Abstract
Biochar can change the availability and morphology of soil Cd. However, the influence of biochar on Cd chemical form and subcellular fraction in rice is poorly understood, particularly under different irrigation methods. A pot experiment of biochar application combined with two irrigation methods (continuous flooding and intermittent irrigation, CF and II) was conducted. The Cd accumulation, chemical form and subcellular fraction in rice organs and the associated physiological responses were examined. Biochar significantly reduced soil available Cd (30.85–47.26% and 32.35–52.35%) under CF and II but increased the Cd content (30.4–63.88% and 13.03–18.59%) in brown rice. Additionally, the Cd content in shoots/grains under II was higher than that under CF. Biochar elevated the Cd soluble fraction in roots while lowered the cell wall fraction under both irrigation methods, whereas the opposite result was observed in leaves. Biochar increased water-, ethanol-, and NaCl-extractable Cd in roots meanwhile increased ethanol-extractable Cd in leaves under both irrigation methods. Moreover, the total amount of water-, ethanol-, and NaCl-extractable Cd in rice roots was higher under II than under CF. Related hormones and antioxidant enzymes may also be involved in biochar-mediated Cd accumulation in rice grains. Thus, changes in Cd chemical form and subcellular fraction in the root and leaf are the main mechanisms of biochar-induced rice grains Cd accumulation.
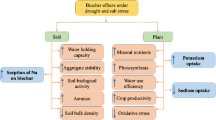
Graphical Abstract

Highlights
-
Biochar decreased alkaline soil available Cd but increased rice Cd accumulation due to increased functional groups in roots
-
Biochar increased soluble fractions and active Cd in rice roots and leaves
-
Hormones, subcellular distribution and chemical forms act together on rice grain Cd accumulation
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cd is a hazardous metal element for living organisms (Riaz et al. 2021). Agricultural soil Cd pollution is ubiquitous and is a serious problem that needs to be addressed worldwide. Nearly 280,000 ha of farmland in China is polluted by Cd (Li et al. 2017). FAO (2017) documented that cereals, especially rice, are the principal source of Cd ingested by people. More than 50% of people worldwide eat rice as their staple food, particularly in China (FAO 2017). Hence, adopting effective strategies to deter rice Cd pollution in rice grains is imperative.
Ameliorant addition and agronomic regulation are the common methods for reducing heavy metal accumulation in crops (Tang et al. 2016). Studies have documented that biochar addition and water management are effective measures for deterring Cd contamination in rice and that a combination of the two is more effective (Rizwan et al. 2018). Biochar decreases soil Cd bioavailability, reducing rice uptake (Chen et al. 2020a, 2022). A similar effect also occurs in other plants (Nigam et al. 2019). The inhibition of Cd uptake in rice by biochar is mainly attributed to the increase in soil pH and the transformation of available Cd into stable complex states (Sun et al. 2023). Many studies have shown that the fixation effect of biochar on Cd is closely related to the type and properties of biochar (Yin et al. 2017; Li et al. 2019a), and is affected by soil properties (Yu et al. 2021c). Flooded environment induces the formation of CdS and iron plaque, which further prevents soil Cd uptake by roots (Yan et al. 2021; Zhang et al. 2019a). However, previous studies on the mechanism of decreased Cd accumulation in rice grains have mainly focused on the soil level, and the underlying physiological mechanism of biochar regulating the Cd transport from roots to shoot sand grains is unclear.
Cd accumulation in grains mainly occurs through four physiological stages: (i) root uptake the soil Cd; (ii) Cd in rice root cells enters the xylem and is transported from roots to shoots; (iii) Cd transport to different tissues and organs is achieved at the nodes; and (iv) Cd accumulated in leaves and other organs is reactivated and transferred to grains through the phloem (Uraguchi and Fujiwara 2012). In particular, the direct translocation of Cd from roots to grains through the xylem is key to grain Cd accumulation (Uraguchi et al. 2009). Studies have suggested that Cd-rich rice varieties are caused by chemical forms and subcellular distribution (Yu et al. 2021a). Xin et al. (2017) showed that soluble fractions in cells and Cd extracted from water, ethanol, and NaCl had strong plant mobility. Additionally, Chen et al. (2020b) observed that biochar altered the Cd chemical forms in vegetables and varied by species. Therefore, we hypothesised that biochar regulates Cd accumulation in rice grains by mediating changes in the chemical form and subcellular fraction of Cd. Moreover, water management may also affect the role of biochar.
Phytohormones and antioxidant enzymes are also directly or indirectly involved in the metal translocation in plants (Ahmad et al. 2017; Wang et al. 2021). Previous studies have reported that biochar induces corresponding changes in certain hormone level and antioxidant enzyme activity in some crops (Kamran et al. 2019; Farhangi-Abriz and Torabian 2018). Thus, biochar was added to the Cd-contaminated soil where rice was grown, and different water management practices were performed to (a) investigate whether biochar alters the Cd chemical form and subcellular fraction in rice and whether the effects are consistent under different irrigation methods; (b) examine the role of Cd chemical form and subcellular fraction in leaf, stem, and root on rice grain Cd accumulation, and (c) elucidate the effect of biochar on rice physiological traits under different irrigation methods and its relationship with grain Cd accumulation. This study contributes to elucidating the physiological mechanism by which biochar regulates the transport of Cd from rice roots to shoots and grains.
2 Materials and methods
2.1 Soil and biochar properties
The soil was obtained from a rice field on the campus of Yangzhou University, Jiangsu Province, China. The soil was air-dried and sieved for chemical analysis. Soil pH was measured with the pH-water (1:5 w/v) method using a pH meter (Li et al. 2019b). Soil total nitrogen was determined using the Kjeldahl Nitrogen Method. The detailed determination methods of soil organic matter, cation exchange capacity, total nitrogen, available phosphorus, and available potassium can be traced to our previous research (Chen et al. 2020c). The metal elements in the soil (or biochar) were digested by microwave digester, and the concentration was determined by ICP-MS (Chen et al. 2020a). The basic properties of the tested soils are exhibited in Table 1. The slow pyrolysis of wheat straw produced biochar at 550 °C for 2 h, with a heating rate of 10 °C min–1, and its physicochemical properties are exhibited in Table 1. The pH of biochar was measured with the pH-water (1:20 w/v) method using a pH meter. The ash content and volatile matter of biochar were determined according to the method described by Lapczynska-Kordon et al. (2022). The available phosphorus and potassium in biochar were determined in detail as described by Chen et al. (2020c). The elemental composition of biochar was determined using an elemental analyzer. The SEM and FTIR spectra of biochar are shown in Additional file 1: Fig. S1. The soil properties after rice harvest are presented in Additional file 1: Table S1 and Fig. S3.
2.2 Experiment treatment
The experiment was conducted on the experimental field of Yangzhou University, China (32.39′N, 119.42′E). According to the soil environmental quality standard (GB 15618-2018), the critical value of soil total Cd is 0.8 mg kg−1 when soil pH > 7.5 in farmland, exceeding this value, there may be risks to the quality and safety of agricultural products, crop growth and soil ecological environment. In addition, according to previous studies (Zhang et al. 2019b), 2.5 mg kg−1 of Cd was added to soil in this experiment. Eight kg of air-dried soil was weighed per bucket (27 cm height, 28 cm diameter), and CdCl2 was added to give a final concentration of 2.75 mg kg−1 for soil Cd. Each bucket of soil was equilibrated by water immersion for half a month. The trial was performed using a two-factor randomised block design. A combination of three biochar addition amounts (0, 2.5%, and 5.0%; recorded as CK, BC2.5, and BC5.0), and two water management (continuous flooding and intermittent irrigation; recorded as CF and II). There were six treatments, with 15 buckets per treatment. Continuous flooding means maintaining a 20–30 mm water layer after rice transplanting until the tillering stage and a 40–50 mm water layer until the maturity stage. Intermittent irrigation maintains a 20–30 mm water layer after transplanting until greening, then implements 30 mm–0 (for 1 d)–30 mm irrigation until the tillering stage, and then implements 50 mm–0 (for 1 d)–50 mm irrigation until the maturity stage. The tested rice variety was Yangxianyou 918, with a growth period of 139 days. Each bucket was transplanted with six seedlings divided equally into three holes. A total of 3.59 g urea per pot was split and applied as base, tillering, and panicle fertilizer (4:2:4). Exactly 7.5 g of calcium magnesium phosphate fertilizer was used per pot as a base fertilizer. Exactly 3.0 g potassium chloride was applied per pot, split into base fertilizer and panicle fertilizer (5:5). Diseases, and pests were managed following high field specifications.
2.3 Sampling
Fresh rice plant samples were collected during the filling stage and separated into leaves, stems, and roots. All samples were snap-frozen in liquid nitrogen and stored at − 80 °C to determine chemical form and subcellular fractions. In addition, only flag leaves were used for the endogenous hormone and enzyme assays. Soil and plant samples were investigated at maturity. The soil was air-dried and sieved to measure the available Cd content. The plants were washed with deionised water, separated into grains, leaves, stems, and roots, and dried to determine Cd content. All samples were analysed in triplicate.
2.4 Available Cd and tissue Cd measurement
The available Cd was extracted using the DTPA reagent (Lindsay and Norvell 1978), and the plant Cd was dissolved in HNO3 and H2O2 (4:1, v:v; 10 mL; Microwave apparatus: MASTER 40, SINEO). The ICP-MS (iCAP Q, Thermo Fisher SCIENTIFIC) was adopted for Cd concentration measurement. Our previous article describes the specific method (Chen et al. 2022).
2.5 Subcellular distribution
Subcellular fractions of rice leaves, stems, and roots were isolated by differential centrifugation to obtain soluble fractions (mainly cytoplasm and vacuoles), organelles, and cell wall. The specific separation process was referred to the procedures described by Xin et al. (2017). All fractions were heated, evaporated to near dryness, then digested by adding 68% HNO3 and 30% H2O2 (4:1, v:v; 10 mL) in a microwave digestion apparatus. Finally, Cd concentration was analysed using ICP-MS.
2.6 Chemical form extraction
The Cd chemical forms were gradually extracted using chemical reagents with different polarities. The detailed procedures have been described by Xin et al. (2017). The five reagents were added in sequence: (a) 80% ethanol, extraction of inorganic Cd with strong mobility (F-ethanol); (b) deionised water, extraction of water-soluble Cd (F-water); (c) 1.0 mol L−1 NaCl, extraction of protein and pectate-binding Cd (F-NaCl); (d) 2% acetic acid, extraction of phosphate-Cd complexes (F-HAC); (e) 0.6 mol L−1 HCl, extraction of oxalate-binding Cd (F-HCl); and (f) residual Cd (F-residual). The six extracts were heated, evaporated to dryness, and then digested with 30% H2O2 and 68% HNO3. The Cd concentration was analysed using an atomic absorption spectrometer.
2.7 Jasmonic acid (JA), salicylic acid (SA), and abscisic acid (ABA) assays
Extraction and pretreatment of ABA, JA and SA were performed based on the protocol of Pérez-Jiménez et al. (2014). The hormone content was assayed by HPLC (Waters2695, USA).
2.8 Malondialdehyde (MDA), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) assays
CAT, POD, and SOD assays were conduced as recorded by Liu et al. (2022). The enzyme activity was measured using a corresponding test kit (Suzhou Mengxi Biomedical Technology Co., LTD, China). The MDA level was analysed by thiobarbituric acid colorimetric method, with detailed steps described by Guo et al. (2019).
2.9 Functional group analysis
The functional groups in the rice root, stem, and leaf were analysed using Fourier transform infrared spectroscopy (Thermo Scientific Nicolet iS20, America).
2.10 Data analysis
SPSS17.0 (IBM) and Excel 2010 (Microsoft) were used to process the data and draw graphs. A plot of the standardized residual was used for checking the normality assumption. Where necessary, the data were log10-transformed to meet the homogeneity of variance and normality assumptions of the ANOVAs. We analyzed soil and plant relevant indicators using two-way analyses of variance. The LSD-Test was employed to compare the differences between treatments at a probability of 0.05.
3 Results
3.1 Rice Cd concentration
Biochar observably elevated Cd concentration in all rice tissues under both CF and II irrigation conditions, and its promotion effect was stronger in the CF treatment (Fig. 1). Compared to CK, Cd in brown rice treated with BC2.5 and BC5.0 increased by 63.88% and 30.4%, 149.82% and 165.2% in leaves, 106.87% and 113.19% in stems, and 77.41% and 104.94% in roots under CF, respectively. Similarly, it increased by 18.59% and 13.03% in brown rice, 44.13% and 102.01% in leaves, 38.31% and 33.15% in stems, and 48.55% and 44.09% in roots under II, respectively. In addition, the Cd concentration in tissues was higher under II relative to under CF, regardless of whether biochar was applied, and was more pronounced in rice roots and grains.
3.2 Soil available Cd
Biochar decreased the available Cd content under different irrigation conditions, especially at high biochar dosages (Fig. 2). The available Cd content in the BC5.0 treatment under CF and II decreased by 47.26% and 52.35% relative to CK, respectively. In addition, this study observed that the soil available Cd content was higher under CF than under II.
Biochar induced changes of soil available Cd under different water management. CF, continuous flooding; II, intermittent irrigation. CK, BC2.5 and BC5.0 refer to biochar dosage of 0, 2.5% and 5.0%, respectively. Error bars are ± SD, n = 3. The different lowercase letters indicate significant difference between treatments (p < 0.05)
3.3 Cd subcellular distribution
Nearly 70% of Cd in rice roots was integrated with the cell wall (Fig. 3). Biochar increased the Cd-soluble fraction in roots while decreasing the organelle and cell wall fractions under both irrigation conditions. Compared to CK, the Cd-soluble fraction in roots induced by biochar under CF and II increased by 9.11–36.39% and 49.9–63.74%, respectively. Under CF, biochar decreased the Cd-soluble fraction in the stem by 14.76–17.36% but increased the cell wall fraction by 18.07–23.39% and the organelle fraction by 61.94–77.61%. On the contrary, biochar under II increased the Cd-soluble fraction in the stem by 13.58–14.99%, whereas the organelle and cell wall fractions decreased. In the CF and II treatments, biochar reduced the Cd-soluble fraction in the leaf by 15.14–21.35% and 14.26–19.73%, whereas the Cd cell wall fraction increased by 29.91–41.84% and 5.11–7.23%, respectively. The biochar decreased its distribution under CF for the organelle fraction, while it elevated under II. Additionally, the Cd-soluble fraction in stems and leaves was significantly higher under CF than under II, whereas the opposite was observed for the cell wall fraction.
3.4 Cd chemical forms
The high proportion of Cd extracted from NaCl and acetic acid in rice roots indicated that Cd existed mainly in phosphate, pectate, and protein-bound forms (Fig. 4). As a result of biochar application, the ethanol-extractable (212.6–239.0% and 105.5–170.7%), water-extractable (46.6–54.0% and 41.1–102.2%), and NaCl-extractable (25.8–34.9% and 36.4–51.9%) Cd increased in roots under CF and II. In contrast, the acetic acid - extractable, HCl-extractable, and residual Cd decreased. This indicates that biochar promoted the transformation of the phosphate-bound, oxalate-bound, and residual-state Cd to a form with strong mobility in rice roots.
In rice stems, Cd in water-extractable, acetic acid-extractable, and ethanol-extractable forms accounted for a higher proportion (Fig. 4). The ethanol-extractable Cd in rice stems under CF was remarkably higher than that under II. In contrast, the opposite was observed for NaCl-extractable and HCl-extractable Cd. Biochar decreased the ethanol-extractable (34.6–35.77% and 1.98–12.66%) and water-extractable (6.65–16.96% and 23.03–27.53%) forms of Cd in stems under both CF and II but increased the Cd in residual, HCl-extractable, acetic acid-extractable, and NaCl-extractable forms. This indicated that biochar promoted the transformation of water-soluble and inorganic Cd to forms with weak mobility in the stem, whereas water management affected the transformation degree.
In rice leaves, the proportion of HCl-extractable Cd was the largest, followed by ethanol-extractable Cd (Fig. 4). Under CF and II, biochar increased the ethanol-extractable Cd (38.01–45.54% and 25.43–84.22%) in leaves, but decreased the water-extractable Cd (18.62–32.46% and 5.56–30.27%) and acetic acid-extractable Cd (46.34–46.99% and 34.2–38.45%). For the NaCl-extractable and residual Cd, the biochar content decreased under the CF condition but increased under the II condition. For HCl-extractable Cd, biochar increased its content under CF but decreased under II.
3.5 Hormone changes in rice leaves
Different types of endogenous hormones in rice leaves showed inconsistent responses to biochar and were regulated by water management (Fig. 5). Under CF, biochar increased the contents of SA (28.33–122.2%) and JA (1.39–28.17%) in rice leaves, but decreased the content of ABA (37.9–57.17%). In contrast, under II, biochar application decreased SA (17.58–29.0%) and JA (22.01–47.14%) levels, whereas ABA increased by 13.99–35.15%.
Biochar induced hormone changes in rice leaves under different water management. CF, continuous flooding; II, intermittent irrigation. CK, BC2.5 and BC5.0 refer to biochar dosage of 0, 2.5% and 5.0%, respectively. Error bars are ± SD, n = 3. The different lowercase letters indicate significant difference between treatments (p < 0.05)
3.6 MDA and antioxidant enzyme activity in rice leaves
Water management determines whether biochar has a negative or positive influence on antioxidant enzyme activity and MDA content (Fig. 6). Under CF, the activities of SOD (2.2–14.43%) and POD (35.94–57.29%) and the content of MDA (22.96–23.51%) in leaves were increased by biochar. However, biochar markedly decreased the activity of these two enzymes and the MDA content under II. For CAT, biochar decreased its activity under CF and II by 34.08–52.87% and 28.52–54.46%, respectively.
Biochar induced changes of MDA and antioxidant enzyme activity in rice leaves under different water management. CF, continuous flooding; II, intermittent irrigation. CK, BC2.5 and BC5.0 refer to biochar dosage of 0, 2.5% and 5.0%, respectively. Error bars are ± SD, n = 3. The different lowercase letters indicate significant difference between treatments (p < 0.05)
3.7 Biochar-induced changes of functional groups in rice roots, stems and leaves
FTIR spectra showed abundant functional groups in rice roots, stems, and leaves, especially oxygen-containing functional groups such as carboxyl, hydroxyl, Si–O, Fe–O, Mn–O, P–O, and aromatic C–O (Fig. 7). Compared with CK, biochar observably increased the hydroxyl, carboxyl, P–O, and aromatic C–O contents in rice roots under both water management conditions. In contrast, biochar treatment resulted in a decrease in these functional groups in rice stems. Biochar decreased oxygen-containing functional groups in rice leaves under CF and induced an increase under II.
3.8 Correlation analysis of hormones, antioxidant enzymes with Cd chemical forms and subcellular fractions
Correlation analysis showed that water management could influence the positive and negative effects of hormones on chemical forms and subcellular fractions (Table 2). Under CF, ABA was significantly negatively correlated with the Cd cell wall fraction and positively correlated with the soluble fraction but inversely (though not significantly) correlated under II. Under CF, JA/ABA was significantly positively correlated with cell wall fraction and HCl-extractable Cd, whereas significantly negatively correlated with the soluble fraction. A significant negative correlation existed between MDA and organelle fraction, acetic acid-extractable Cd, and NaCl-extractable Cd. Additionally, POD was negatively correlated with water-extractable Cd. CAT was significantly negatively correlated with the cell wall fraction but positively correlated with the soluble fraction. Under II, ABA and SA were significantly negatively correlated with HCl-extractable and residual Cd, respectively. The SA/ABA ratio was significantly positively correlated with acetic acid-extractable Cd. SOD was significantly negatively correlated with organelle fraction. In addition, MDA was significantly correlated with ethanol-extractable (negative) and water-soluble Cd (positive).
3.9 Factors associated with Cd accumulation in brown rice
Principal component analysis (PCA) was performed on soil and rice plant characteristics (Fig. 8). The cumulative contribution rate of PC1 and PC2 was 71.5%. Compared with treatments without biochar, the mapping value of PC1 axis was larger in the biochar treatments (Fig. 8), indicating that biochar promoted Cd accumulation in rice under both irrigation methods (Fig. 1; Additional file 1: Fig. S4a). Similarly, the mapping value of the II treatment on the PC1 axis was larger than that of the CF treatment at the same biochar level (Fig. 8). Cd accumulation in brown rice was positively correlated with MDA, SA, active Cd (soluble fraction, ethanol extractable, water extractable and NaCl extractable) in roots, NaCl extractable Cd in stems and ethanol extractable Cd in leaves.
Principal component analysis (PCA) of rice Cd accumulation, subcellular distribution, chemical forms, physiological characteristics, and soil properties under different treatments. CF, continuous flooding; II, intermittent irrigation. CK, BC2.5 and BC5.0 refer to biochar dosage of 0, 2.5% and 5.0%, respectively. R, root; S, stem; L, leaf. Fa, soluble fraction; Fb, non-soluble fraction. F1, ethanol-extractable Cd; F2, water-extractable Cd; F3, NaCl-extractable Cd; F4, stable bound Cd
4 Discussion
4.1 Biochar decreased soil available Cd but increased rice accumulation
Many literatures have recorded that biochar reduces rice Cd accumulation because of reduced soil available Cd content (Chen et al. 2022; Liu et al. 2021). Biochar significantly reduced the soil available Cd, meanwhile increased the rice Cd accumulation (Figs. 1 and 2). Similar findings were showed by Li et al. (2019b), who suggested that the high chloride ion content in biochar might cause the increase in rice Cd accumulation. The higher Cd accumulation in rice under intermittent irrigation than under continuous flooding observed in this study is consistent with previous results (Chen et al. 2022). However, the available Cd under II was significantly lower than that under CF, which differs from the previously recorded results. This may be because they occur in alkaline soils (Table 1). In addition, Liu et al. (2017) showed that root functional groups play a significant role in Cd absorption by roots. We found that biochar increased various oxygen-containing functional groups (–OH and –COOH in particular) in rice roots (Fig. 7), which might be why biochar caused Cd accumulation in roots. The fixation mechanism of biochar and water management on soil Cd was discussed in detail in previous study (Chen et al. 2022). Therefore, the availability of soil Cd cannot explain the cause of Cd accumulation in grains.
4.2 Biochar increased Cd accumulation in rice grains via altering chemical forms and subcellular fractions
However, the influences of biochar on the Cd chemical form and subcellular fraction in rice have rarely been reported. Subcellular distribution and chemical form are important factors affecting Cd migration in plants, which has been confirmed in some crops, such as watercress (Wang et al. 2015), peanut (Shi et al. 2017) and rice (Yu et al. 2021a).
Vacuolar sequestration and cell wall retention are pivotal mechanisms of Cd detoxification in plant cells (Wei et al. 2021). Indeed, we observed that Cd was mainly distributed in the cell wall and soluble fractions of roots, stems, and leaves, especially in rice roots, where the cell wall fractions accounted for 70% (Fig. 3). In addition, we found that both biochar and water management affected the subcellular fractions, with large differences among the organs. For example, biochar decreased the Cd cell wall fraction while increased the soluble fraction in roots, whereas the opposite was observed in the leaf (Fig. 3). Water management also had a greater influence on subcellular distribution in stems and leaves than in the root. Liu et al. (2014) reported that the soluble fraction was a pivotal source of Cd accumulation in rice grains. Therefore, our results indicate that biochar-induced Cd accumulation in rice grains was mainly derived from the soluble fraction in the roots. Likewise, Lei et al. (2022) found that biochar addition increased the Cd-soluble fraction in rape roots. Biochar and water management alter subcellular distribution, probably by affecting cell wall composition and organic ligand formation in the vacuoles. Plant vacuoles contain glutathione and phytochelin, which can form complexes with Cd (Huang et al. 2021). The cell wall is mainly composed of pectin, cellulose, and hemicellulose, among which pectin and hemicellulose contain abundant negatively charged functional groups, a key mechanism of Cd retention (Yu et al. 2021b).
Additionally, the Cd translocation from roots to shoots also depends on its chemical form (Xin et al. 2017). In the present research, a large percentage of acetic acid- and NaCl-extractable Cd was observed in rice roots (Fig. 4), implying that Cd was mainly combined with pectate and protein as well as formed insoluble phosphates. This phenomenon is similar to that of Xin et al. (2017). However, it was primarily present in oxalate and active inorganic Cd in leaves (Fig. 4). Both biochar and irrigation induced changes in the chemical form of Cd in rice organs, probably by altering the amount of the corresponding ligand in the cells. Studies have shown that Cd extracted from ethanol, water, and NaCl has a strong migration ability in plants, especially the former two (Li et al. 2014). Therefore, the increase in rice grain Cd content caused by biochar was because of the significant increase in water-, ethanol-, and NaCl-extractable Cd in roots as well as ethanol-extractable Cd in leaves (Fig. 4). Similarly, the higher brown rice Cd content under intermittent irrigation was mainly attributed to the active forms of Cd in the rice roots (Fig. 4).
4.3 Synergistic effects of hormones and antioxidant enzymes with chemical forms and subcellular distribution on Cd accumulation in rice grains
Correlation analysis showed that endogenous hormone and antioxidant enzyme activities were significantly correlated with Cd chemical form and subcellular distribution (Table 2). A close association between hormones, antioxidant enzymes, and plant Cd accumulation has been reported (Guo et al. 2019; Singh et al. 2019; Wang et al. 2021). Studies have revealed that SA regulates cell wall polysaccharide synthesis through nitrous oxide signalling, such as the synthesis of pectin and lignin to fix Cd (Pan et al. 2021). Guo et al. (2007) indicated that SA mainly mediates the binding of non-protein thiols to Cd in rice roots. Additionally, SA and ABA mediate Cd translocation in plants by regulating the expression of genes involved in Cd transport (Fan et al. 2014; Wang et al. 2021). This suggests that biochar may mediate polysaccharide synthesis and ligand formation by altering the contents of JA, SA, and ABA, thus affecting the Cd chemical form and subcellular fraction. Furthermore, our results suggested that two or more hormones are synergistically involved in the Cd chemical morphogenesis and subcellular partitioning (Table 2). Therefore, the main mechanism of biochar-mediated Cd accumulation in rice grains involves changes in subcellular distribution, chemical forms, endogenous hormones, and antioxidant enzyme activities.
5 Conclusion
Biochar significantly reduced soil-available Cd content under continuous and intermittent irrigation but significantly increased brown rice Cd content. Both biochar and water management changed the Cd chemical form and subcellular fraction in various rice organs, and interactive effects were observed. Biochar-induced Cd accumulation in rice grains was attributed to the increase in soluble fractions and water-, ethanol-, and NaCl-extractable Cd in roots and the increase in ethanol-extractable Cd in leaves, as well as the increase in NaCl-extractable Cd in stem. The Cd accumulation in shoots and grains under intermittent irrigation was higher than that under continuous flooding, possibly because the total amount of water-, ethanol-, and NaCl-extracted Cd was higher in rice roots under intermittent irrigation. In addition, changes in related hormone and enzyme activities induced by biochar might play a synergistic role in Cd accumulation in rice grains. Therefore, the Cd chemical form and subcellular fraction in rice roots and leaves, and their corresponding physiological responses are the main mechanisms of biochar-induced Cd accumulation in rice grains. This study revealed from physiological and biochemical perspectives the reason why biochar immobilizes soil Cd but still promotes rice accumulation. Thus, biochar remediation of heavy metal contaminated soil requires crops to evaluate its effect, rather than simply soil status.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon request.
References
Ahmad P, Alyemeni MN, Wijaya L, Alam P, Ahanger MA, Alamri SA (2017) Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch Agron Soil Sci 63:1889–1899. https://doi.org/10.1080/03650340.2017.1313406
Chen L, Guo L, Zhou Q, Liu M, Zhan S, Pan X, Zeng Y (2020a) Response of soil fertility and Cu and Cd availability to biochar application on paddy soils with different acidification levels. Biomass Convers Bior 12:1493–1502. https://doi.org/10.1007/s13399-020-00917-5
Chen K, Pai C, Lai H (2020b) Amendment of husk biochar on accumulation and chemical form of cadmium in Lettuce and Pak-Choi grown in contaminated soil. Water 12:868. https://doi.org/10.3390/w12030868
Chen L, Liu M, Ali A, Zhou Q, Zhan S, Chen Y, Pan X, Zeng Y (2020c) Effects of biochar on paddy soil fertility under different water management modes. J Soil Sci Plant Nut 20:1010–1818. https://doi.org/10.1007/s42729-020-00252-8
Chen L, Guo L, Liao P, Xiong Q, Deng X, Gao H, Wei H, Dai Q, Pan X, Zeng Y, Zhang H (2022) Rice straw biochar reduces Cd accumulation and promotes Cu accumulation in rice: irrigation regime is the driving factor. J Soil Sediment 23:193–205. https://doi.org/10.1007/s11368-022-03332-7
Fan SK, Fang XZ, Guan MY, Ye YQ, Lin XY, Du ST, Jin CW (2014) Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front Plant Sci 5:721. https://doi.org/10.3389/fpls.2014.00721
Farhangi-Abriz S, Torabian S (2018) Biochar increased plant growth-promoting hormones and helped to alleviates salt stress in common bean seedlings. J Plant Growth Regul 37:591–601. https://doi.org/10.1007/s00344-017-9756-9
Food and Agriculture Organization of the United Nations (FAO) (2017). http://www.fao.org/3/I8317EN/I8317EN.pdf
Guo B, Liang Y, Li Z, Guo W (2007) Role of salicylic acid in alleviating cadmium toxicity in rice roots. J Plant Nutr 30:427–439. https://doi.org/10.1080/01904160601171835
Guo J, Qin S, Rengel Z, Gao W, Nie Z, Liu H, Li C, Zhao P (2019) Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotox Environ Safe 172:380–387. https://doi.org/10.1016/j.ecoenv.2019.01.069
Huang H, Li M, Rizwan M, Dai Z, Yuan Y, Hossain MM, Cao M, Xiong S, Tu S (2021) Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J Hazard Mater 401:123393. https://doi.org/10.1016/j.jhazmat.2020.123393
Kamran M, Malik Z, Parveen A, Zong Y, Abbasi GH, Rafiq MT, Shaaban M, Mustafa A, Bashir S, Rafay M, Mehmood S, Ali M (2019) Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J Environ Manage 250:109500. https://doi.org/10.1016/j.jenvman.2019.109500
Lapczynska-Kordon B, Slipek Z, Slomka-Polonis K, Styks J, Hebda T, Francik S (2022) Physicochemical properties of biochar produced from goldenrod plants. Materials 15:2615–2615. https://doi.org/10.3390/ma15072615
Lei M, Li Z, Zhang B, Wang X, Tie B, Ayaz T, Lu X (2022) Mechanisms of stress alleviation after lime and biochar applications for Brassica napus L. in cadmium-contaminated soil. Adsorpt Sci Technol 2022:4195119. https://doi.org/10.1155/2022/4195119
Li C, Dang F, Cang L, Zhou C, Zhou D (2014) Integration of metal chemical forms and subcellular partitioning to understand metal toxicity in two lettuce (Lactuca sativa L.) cultivars. Plant Soil 384:201–212. https://doi.org/10.1007/s11104-014-2194-6
Li H, Luo N, Li Y, Cai Q, Li H, Mo C, Wong MH (2017) Cadmium in rice: transport mechanisms, influencing factors, and minimizing measures. Environ Pollut 224:622–630. https://doi.org/10.1016/j.envpol.2017.01.087
Li H, Li Z, Khaliq MA, Xie T, Chen Y, Wang G (2019a) Chlorine weaken the immobilization of Cd in soil-rice systems by biochar. Chemosphere 235:1172–1179. https://doi.org/10.1016/j.chemosphere.2019.06.203
Li H, Yu Y, Chen Y, Li Y, Wang M, Wang G (2019b) Biochar reduced soil extractable Cd but increased its accumulation in rice (Oryza sativa L.) cultivated on contaminated soils. J Soil Sediment 19:862–871. https://doi.org/10.1007/s11368-018-2072-6
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Liu J, Qu P, Zhang W, Dong Y, Li L, Wang M (2014) Variations among rice cultivars in subcellular distribution of Cd: the relationship between translocation and grain accumulation. Environ Exp Bot 107:25–31. https://doi.org/10.1016/j.envexpbot.2014.05.004
Liu ZD, Zhou Q, Hong ZN, Xu RK (2017) Effects of surface charge and functional groups on the adsorption and binding forms of Cu and Cd on roots of Indica and Japonica rice cultivars. Front Plant Sci 8:1489. https://doi.org/10.3389/fpls.2017.01489
Liu Y, Luo H, Tie B, Li D, Liu S, Lei M (2021) The long-term effectiveness of ferromanganese biochar in soil Cd stabilization and reduction of Cd bioaccumulation in rice. Biochar 3:499–509. https://doi.org/10.1007/s42773-021-00113-2
Liu X, Wei Z, Hou J, Wan H, Zhang Q, Ma Y, Liu F (2022) Partial root-zone drying irrigation improves growth and physiology of tobacco amended with biochar by modulating phytohormonal profle and antioxidant system. Plant Soil 474:561–579. https://doi.org/10.1007/s11104-022-05359-8
Nigam N, Khare P, Yadav V, Mishra D, Jain S, Karak T, Panja S, Tandon S (2019) Biochar-mediated sequestration of Pb and Cd leads to enhanced productivity in Mentha arvensis. Ecotox Environ Safe 172:411–422. https://doi.org/10.1016/j.ecoenv.2019.02.006
Pan J, Guan M, Xu P, Chen M, Cao Z (2021) Salicylic acid reduces cadmium (Cd) accumulation in rice (Oryza sativa L.) by regulating root cell wall composition via nitric oxide signaling. Sci Total Environ 797:149202. https://doi.org/10.1016/j.scitotenv.2021.149202
Pérez-Jiménez M, Cantero-Navarro E, Pérez-Alfocea F, Cos-Terrer J (2014) Endogenous hormones response to cytokinins with regard to organogenesis in explants of peach (Prunus persica L. Batsch) cultivars and rootstocks (P. persica × Prunus dulcis). Plant Physiol Bioch 84:197–202. https://doi.org/10.1016/j.plaphy.2014.09.014
Riaz M, Kamran M, Rizwan M, Ali S, Parveen A, Malik Z, Wang X (2021) Cadmium uptake and translocation: selenium and silicon roles in Cd detoxification for the production of low Cd crops: a critical review. Chemosphere 273:129690. https://doi.org/10.1016/j.chemosphere.2021.129690
Rizwan M, Ali S, Abbas T, Adrees M, Zia-Ur-Rehman M, Ibrahim M, Abbas F, Qayyum F, Nawaz R (2018) Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa, L.) under Cd stress with different water conditions. J Environ Manage 206:676–683. https://doi.org/10.1016/j.jenvman.2017.10.035
Shi G, Zhang Z, Liu C (2017) Silicon influences cadmium translocation by altering subcellular distribution and chemical forms of cadmium in peanut roots. Arch Agron Soil Sci 63:117–123. https://doi.org/10.1080/03650340.2016.1189075
Singh S, Singh VP, Prasad SM, Sharma S, Ramawat N, Dubey NK, Tripathi DK, Chauhan DK (2019) Interactive efect of silicon (Si) and salicylic acid (SA) in maize seedlings and their mechanisms of cadmium (Cd) toxicity alleviation. J Plant Growth Regul 38:1587–1597. https://doi.org/10.1007/s00344-019-09958-1
Sun X, Wang J, Zhang M, Liu Z, Yang E, Lan Y, He T, Meng J (2023) Effects of biochar on the Cd uptake by rice and the Cd fractions in paddy soil: a 3-year field experiment. Agronomy 13:1335. https://doi.org/10.3390/agronomy13051335
Tang X, Li Q, Wu M, Lin L, Scholz M (2016) Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J Environ Manage 181:646–662. https://doi.org/10.1016/j.jenvman.2016.08.043
Uraguchi S, Fujiwara T (2012) Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice 5:5. https://doi.org/10.1186/1939-8433-5-5
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688. https://doi.org/10.1093/jxb/erp119
Wang J, Su L, Yang J, Yuan J, Yin A, Qiu Q, Zhang K, Yang Z (2015) Comparisons of cadmium subcellular distribution and chemical forms between low-Cd and high-Cd accumulation genotypes of watercress (Nasturtium officinale L. R. Br.). Plant Soil 396:325–337. https://doi.org/10.1007/s11104-015-2580-8
Wang F, Tan H, Zhang Y, Huang L, Bao H, Ding Y, Chen Z, Zhu C (2021) Salicylic acid application alleviates cadmium accumulation in brown rice by modulating its shoot to grain translocation in rice. Chemosphere 263:128034. https://doi.org/10.1016/j.chemosphere.2020.128034
Wei W, Peng H, Xie Y, Wang X, Huang R, Chen H, Ji X (2021) The role of silicon in cadmium alleviation by rice root cell wall retention and vacuole compartmentalization under different durations of Cd exposure. Ecotox Environ Safe 226:1128110. https://doi.org/10.1016/j.ecoenv.2021.112810
Xin J, Zhao X, Tan Q, Sun X, Hu C (2017) Comparison of cadmium absorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L.). Ecotox Environ Safe 145:258–265. https://doi.org/10.1016/j.ecoenv.2017.07.042
Yan J, Fischel M, Chen H, Siebecker MG, Wang P, Zhao F, Sparks DL (2021) Cadmium speciation and release kinetics in a paddy soil as affected by soil amendments and flooding-draining cycle. Environ Pollut 268:115944. https://doi.org/10.1016/j.envpol.2020.115944
Yin D, Wang X, Peng B, Tan C, Ma LQ (2017) Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 186:928–937. https://doi.org/10.1016/j.chemosphere.2017.07.126
Yu H, Wang K, Huang H, Zhang X, Li T (2021a) The regulatory role of root in cadmium accumulation in a high cadmium-accumulating rice line (Oryza sativa L.). Environ Sci Pollut R 28:25432–25441. https://doi.org/10.1007/s11356-021-12373-3
Yu H, Yang A, Wang K, Li Q, Ye D, Huang H, Zhang X, Wang Y, Zheng Z, Li T (2021b) The role of polysaccharides functional groups in cadmium binding in root cell wall of a cadmium-safe rice line. Ecotox Environ Safe 226:112818. https://doi.org/10.1016/j.ecoenv.2021.112818
Yu X, Zhou H, Ye X, Wang H (2021c) From hazardous agriculture waste to hazardous metal scavenger: tobacco stalk biochar-mediated sequestration of Cd leads to enhanced tobacco productivity. J Hazard Mater 413:125303. https://doi.org/10.1016/j.jhazmat.2021.125303
Zhang Q, Chen H, Huang D, Xu C, Zhu H, Zhu Q (2019a) Water managements limit heavy metal accumulation in rice: dual effects of iron-plaque formation and microbial communities. Sci Total Environ 687:790–799. https://doi.org/10.1016/j.scitotenv.2019.06.044
Zhang W, Long J, Li J, Zhang M, Xiao G, Ye X, Chang W, Zeng H (2019b) Impact of ZnO nanoparticles on Cd toxicity and bioaccumulation in rice (Oryza sativa L.). Environ Sci Pollut Res 26:23119–23128. https://doi.org/10.1007/s11356-019-05551-x
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by the National Natural Science Foundation of China (32201889); the Jiangsu Funding Program for Excellent Postdoctoral Talent (2022ZB627); the Jiangsu Technical System of Rice Industry (JATS[2021]485); and the Special Funds of the Rice Industry System of Jiangxi Province (JXARS-02-03).
Author information
Authors and Affiliations
Contributions
LC: Conceptualization, Investigation, Data curation, Funding acquisition, Writing—original draft. LG: Formal analysis, Writing—review & editing. Q X: Investigation. PL: Investigation. XD: Resources. XP: Writing—review & editing. XT: Methodology. XX: Writing—review & editing. QD: Writing—review & editing. HG: Methodology. HW: Data curation. YZ: Conceptualization, Supervision, Funding acquisition, Writing—review & editing. HZ: Conceptualization, Project administration, Funding acquisition, Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling editor: Hailong Wang
Supplementary Information
Additional file 1. Table S1.
Effects of biochar on soil chemical properties under different irrigation regimes. Table S2. Effects of biochar on chloride content in rice plants under different irrigation regimes. Fig. S1. SEM (a) and FTIR spectra (b) of biochar. Fig. S2. Dry matter production and grain yield of rice at maturity. Fig. S3. Effects of biochar on soil Cd forms under different water management. Fig. S4. Parallel coordinate plots of rice Cd accumulation, soil properties and physiological characteristics under different treatments (a); Parallel coordinate plots of brown rice Cd accumulation, subcellular distribution and chemical form under different treatments (b).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, L., Guo, L., Xiong, Q. et al. Biochar-mediated Cd accumulation in rice grains through altering chemical forms, subcellular distribution, and physiological characteristics. Biochar 5, 48 (2023). https://doi.org/10.1007/s42773-023-00248-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00248-4