that influence CO2 capture, using the adsorption amount of CO2 against SSA, PV, and MV (Fig. 2). The R2 of SSA, PV and MV were 0.9427, 0.8343 and 0.9204, respectively, indicating all the selected pore structure parameters had a linear relationship with CO2 uptake. This confirmed that pore structure was the dominant factor for CO2 capture. Notably, the R2 of MV was higher than that of PV, indicating that CO2 adsorption process was mainly influenced by micropore structure. This is in accordance with the findings of previous studies. For example, Zhang et al. (2022b) found the CO2 adsorption was more influenced by micropore volume other than total pore volume. It was worth noting that although the pore structure of LBB20 was superior to that of LBB15, the adsorption amount of LBB20 was still lower than that of LBB15. This could be attributed to the decrease of basicity. As a Lewis base, lignin can enhance the alkalinity of biochar, making it easier for the Lewis acid gas CO2 to be captured by lignin-impregnated biochar. The total basicity decreased with reducing the impregnation ratio, weakening biochar's ability to capture CO2 by Lewis acid–base reaction. Hence, although the SSA, PV and MV of LBB20 increased by 10.87%, 9.40%, and 9.40% compared with those of LBB15, respectively, its basicity decreased by 54.07%. Therefore, the CO2 uptake of LBB20 still decreased due to the contradicting effects of pore structure and basicity.
Temperature plays a critical role in CO2 adsorption, and its influence on CO2 adsorption was evaluated by changing the adsorption temperature from 0 °C to 65 °C (Fig. 2a). Notably, the CO2 adsorption amount on all the biochar decreased with elevating temperature from 0 °C to 65 °C. That was because the Brownian motion of CO2 molecule intensified with increasing temperature, making it harder to be captured by the biochar. The significant decrease of CO2 adsorption amount indicates that CO2 adsorption is an overwhelming exothermic process (Zhang et al. 2022b).
3.3 Adsorption kinetics and activation energy
Pseudo-first-order, pseudo-second-order, and Avrami model were used to fit CO2 capture on biochar. Pseudo-first-order model is mainly used to describe pure physical adsorption (Ding and Liu 2020), while pseudo-second-order model is applicable when the chemisorption is the rate-limiting step (Cho et al. 2017). Avrami model is favorable to predict particle nucleation and crystal growth, which can account for both physical and chemical adsorption (Raganati et al. 2019).
The R2 values of aforementioned three models at 25 °C were 0.951–0.972, 0.925–0.973, and 0.968–0.984, respectively (Table 2 and Fig. 3). Obviously, Avrami fractional model had the highest, R2 values mostly and described the CO2 adsorption process well. Nevertheless, the R2 of the pseudo-second-order model was slightly larger than that of Avrami model for LBB3. This indicated that excessive lignin impregnation made the biochar exhibit certain chemisorption properties. All the Avrami exponents (n) were higher than 1, indicating that CO2 adsorption on biochar was not homogeneous (Zhang et al. 2022b; Zubbri et al. 2020). This might be attributed to the deviations in the uniformity of adsorption sites, and different regions had different probabilities to capture CO2 (Raganati et al. 2019). KA is adsorption rate constant and all the impregnated biochar had lower KA than BB, which implied lignin impregnation could reduce the CO2 adsorption kinetic rate. That was because lignin impregnation reduced biochar’s pore size and increased the diffusion resistance of CO2 in the narrow pores (Zhang et al. 2017). Besides, with the elevation of adsorption temperature, the adsorption rate KA of each biochar increased (Additional file 1: Table S1), indicating the acceleration of adsorption (Raganati et al. 2019).
Arrhenius equation was used to calculate the activation energy of CO2 adsorbed on biochar (Li et al. 2015; Raganati et al. 2019), which ranged from 1.23 kJ mol−1 to 5.05 kJ mol−1 (Fig. 4). As reported, the physical adsorption activation energy is less than 4.184 kJ mol−1, while it ranges 40–800 kJ mol−1 for pure chemical adsorption (Devi and Saroha 2015b; Huang et al. 2015). In this study, the BB had the lowest activation energy of 1.23 kJ mol−1. After lignin impregnation, the activation energy increased up to 3.54–5.05 kJ mol−1, suggesting the lignin impregnation could promote the CO2 adsorption on biochar by chemical adsorption. Moreover, the more lignin impregnated, the higher activation energy of biochar had. For instance, LBB3 had the highest activation energy of 5.05 kJ mol−1, and it decreased gradually to 4.75 kJ mol−1 on LBB5, and 3.64 kJ mol−1 on LBB20. All in all, the calculation of adsorption activation energy indicated that CO2 capture on lignin impregnated biochar was dominated by physisorption accompanying with slight chemisorption.
3.4 Adsorption isotherms
Langmuir and Freundlich models could fit the adsorption isotherm of CO2 on biochar, and the R2 values were 0.990–0.993 and 0.982–0.993, respectively (Table 3 and Fig. 5). Langmuir model fitted the process slightly better than Freundlich model, indicating CO2 adsorption on biochar was mainly monolayer (Igalavithana et al. 2020b). That was because after lignin impregnation and microwave irradiation, uniform micropores were formed in the biochar. As a result, the CO2 adsorption isotherms tended to exhibit monolayer adsorption.
3.5 Reusability
The reusability of biochar was evaluated by ten consecutive adsorption/desorption cycles. The reuse efficiency was 89.79–99.06% after 10 cycles, indicating the biochar had excellent reusability for CO2 adsorption (Fig. 6). Notably, the highest CO2 adsorption amount of each cycle happened at the first adsorption/desorption process, which is consistent with the results of previously studies (Ding and Liu 2020; Gong et al. 2019; Zhang et al. 2022b). This phenomenon can be attributed to the irreversible interaction between adsorbate and adsorbent (Ding and Liu 2020). It is worth noting that LBB3 had the lowest reuse efficiency. This may be caused by the strong bonding of CO2 and the adsorbent. LBB3 had the highest basicity, and thus in the first few cycles, it could adsorb CO2 by strong Lewis acid–base reaction. Nevertheless, the number of alkaline adsorption sites on LBB3 decreased gradually after several cycles, resulting in a decrease in CO2 adsorption. Whereas the bonding between CO2 and other biochar with low alkalinity was mainly through reversible physisorption, thus the adsorbed CO2 could be more easily desorbed, making their reusability higher than that of LBB3. To sum, lignin-impregnated biochar had excellent reusability for CO2 adsorption.
3.6 Multicomponent competitive adsorption
Competitive adsorption was performed to investigate lignin-impregnated biochar’s selectivity for CO2 in gas mixture. The typical flue gas from coal-fired power plants usually contains 10–16% CO2 and small traces of NOx and SOx (Ghanbarpour Mamaghani et al. 2023), so the volume concentrations of CO2, NO, and SO2 used in multicompetent competitive adsorption experiment were 12 vol%, 0.4 vol%, and 0.4 vol%, respectively.
The adsorption amount of single CO2, NO and SO2 was 28.50 mg g−1, 0.24 mg g−1, 50.10 mg g−1, respectively (Additional file 1: Table S2). When three gases went through the adsorbent simultaneously, their adsorption amount decreased to 23.70 mg g−1, 0.14 mg g−1, and 45.06 mg g−1, respectively. NO has small molecular mass and dynamic diameter, so it passed through the column faster. However, CO2 and SO2, which reached the adsorption site more slowly, would replace some unsteadily adsorbed NO. As a result, there was a sudden increase in the outlet concentration of NO (Fig. 7). The presence of SO2 had an adverse effect on CO2 adsorption because it could form acidic sites on the biochar to repel CO2. However, CO2 occupied the adsorption site faster than SO2, making it decreased only by 16.8% under multicomponent competitive adsorption. Therefore, the lignin-impregnated biochar had excellent selectivity for CO2, making it a promising CO2 adsorbent for practical applications.
4 Conclusion
The aperture of biochar was modulated through lignin impregnation and microwave irradiation. Excessive lignin impregnation reduced the biochar’s SSA, PV and MV because of pore coverage and blockage. However suitable lignin impregnation improved the porosity of biochar. The uptake of CO2 on lignin-impregnated biochar was influenced by pore structure, alkalinity, and temperature, and could reach up to 134.46 mg g−1. The results of kinetic model fittings and activation energy calculation denoted the adsorption process of CO2 on lignin-impregnated biochar was mainly physisorption assisted with slight chemisorption. And the adsorption isotherm fittings showed that the CO2 was mainly adsorbed on the biochar through monolayer adsorption. The reusability of biochar was confirmed (89.79–99.06%) by 10 successive adsorption/desorption cycles. Multicomponent competitive adsorption demonstrated that lignin-impregnated biochar had excellent CO2 selectivity. In a word, lignin impregnation together with microwave irradiation is an effective method to modulate biochar’s pores and the resulting lignin-impregnated biochar is a promising adsorbent for CO2 capture.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Atinafu DG, Yeol Yun B, Uk Kim Y, Wi S, Kim S (2021) Introduction of eicosane into biochar derived from softwood and wheat straw: Influence of porous structure and surface chemistry. Chem Eng J 415:128887. https://doi.org/10.1016/j.cej.2021.128887
Baghery R, Riahi S, Abbasi M, Mohammadi-Khanaposhtani M (2019) Investigation of the CO2 absorption in pure water and MDEA aqueous solution including amine functionalized multi-wall carbon nano tubes. J Mol Liq 293:111431. https://doi.org/10.1016/j.molliq.2019.111431
Cao L, Zhang X, Xu Y, Xiang W, Wang R, Ding F, Hong P, Gao B (2022) Straw and wood based biochar for CO2 capture: adsorption performance and governing mechanisms. Sep Purif Technol 287:120592. https://doi.org/10.1016/j.seppur.2022.120592
Cao W, Xu H, Zhang X, Xiang W, Qi G, Wan L, Gao B (2023) Novel post-treatment of ultrasound assisting with acid washing enhance lignin-based biochar for CO2 capture: adsorption performance and mechanism. Chem Eng J 471:144523. https://doi.org/10.1016/j.cej.2023.144523
Chang C-W, Kao Y-H, Shen P-H, Kang P-C, Wang C-Y (2020) Nanoconfinement of metal oxide MgO and ZnO in zeolitic imidazolate framework ZIF-8 for CO2 adsorption and regeneration. J Hazard Mater 400:122974. https://doi.org/10.1016/j.jhazmat.2020.122974
Chio C, Sain M, Qin W (2019) Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sustain Energy Rev 107:232–249. https://doi.org/10.1016/j.rser.2019.03.008
Cho D-W, Kwon G, Yoon K, Tsang YF, Ok YS, Kwon EE, Song H (2017) Simultaneous production of syngas and magnetic biochar via pyrolysis of paper mill sludge using CO2 as reaction medium. Energy Convers Manage 145:1–9. https://doi.org/10.1016/j.enconman.2017.04.095
Chu G, Zhao J, Chen F, Dong X, Zhou D, Liang N, Wu M, Pan B, Steinberg CEW (2017) Physi-chemical and sorption properties of biochars prepared from peanut shell using thermal pyrolysis and microwave irradiation. Environ Pollut 227:372–379. https://doi.org/10.1016/j.envpol.2017.04.067
Creamer AE, Gao B, Zhang M (2014) Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J 249:174–179. https://doi.org/10.1016/j.cej.2014.03.105
Dai Q, Liu Q, Zhang X, Cao L, Hu B, Shao J, Ding F, Guo X, Gao B (2022) Synergetic effect of co-pyrolysis of sewage sludge and lignin on biochar production and adsorption of methylene blue. Fuel 324:124587. https://doi.org/10.1016/j.fuel.2022.124587
Devi P, Saroha AK (2015a) Effect of pyrolysis temperature on polycyclic aromatic hydrocarbons toxicity and sorption behaviour of biochars prepared by pyrolysis of paper mill effluent treatment plant sludge. Biores Technol 192:312–320. https://doi.org/10.1016/j.biortech.2015.05.084
Devi P, Saroha AK (2015b) Simultaneous adsorption and dechlorination of pentachlorophenol from effluent by Ni–ZVI magnetic biochar composites synthesized from paper mill sludge. Chem Eng J 271:195–203. https://doi.org/10.1016/j.cej.2015.02.087
Ding S, Liu Y (2020) Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 260:116382. https://doi.org/10.1016/j.fuel.2019.116382
Dissanayake PD, Choi SW, Igalavithana AD, Yang X, Tsang DCW, Wang C-H, Kua HW, Lee KB, Ok YS (2020) Sustainable gasification biochar as a high efficiency adsorbent for CO2 capture: a facile method to designer biochar fabrication. Renew Sustain Energy Rev 124:109785. https://doi.org/10.1016/j.rser.2020.109785
Foong SY, Liew RK, Yang Y, Cheng YW, Yek PNY, Wan Mahari WA, Lee XY, Han CS, Vo D-VN, Van Le Q, Aghbashlo M, Tabatabaei M, Sonne C, Peng W, Lam SS (2020) Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: progress, challenges, and future directions. Chem Eng J 389:124401. https://doi.org/10.1016/j.cej.2020.124401
Ghanbarpour Mamaghani Z, Hawboldt KA, MacQuarrie S (2023) Adsorption of CO2 using biochar—review of the impact of gas mixtures and water on adsorption. J Environ Chem Eng 11(3):109643. https://doi.org/10.1016/j.jece.2023.109643
Goel C, Mohan S, Dinesha P (2021) CO2 capture by adsorption on biomass-derived activated char: a review. Sci Total Environ 798:149296. https://doi.org/10.1016/j.scitotenv.2021.149296
Gong H, Tan Z, Zhang L, Huang Q (2019) Preparation of biochar with high absorbability and its nutrient adsorption–desorption behaviour. Sci Total Environ 694:133728. https://doi.org/10.1016/j.scitotenv.2019.133728
Guo T, Tian W, Wang Y (2022) Effect of pore structure on CO2 adsorption performance for ZnCl2/FeCl3/H2O(g) co-activated walnut shell-based biochar. Atmosphere 13(7):1110. https://doi.org/10.3390/atmos13071110
Hong WY (2022) A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci Technol 3:100044. https://doi.org/10.1016/j.ccst.2022.100044
Huang Y-F, Chiueh P-T, Shih C-H, Lo S-L, Sun L, Zhong Y, Qiu C (2015) Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 84:75–82. https://doi.org/10.1016/j.energy.2015.02.026
Igalavithana AD, Choi SW, Dissanayake PD, Shang J, Wang C-H, Yang X, Kim S, Tsang DCW, Lee KB, Ok YS (2020a) Gasification biochar from biowaste (food waste and wood waste) for effective CO2 adsorption. J Hazard Mater 391:121147. https://doi.org/10.1016/j.jhazmat.2019.121147
Igalavithana AD, Choi SW, Shang J, Hanif A, Dissanayake PD, Tsang DCW, Kwon J-H, Lee KB, Ok YS (2020b) Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: effect of porous structure and surface chemistry. Sci Total Environ 739:139845. https://doi.org/10.1016/j.scitotenv.2020.139845
Jellali S, El-Bassi L, Charabi Y, Usman M, Khiari B, Al-Wardy M, Jeguirim M (2022) Recent advancements on biochars enrichment with ammonium and nitrates from wastewaters: a critical review on benefits for environment and agriculture. J Environ Manage 305:114368. https://doi.org/10.1016/j.jenvman.2021.114368
Jung S, Park Y-K, Kwon EE (2019) Strategic use of biochar for CO2 capture and sequestration. J CO2 Utilization 32:128–139. https://doi.org/10.1016/j.jcou.2019.04.012
Karimi M, Shirzad M, Silva JAC, Rodrigues AE (2022) Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: a review and prospects for future directions. J CO2 Utilization 57:101890. https://doi.org/10.1016/j.jcou.2022.101890
Kim H-B, Kim J-G, Kim T, Alessi DS, Baek K (2020) Mobility of arsenic in soil amended with biochar derived from biomass with different lignin contents: relationships between lignin content and dissolved organic matter leaching. Chem Eng J 393:124687. https://doi.org/10.1016/j.cej.2020.124687
Kostas ET, Beneroso D, Robinson JP (2017) The application of microwave heating in bioenergy: a review on the microwave pre-treatment and upgrading technologies for biomass. Renew Sustain Energy Rev 77:12–27. https://doi.org/10.1016/j.rser.2017.03.135
Kumar KV, Preuss K, Lu L, Guo ZX, Titirici MM (2015) Effect of nitrogen doping on the CO2 adsorption behavior in nanoporous carbon structures: a molecular simulation study. J Phys Chem C 119(39):22310–22321. https://doi.org/10.1021/acs.jpcc.5b06017
Li G, Shen B, Li Y, Zhao B, Wang F, He C, Wang Y, Zhang M (2015) Removal of element mercury by medicine residue derived biochars in presence of various gas compositions. J Hazard Mater 298:162–169. https://doi.org/10.1016/j.jhazmat.2015.05.031
Li J, Dai J, Liu G, Zhang H, Gao Z, Fu J, He Y, Huang Y (2016) Biochar from microwave pyrolysis of biomass: a review. Biomass Bioenerg 94:228–244. https://doi.org/10.1016/j.biombioe.2016.09.010
Li J, Liang N, Jin X, Zhou D, Li H, Wu M, Pan B (2017) The role of ash content on bisphenol A sorption to biochars derived from different agricultural wastes. Chemosphere 171:66–73. https://doi.org/10.1016/j.chemosphere.2016.12.041
Li H, Li J, Fan X, Li X, Gao X (2019) Insights into the synergetic effect for co-pyrolysis of oil sands and biomass using microwave irradiation. Fuel 239:219–229. https://doi.org/10.1016/j.fuel.2018.10.139
Liao W, Tang C, Zheng H, Ding J, Zhang K, Wang H, Lu J, Huang W, Zhang Z (2022) Tuning activity and selectivity of CO2 hydrogenation via metal-oxide interfaces over ZnO-supported metal catalysts. J Catal 407:126–140. https://doi.org/10.1016/j.jcat.2022.01.037
Lin J, Sun S, Xu D, Cui C, Ma R, Luo J, Fang L, Li H (2022) Microwave directional pyrolysis and heat transfer mechanisms based on multiphysics field stimulation: design porous biochar structure via controlling hotspots formation. Chem Eng J 429:132195. https://doi.org/10.1016/j.cej.2021.132195
Ma Y, He X, Tang S, Xu S, Qian Y, Zeng L, Tang K (2022) Enhanced 2-D MOFs nanosheets/PIM-PMDA-OH mixed matrix membrane for efficient CO2 separation. J Environ Chem Eng 10(2):107274. https://doi.org/10.1016/j.jece.2022.107274
Mankar JS, Rayalu SS, Balasubramanian R, Krupadam RJ (2021) High performance CO2 capture at elevated temperatures by using cenospheres prepared from solid waste, fly ash. Chemosphere 284:131405. https://doi.org/10.1016/j.chemosphere.2021.131405
Mašek O, Budarin V, Gronnow M, Crombie K, Brownsort P, Fitzpatrick E, Hurst P (2013) Microwave and slow pyrolysis biochar—comparison of physical and functional properties. J Anal Appl Pyrol 100:41–48. https://doi.org/10.1016/j.jaap.2012.11.015
Mushtaq F, Mat R, Ani FN (2014) A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew Sustain Energy Rev 39:555–574. https://doi.org/10.1016/j.rser.2014.07.073
Panwar NL, Pawar A (2022) Influence of activation conditions on the physicochemical properties of activated biochar: a review. Biomass Conversion Biorefinery 12(3):925–947. https://doi.org/10.1007/s13399-020-00870-3
Petrovic B, Gorbounov M, Masoudi Soltani S (2021) Influence of surface modification on selective CO2 adsorption: a technical review on mechanisms and methods. Microporous Mesoporous Mater 312:110751. https://doi.org/10.1016/j.micromeso.2020.110751
Raganati F, Alfe M, Gargiulo V, Chirone R, Ammendola P (2019) Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite-based sorbent. Chem Eng J 372:526–535. https://doi.org/10.1016/j.cej.2019.04.165
Rashidi NA, Yusup S (2016) An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J CO2 Utilization 13:1–16. https://doi.org/10.1016/j.jcou.2015.11.002
Salema AA, Ani FN, Mouris J, Hutcheon R (2017) Microwave dielectric properties of Malaysian palm oil and agricultural industrial biomass and biochar during pyrolysis process. Fuel Process Technol 166:164–173. https://doi.org/10.1016/j.fuproc.2017.06.006
Santos JL, Mäki-Arvela P, Monzón A, Murzin DY, Centeno MÁ (2020) Metal catalysts supported on biochars: part I synthesis and characterization. Appl Catal B 268:118423. https://doi.org/10.1016/j.apcatb.2019.118423
Shafawi AN, Mohamed AR, Lahijani P, Mohammadi M (2021) Recent advances in developing engineered biochar for CO2 capture: an insight into the biochar modification approaches. J Environ Chem Eng 9(6):106869. https://doi.org/10.1016/j.jece.2021.106869
Shirvanimoghaddam K, Czech B, Abdikheibari S, Brodie G, Kończak M, Krzyszczak A, Al-Othman A, Naebe M (2022) Microwave synthesis of biochar for environmental applications. J Anal Appl Pyrol 161:105415. https://doi.org/10.1016/j.jaap.2021.105415
Siddique IJ, Salema AA, Antunes E, Vinu R (2022) Technical challenges in scaling up the microwave technology for biomass processing. Renew Sustain Energy Rev 153:111767. https://doi.org/10.1016/j.rser.2021.111767
Sreedhar I, Vaidhiswaran R, Kamani BM, Venugopal A (2017) Process and engineering trends in membrane based carbon capture. Renew Sustain Energy Rev 68:659–684. https://doi.org/10.1016/j.rser.2016.10.025
Sun Y, Wang T, Sun X, Bai L, Han C, Zhang P (2021) The potential of biochar and lignin-based adsorbents for wastewater treatment: comparison, mechanism, and application—a review. Ind Crops Prod 166:113473. https://doi.org/10.1016/j.indcrop.2021.113473
Torrisi A, Bell RG, Mellot-Draznieks C (2010) Functionalized MOFs for enhanced CO2 capture. Cryst Growth Des 10(7):2839–2841. https://doi.org/10.1021/cg100646e
Tsechansky L, Graber ER (2014) Methodological limitations to determining acidic groups at biochar surfaces via the Boehm titration. Carbon 66:730–733. https://doi.org/10.1016/j.carbon.2013.09.044
Xiang W, Zhang X, Cao C, Quan G, Wang M, Zimmerman AR, Gao B (2022a) Microwave-assisted pyrolysis derived biochar for volatile organic compounds treatment: characteristics and adsorption performance. Biores Technol 355:127274. https://doi.org/10.1016/j.biortech.2022.127274
Xiang W, Zhang X, Luo J, Li Y, Guo T, Gao B (2022b) Performance of lignin impregnated biochar on tetracycline hydrochloride adsorption: governing factors and mechanisms. Environ Res 215:114339. https://doi.org/10.1016/j.envres.2022.114339
Zhang J, Liu J, Liu R (2015) Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Biores Technol 176:288–291. https://doi.org/10.1016/j.biortech.2014.11.011
Zhang X, Gao B, Creamer AE, Cao C, Li Y (2017) Adsorption of VOCs onto engineered carbon materials: a review. J Hazard Mater 338:102–123. https://doi.org/10.1016/j.jhazmat.2017.05.013
Zhang X, Miao X, Xiang W, Zhang J, Cao C, Wang H, Hu X, Gao B (2021) Ball milling biochar with ammonia hydroxide or hydrogen peroxide enhances its adsorption of phenyl volatile organic compounds (VOCs). J Hazard Mater 403:123540. https://doi.org/10.1016/j.jhazmat.2020.123540
Zhang J, Huang D, Shao J, Zhang X, Zhang S, Yang H, Chen H (2022a) A new nitrogen-enriched biochar modified by ZIF-8 grafting and annealing for enhancing CO2 adsorption. Fuel Process Technol 231:107250. https://doi.org/10.1016/j.fuproc.2022.107250
Zhang X, Cao L, Xiang W, Xu Y, Gao B (2022b) Preparation and evaluation of fine-tuned micropore biochar by lignin impregnation for CO2 and VOCs adsorption. Sep Purif Technol 295:121295. https://doi.org/10.1016/j.seppur.2022.121295
Zhang X, Xiang W, Miao X, Li F, Qi G, Cao C, Ma X, Chen S, Zimmerman AR, Gao B (2022c) Microwave biochars produced with activated carbon catalyst: characterization and sorption of volatile organic compounds (VOCs). Sci Total Environ 827:153996. https://doi.org/10.1016/j.scitotenv.2022.153996
Zhang Y, Wang S, Feng D, Gao J, Dong L, Zhao Y, Sun S, Huang Y, Qin Y (2022d) Functional biochar synergistic solid/liquid-phase CO2 capture: a review. Energy Fuels 36(6):2945–2970. https://doi.org/10.1021/acs.energyfuels.1c04372
Zubbri NA, Mohamed AR, Kamiuchi N, Mohammadi M (2020) Enhancement of CO2 adsorption on biochar sorbent modified by metal incorporation. Environ Sci Pollut Res 27(11):11809–11829. https://doi.org/10.1007/s11356-020-07734-3
Acknowledgements
The authors sincerely acknowledge the anonymous reviewers for their insights and comments to further improve the quality of the manuscript.
Funding
The research leading to these results received funding from Natural Science Foundation of Jiangsu Province (BK20201151) and Science and Technology Plan Projects of Xuzhou (KC21288).
Author information
Authors and Affiliations
Contributions
XZ: Conceptualization, Methodology, Formal analysis, Investigation, Writing—review & editing, Project administration, Funding acquisition. HX: Conceptualization, Methodology, Formal analysis, Writing—original draft. WX: Formal analysis, Writing—review & editing, Funding acquisition. XY: Investigation, Validation. HD: Investigation, Validation. BG: Formal analysis, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Handling editor: Lukáš Trakal
Supplementary Information
Additional file 1: Table S1.
Adsorption kinetic parameters of CO2 on biochar at 35–65 oC. Table S2. Multicomponent adsorption experiment on impregnated biochar at 25 oC.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Xu, H., Xiang, W. et al. Lignin-impregnated biochar assisted with microwave irradiation for CO2 capture: adsorption performance and mechanism. Biochar 6, 22 (2024). https://doi.org/10.1007/s42773-024-00310-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-024-00310-9