Abstract
Biochar has various agricultural applications, including the promising use as a carrier for beneficial microorganisms. However, most recent research has demonstrated the possible attachment or immobilization of a single bacterial species onto biochar rather than a consortium of microbes for biotechnological applications. Thus, an assessment on the potential of oil palm kernel shell (OPKS) biochar as a biofilm-producing Bacillus consortium carrier through optimization study on the operating and environmental factors influencing the biofilm adhesion was conducted using response surface methodology (RSM) and the subsequent soil stability and storage potential of the formulation. The highest Bacillus population was observed at temperature 33 °C, agitation speed of 135 rpm, at a neutral pH of 7.5 with 10% (w/w) of sago starch as the co-carbon source. The adhesion of Bacillus on OPKS biochar following the optimized conditions fitted pseudo-second order (PSO) of kinetic modelling (R2 = 0.998). The optimized formulation was subjected to storage in different temperatures and in vitro soil incubation which revealed that the Bacillus biofilm-adhered OPKS biochar may be stored up to 4 months with minimum range of live Bacillus viability reaching 107 CFU g-1 of biochar which is within the minimum range of acceptable biofertilizer viability (106 CFU mL-1). Formulation that is viable in room storage can be easily incorporated into current agricultural distribution networks that do not have refrigeration. This work highlighted the physicochemical and soil stability qualities of optimized Bacillus consortium adhesion on biochar for agricultural usage.
Article Highlights
-
Integration of biochar with Bacillus consortium biofilms served as novel organic fertilizer in agriculture.
-
The biochar-integrated Bacillus biofilms persisted in challenging temperature and environment.
-
Biochar-integrated Bacillus biofilm fertilizer fostered the attainment of the Sustainable Development Goals
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bacteria colonization and biofilm development on a material begin with an initial adhesion. Biofilms may be harmful to human health and industrial operations, producing infection, pathogen contamination, and slime production, for example, while also being useful in environmental technologies and bioprocesses. Wild-type bacteria have evolved numerous cooperative skills, such as the capacity to swarm or form surface-attached colonies known as biofilms, as a result of their evolution in changing settings and competition for limited resources. These 'city of microbes' are created in response to a variety of stimuli and have been demonstrated to give an advantage in a variety of scenarios, ranging from nutrition retention to predator protection via greater antibiotic resistance (Moradali and Rehm 2019). As the biofilm matures, its inhabitants begin to generate an adhesive matrix, which allows cells to adhere to one another and form a layered biofilm. The matrix, also known as extra polymeric material (EPS), is mostly made up of exopolysaccharide, protein, and DNA (Hamon and Lazazzera 2001; Abdullah et al. 2021).
The capacity of creating a biofilm relies on the presence of bacteria that can release extracellular polymeric substances (EPSs), which 'glue' cells together and onto the surface, inside the community (Dervaux et al. 2014). Adhesion mechanisms must be explored in order to regulate and use bacterial adhesion and biofilms (Ajeng et al. 2021c, 2021a). In the development of a biofertilizer, the selection of bacterial species is an important component towards a successful intended applications in the soil. However, due to the heterogeneity of soil microbes and resident pathogens, the intended application of biofertilizer without any carrier is often unsuccessful due to the grazing by the pathogen and competition with the resident soil microbes. That is why the recent emerging biofilm immobilization on a carrier method is becoming popular for these reasons.
Biofilmed Biofertilizer (BBF) is an emerging technique in biofertilizer development which is based on the biofilm formation of beneficial plants probiotics on certain carrier which allows for a safe delivery into the soil, to avoid grazing by soil pathogen and competition with resident soil microbiota (Dervaux et al. 2014; Otaiku et al. 2022). B. subtilis has been used to research Gram-positive bacterial physiology as a model organism. B. subtilis produces adherent biofilms on inert surfaces under the influence of a number of transcription factors, according to early studies (Hamon and Lazazzera 2001; Stanley et al. 2003). There is a clear recognition that biosurfactant synthesis not only influences biofilm architecture but also influences bacteria adhesion to surfaces (Davey et al. 2003). The effectiveness of the biofertilizer often depends on several factors including types and abundance of carbon and nitrogen sources, temperature, pH, aeration, and incubation period (Barin et al. 2022). Therefore, different formulations are optimized following these factors, in order to have the most efficient biofertilizers.
Biochar is an established organic soil amendment produced via the pyrolysis of biomass under limited oxygen (Lehmann and Joseph 2015; Ajeng et al. 2020). The growing evidence of biochar-immobilized microbes in various biotechnological sectors such as the bioremediation provides better insights into the interaction between the materials and the microorganisms (Li et al. 2022). However, the persistence of the biofilm generated on biochar and the subsequent evolution of the immobilized microorganisms received little attention particularly in the biofertilizer development. When developing a bioformulation with any type of carrier material, several factors governing bacterial growth such as pH, temperature, culture medium, and time should be considered to achieve an optimised microbial growth with viable cell count that is within the minimum range of 106—107 CFU per gramme of the material (Vanek et al. 2016; Abdulrasheed et al. 2020). Many variables impact the physical foundation of bacterial biofilm development on surfaces such as biochar, including electrostatics, metal ion concentration, carrier zeta potential, and bacterial motility. According to Hong et al. (2012), hydrophobicity exhibited no discernible relationship with other adhesion characteristics, however the surface electrical property dominated the affinity between Bacillus subtilis and six soil minerals. On the other hand, Bejarano et al. (2017) showed that the zeta potential had only a little impact on the effectiveness of Paraburkholderia phytofirmans PsJN's adsorption on talc and a small number of other carriers. According to Zhang et al. (2021), bacteria from activated sludge are more likely to form biofilms on cationic polyacrylamide-derived hydrophilic group-modified material. According to Bejarano et al. (2017), bacteria can build up more readily on hydrophobic carriers than on hydrophilic mineral surfaces, which is the case for most biochars. Furthermore, a new study shows that single bacterial species may be immobilized on biochar for biotechnological purposes rather than a community of bacteria, which is required in the production of an efficient biofertilizer(Cheng et al. 2020; Ajeng 2021). Thus, the aim of this study is to optimize the operating conditions for the immobilization of Bacillus consortium biofilm formation on palm kernel shell biochar. We hypothesized that addition of co-carbon source such as starch during the optimization process would positively affect the immobilization and encourage biofilm formation on palm kernel shell biochar.
2 Materials and methods
2.1 Chemicals and reagents
Phosphate buffered saline (PBS)(Sigma Aldrich), Brain heart infusion (BHI)(Sigma Aldrich), Sago Starch (Food Grade), sodium hydroxide (NaOH) tablets (Sigma Aldrich), 5% hydrochloric acid (HCl) (Ajeng et al. 2021c).
2.2 Biochar preparation and Bacillus consortium preparation
Oil palm kernel shell biochar (OPKSB) was obtained from Malaysia Palm Oil Berhad (MPOB) which was produced via slow pyrolysis process at 400 °C. The characterization and properties of OPKSB were described previously (Ajeng et al. 2021c). OPKSB biochar was rinsed with water to remove water soluble fractions which could potentially possess toxicity towards microbes. The OPKSB was then dried in an oven at 100 °C to remove remaining moisture prior to grinding into coarse particles using pestle and mortar (Vejan et al. 2022). The ground OPKSB was sieved to pass a 2 mm sieve.
Bacillus Strain Salmah Ismail (SI) 139SI was obtained from the Universiti Malaya Molecular Bacteriology and Toxicology Laboratory (UMBTL) and was first isolated from soil taken from a private farm in Selangor, Malaysia (2.99917°N 101.70778°E) (Ismail and Dadrasnia 2015). Meanwhile, B. amyloliquefaciens NBRC15535 and B. cereus ATCC14579 were isolated from a biofertilizer for oil palms (Isolation and identification data were shown). These strains were routinely cultivated on BHI blood agar and kept at -80 °C in a glycerol solution (25% w/v) and at ambient temperature on BHI-slant agar. In vitro assays were used to determine the compatibility of bacterial species, according to Sundaramoorthy et al. (2012) (Sundaramoorthy et al. 2012). Cell suspensions were prepared and spread on BHI plates in all possible combinations of the three species. In relation to each other, each pair of species was spread out in perpendicular lines. Plates were incubated for 72 h at 28 °C before being examined for inhibition zones. Compatible strains were able to grow over each other, and inhibition zones present between incompatible combinations. The experiment was repeated twice for each culture medium, with four replicates per treatment. All three Bacillus species were compatible with each other (Fig. 1). A standard consortium suspension was then prepared by mixing equal volume from each Bacillus grown in BHI medium (1:1:1) into sterile PBS (Ajeng et al. 2021c).
2.3 Optimization of operating conditions on Bacillus consortium survivability
The Wijesinghe et al. 2015 (Wijesinghe et al. 2019) method was used to conduct the biofilm growth assay with slight modification. To initiate the first cell adhesion on OPKS biochar, 1000 µL of the standard cell suspension was pipetted into sterilized conical flask containing 0.25 g OPKS biochar and statically incubated for 90 min at 37 °C. The flask was then washed once with 2000 µL of sterile PBS to remove non-adherent cells before being replenished with 1000 µL of sterile BHI broth.
The optimization of Bacillus consortium biofilm adhesion on OPKS biochar was conducted using Response Surface Methodology (RSM) technique. A central composite design (CCD) was applied to find the interaction of each parameter with a response (Design-Expert 13, Stat-ease, Minneapolis, USA). The Bacillus population (CFU/ml) was chosen as the response for this study (Eq. (1)) due to the intended bioformulation application as soil organic amendment where cell viability matters the most in designing an effective carrier (Ajeng et al. 2020; Mitter et al. 2021).
where TC is the total colonies, df is the dilution factor and V is the volume plated in mL.
The Bacillus population was determined using the mathematical model designated by Eq. (2).
where \(y\) is the response of interest (CFU/mL), \(\chi i\) and \(\chi j\) are the parameters (Temperature (°C), agitation speed (rpm), pH, and starch (% w/w), and \(\beta\) is the regression coefficient value of the model. The pH of the medium was adjusted using NaOH or HCl. The results were statistically analyzed using analysis of variance (ANOVA) with p-value < 0.05 to determine optimized Bacillus population. The ranges and levels of independent variables (parameters) are list in Table 1. The conditions of the experiments are shown in Table 2.
2.4 Bacillus consortium adhesion kinetics modelling
The adsorption kinetics was investigated with initial concentrations of 3.0 \(\times\) 108 CFU mL−1 during incubation periods of 0 to 140 min at optimal conditions defined by the RSM analysis. The 100 \(\mathrm{\mu L}\) of the test solutions were centrifuged (3000 rpm) for 5 min (Du et al. 2016) and the supernatant was the unadhered bacteria. The CFU/mL was calculated following Eq. (1) and the attached bacteria was calculated by subtracting the initial concentration with the obtained values. In the current study, three linear kinetic models, namely pseudo-first order, pseudo-second order, and intraparticle diffusion model, were used to investigate the adsorption dynamics of Bacillus spp. onto PKS biochar, where the models allow estimation of the number of bacteria absorbed at the time of processing. The kinetic models also allow for the estimate of sorption rates, which frequently leads to rate expressions that are indicative of putative adhesion processes (Robati 2013). The linearized pseudo-first order (PFO) is expressed as follows:
where Qe (CFU g-1) and Qt (CFU g-1) represent the adsorbate quantity adsorbed at equilibrium and at any t (min), respectively; K1 is the rate constant of adsorption (1 min-1). The value of K1 and Qe were determined from the slope and the intercept of the log (Qe–Qt) against t graph. By drawing the diagrams of log (Qe−Qt) and t/qt against time, the constant parameters of pseudo-first and pseudo-second order kinetic models may be determined and correlation coefficient R2 may also be calculated for laboratory conditions.
The linearized pseudo-second order (PSO) is expressed as follows:
where K2 is the rate constant of the adsorption (CFU/g min). The value of Qe and K2 were determined from the slope and the intercept of the t/Qt against t graph. To compare the efficiency of the kinetic models in describing the adhesion properties, non-linear models of each of the kinetic model were utilized. The non-linear PFO is expressed as follows:
where Qe and Qt are the adsorbate uptake rates per mass of adsorbent at equilibrium and at any time t (min), respectively, and k (min−1) is the rate constant of PFO equation.
And non-linear PSO is expressed as follows:
where Qe (CFU g-1) and Qt (CFU g-1) represent the adsorbate quantity adsorbed at equilibrium and at any t (min), respectively, and K2 (g/CFU min) represents the PSO equation constant rate.
2.5 Bacillus consortium Sago Starch-biochar Adhered Characterization and Storage Potential
The optimized formulation was replicated and left to dry in an aseptic container wrapped in aluminum foil. Equal volume of the formulation was stored in different temperatures (Sun et al. 2016) (-24 °C, 32 °C, and 39 °C) for 4 months and sterilized biochar without inoculants acted as the control. An in vitro soil incubation was also performed following the methods outlined by Husen (2003) with slight modifications. Briefly, three sterilized glass jars containing oxisol were inoculated, mixed with 10% (w/w) of the optimized formulation and kept slightly aired. The water holding capacity of soil was capped at 70% by using sterilized distilled water (Sarkar et al. 2005). The jar was kept at room temperature for 4 months. To obtain bacterial isolations, the protocol described by Mandakovic et al. (Mandakovic et al. 2018), previously used to isolate bacteria from soil samples in the Lejía Lake, was followed with slight modifications. 1 g of the formulation (storage potential) and 1 g of soil (in vitro incubation) were diluted in 900 \(\mathrm{\mu L}\) of sterilized distilled water and vortexed. 100 \(\mathrm{\mu L}\) of the suspension were diluted to a dilution factor (df) of df = 3 or df = 4, and 30 \(\mathrm{\mu L}\) of the final dilution was pipetted on three BHI plates and incubated overnight. The total viable bacteria count was performed using Eq. 1. The results were presented in CFU/ml and visual plates images. Fourier transformed infrared (FTIR) for OPKS biochar served as the control and OPKS biochar adhered Bacillus consortium were conducted. Prior to storage, the formulation was freeze dried and sent for Field Emission Scanning Electron Microscope (FESEM) analysis to visualize the adhesion of cells onto the OPKS biochar.
2.6 Statistical analysis
Experimental data were statistically analyzed using SPSS statistical software (version 19.0, IBM Corp., Armonk, USA). The results for optimization were statistically analyzed using analysis of variance (ANOVA) with p-value < 0.05 to determine optimized Bacillus population. The multiple regression analysis of the model and construction of response surface graphs were performed by using Design-Expert, version 7.1.6 (STATEASE Inc., Minneapolis, MN, USA). The quality of regression equation was determined by the coefficient of determination R2 and its significance was judged by an F-test. The fitted 2nd order polynomial equation was explained in the form of 3D plot graphs to show the relationship between the response and experimental variables. The point optimization method was used to optimize the maximum response of each variable. Moreover, in order to prevent systematic errors, the run order of experiments was randomized. Differences in the bacteria survivability were evaluated by one-way ANOVA (p < 0.05).
3 Results and discussion
3.1 Optimization of Bacillus consortium biofilm adhesion on OPKS biochar using RSM analysis
Bacillus biofilm adhesion on the biochar was performed under different operating conditions (temperature, pH, agitation speed and starch concentration). The optimization of these parameters was conducted to examine the conditions which promoted high bacterial growth and survivability (high CFU/mL) (Fig. 2). Therefore, the basic Bacillus population (CFU/mL) was chosen as a response for CCD analysis. The Bacillus population is presented in Table 2. The model for the relationship of parameters X1, X2, X3, and X4 on Bacillus consortium survival (Y) is shown in Eq. (7). The statistical significance and the goodness of fit for bacterial survival related to the parameters were proven by the R2 and p-values. It was found that the R2 (0.998) of the model was close to R2 adj (0.996). The p-value of lack of fit was higher than 0.05. These statistical values confirmed that the model had adequate precision and was reliable for modeling the Bacillus consortium survival from biofilm adhered on the OPKS biochar.
where y is Bacillus adhesion rate (mg L−1d−1) and X1, X2, X3, and X4 are temperature (°C), agitation speed (rpm), pH, and co-carbon source concentration (%w/w), respectively. This indicates that the alternated model was effective at estimating the Bacillus adhesion. The equation in terms of coded factors may be used to predict response for different amounts of each parameter. The high values of the components are recorded as + 1 by default, while the low levels are coded as -1. By comparing the factor coefficients, the coded equation may be used to determine the relative importance of the components. The F-value of 531.45 for the model indicates that it is significant. Model terms are significant if their p-values are less than 0.05. Significant model terms in this scenario are X1, X22, and X32. The model terms are not significant if their values exceed 0.10. Model reduction may enhance this model if there are a lot of unimportant model terms (except those necessary to maintain hierarchy). The F-value of 0.96 for the lack of fit indicates that it is not significant when compared to the pure error. The model has a significant lack of fit F-value due to noise, which has a 55.58% probability of occurring.
The CCD results of Bacillus population released from the maturation biofilm were analyzed by the ANOVA test. At the 95% confidence range, only the temperature and starch % (w/w) had statistically significant effects on Bacillus survival rate with other parameters showing no clear influence. Low to intermediate operating temperature significantly increased the Bacillus population. This indicates that the biochar-adhered bacterial biofilm is effective in releasing bacterial cells after biofilm maturation within the medium range of temperature tested whereby the highest temperature of 38 °C is detrimental to the bacterial biofilm adhesion, which leads to decreased bacterial survival on biochar ecosystem.
The findings were in agreement with previously published studies (Hoštacká et al. 2010; Rao 2010; Pavlovsky et al. 2015) which suggested that the bacterial biofilm formation is optimum at temperature ranging from 30 to 33 °C. In addition to EPS generation, a second change occurred during biofilm formation, which was flagella synthesis downregulation. de Kievit (2011) suggested that there is an inverse link between EPS production and fla gella through a transcriptomic analyses, which demonstrates that genes involved in the creation of the biofilm matrix and flagella are inversely expressed. The findings from these studies will add to the current knowledge repositories on the effects of temperature on bacterial biofilm formation on material surfaces, where in the medical and food industries, preventing the formation of pathogenic bacterial biofilm is of major important. Meanwhile, the development of bioformulation containing beneficial inoculants intended for agricultural purposes requires a substantial amount of the cells to be safely delivered into the soil to allow a successful interaction with plant roots (Vanek et al. 2016; Ajeng et al. 2020; Egamberdieva et al. 2020; Saleh et al. 2020). It is interesting to note that addition of sago starch as co-carbon source for the Bacillus has a positive effect on the survival of the consortium on OPKS biochar. It is believed that the addition of starch as co-carbon source in the growing medium of the bacteria increases the survival of the strain due to the induced production of biosurfactant (Stancu 2020). Biosurfactant is an emerging useful biotechnological compound secreted by Bacillus species that aids in facilitating the transport and translocation of insoluble hydrophobic substrates (e.g., polyaromatic hydrocarbons, and oils) across the cell membranes (Dadrasnia and Ismail 2015; Stancu 2020). Furthermore, these biocompounds can withstand a broad variety of pH and temperature conditions, and they can influence interfaces to some extent (Diniz Rufino et al. 2014). The potentials of B. salmalaya 139SI in biosurfactant production have been evaluated in several studies (Dadrasnia and Ismail 2015; Ismail and Dadrasnia 2015; Abu Tawila et al. 2018). However the growth and production of biosurfactant from this species in starch addedstarch-added medium have not been reported. From our findings, the addition of sago starch by 20% (w/w) slightly increased the survival of the consortium in low pH and high temperature. Meanwhile, 10% (w/w) of starch addition is adequate to improve the growth of Bacillus consortium on OPKS biochar (108 CFU ml-1).
Other parameters, on the other hand, had a less influence on Bacillus consortium population (agitation and pH). The parameters that were chosen for optimization study were based on real-world operational and environmental circumstances. However the ranges may not be critical, resulting in a negligible effect on Bacillus population from the adhered biofilm. According to Table 2, an increase in Bacillus population was observed in neutral pH (7.5), with less growth noticed in pH 3 and pH 12. Enhanced bacterial growth in neutral pH ranges could be due to the higher alginate exopolysaccharides production (Boyd and Chakrabarty 1994; Moradali and Rehm 2019). These natural polymers are important polymeric substances contributing to the formation and development of biofilm matrixes of numerous bacteria enhancing their persistence under various environmental stresses (Ajeng et al. 2021b; Ismail et al. 2022).
3.2 Kinetics properties of Bacillus Consortium on OPKS Biochar and its adhesion characterization
From the FESEM analysis shown in Fig. 3, a network of rich Bacillus biofilm and formation of Bacillus colonies on OPKS biochar can be observed. The biofilm is seen to encapsulate the biochar surfaces (Fig. 3c) including its pores. The bioencapsulation of carrier material is preferable in the development of an inoculant carrier for soil application over alternative solid and liquid formulations (Schoebitz et al. 2013). The problems pertaining to the carrier material such as the ineffectiveness to deliver substantial amount of biofertilizer into the soil can be addressed by the immobilization microbe biofilm-based formulations (Vassilev et al. 2020). Therefore, this biofilm-biochar technology is deemed to revolutionize not only the wastewater treatment and bioremediation but also the biofertilizer technology over the conventional formulation types (Dalahmeh et al. 2019; Jayakumar et al. 2021; Xing et al. 2021; Ajeng et al. 2022).
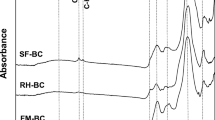
In a study conducted by Ajeng et al. (2021c), the author suggested that oxygen-containing functional groups play an important role in polymer adherence to surfaces. EPS are polymers produced by a variety of bacteria, including the Bacillus species employed in this work. The formation of EPS was demonstrated by the FTIR measurements in Fig. 3e. The -1,4-glycosidic bond, amide II, and uronic acids (1600 – 1200 cm−1) were identified as functional groups corresponding to Bacillus EPS production potentials in the investigated spectra. These peaks were not seen in the OPKS biochar-only as the control sample. The maxima of the amide I band, generally found at around 1650–1660 cm−1, shows the prevalence of helices among the secondary structural components of bacterial cellular proteins under normal growth circumstances (Kamnev 2008). Meanwhile, the functional groups of polysaccharides and nucleic acids are exhibited by the FTIR spectra in the 900–1,300 cm−1 range.
Non-linear and linear kinetic models were used to determine the adhesion kinetics of Bacillus consortium on the OPKS biochar. The bacteria adhered quickly in the first 60 min, and the greatest adhesion was attained after 120 min when the bacterium concentration was 2.0 × 108 CFU g-1 (Fig. 4). In comparison, the linear pseudo-second order kinetic model describes the adhesion kinetics of the Bacillus consortium well on OPKS biochar. For the adhesion system with contact durations of 140 min (Fig. 4), the correlation coefficients for the linear plots of t/qt against time from the pseudo-second order rate law are more than 0.998. This implies that the sorption system is not a first order reaction, and that the pseudo-second order model, based on the assumption that the rate-limiting step may be chemical sorption or chemisorption. The adsorption may involve valency forces via electron sharing or exchange between sorbent and sorbate, providing the best data correlation (Ho and McKay 1999) where the rate-limiting step is postulated to be the biofilm diffusion.
3.3 Storage potential and in vitro soil incubation of optimized formulation
The limited shelf life of inoculants continues to stymie biofertilizer technologies (Aloo et al. 2022) in which the selection of a better inoculant carrier that can deliver a substantial amount of probiotics or beneficial microbe to the soil is of importance (Ajeng et al. 2020). The Bacillus population from the storage potential at different temperatures and in vitro soil incubation are shown in Figs. 5 and 6, respectively and Fig. 7 shows the BHI plate conditions after incubations.
It was evident from the storage potential results that the optimized formulation consisting of OPKS biochar loaded Bacillus was able to be stored for at least 3 months with substantial amount of live Bacillus (107 CFU g-1) which was above the minimum range of live bacteria within a biofertilizer (106 CFU mL-1) (Vanek et al. 2016). After 4 months, the formulation maintained in the freezer exhibited the lowest activity of Bacillus compared to the formulation stored at ambient temperature (32 °C) and at 39 °C. This could be due to the inactivation of cells caused by the low moisture content (MC) in formulation stored in the freezer (Aloo et al. 2022). The MC of carrier materials has a significant impact on inoculant survival and longevity. This is most likely due to the inoculants' ability to remain dormant, resistant to environmental stressors, and unaffected by contamination in carriers with low MC. Dry formulations with low MC may often extend microbial viability for longer periods of time and at higher temperatures, resulting in lower marketing and maintenance costs because refrigeration is not necessary. Meanwhile, the bacterial activity from the cold storage can be revived before inoculating the formulation as a soil amendment, however a repeated freeze–thaw process of this formulation could be detrimental to the survival of the Bacillus as reported by Walker et al. (2006).
To further improve the storage potential, several manipulations to the bacterial strains can be made such as the genetic engineering of strain by introducing the cspB, which is the Bacillus cold shock gene responsible for the production of antifreeze proteins which protects the strain from the detrimental impact of ice crystallization in extreme cold storage temperature (Willimsky et al. 1992). Since the cspB gene and other antifreeze genes can be found in Bacillus species, the possibility of the horizontal gene transfer (HGT) to the Bacillus strains used in this study is higher and achievable although homogenization of HGT may occur in the field. Although high temperature can compromise the growth of bacteria in biofertilizer, OPKS biochar inoculated with probiotics biofilm can revolutionize the biofertilizer technology by providing sufficient surface area for the biofilm adhesion and protection against extreme environmental stressors. From the finding, formulations that have remained viable in room storage can be simply incorporated into current agricultural distribution systems that do not include refrigeration. The findings of our storage potential of beneficial biofilm loaded on biochar revealed that OPKS biochar is a promising choice as an inoculant carrier.
Another key criterion used to measure the performance of the formulation as a soil amendment is the in vitro soil incubation of the OPKS biochar loaded Bacillus in view of the bacterial survivability in a controlled soil incubation before inoculating them into the field. From the aerobic soil in vitro incubation, it can be noted that all the three replicates exhibited a promising shelf life in the soil for 4 months with a range of bacterial survival 107 CFU g-1 of soil. In comparison to the formulation, bacterial survival from the liquid formulation (control) dropped at the second month of incubation, proving the efficiency of OPKS biochar as an inoculant carrier, providing surface adherence for these strains and protecting them against soil pathogen predation that may present even in an autoclaved soil medium (Baker et al. 2020). Thus, the optimized formulation may be suitable to be applied in soil where there is a rapid accumulation of soil pathogens (Nijjer et al. 2007; Radian et al. 2022) or soil pollutants where OPKS biochar may safely offer a significant amount of probiotics to enrich the soil ecology and boost plant growth. This effect has been reported in the article of Meng et al. (2019) where the author concluded that the addition of biochar improves the survival of the inoculant including the overall soil microbiome and wheat plant performance under herbicide fomesafen stress. In another study by Naveed et al. (2020), the twigs biochar inoculated Burkholderia phytofirmans PsJN reduced the uptake and translocation of the heavy metals in the mung bean (Vigna radiata L.) plant. Future research relevant to the application of optimal formulation of OPKS biochar loaded Bacillus biofilm should include in vitro toxicity assessments of various heavy metals or herbicides, which will contribute to the knowledge repository from this work. Furthermore, according to Vanek et al. (2016) and Ajeng et al. (2020), the physicochemical properties of biochar are among the major factors affecting the potentials of biochar as carriers. The authors stated that the higher pore volume and surface area with functional groups that support the adhesion of microbes are of importance. The high porosity and large surface area of biochar offer a habitat for the development of immobilized microorganisms and protect them from soil faunal predators such as mites, protozoans, nematodes, and Collembola (Głodowska et al. 2017). Nano-pore carriers with a pore diameter of less than 25 nm may not be suitable for bacterial attachment due to the electrostatic force exerted on bacterial cells, as previously demonstrated on an anodic alumina surface, and biochar engineering may be required to ensure the porosity is suitable for the attachment of microbes (Feng et al. 2014). In the case of biochar surface properties, as additional oxygen functional groups are produced when the immobilized bacteria oxidize the biochar, stabilizing the soil's organic matter, adding more oxygen functional groups to the surface of the biochar may be advantageous for its surface qualities (Sun et al. 2016). The fused carbon rings in biochar support the microbial electron shuttle, redox reaction, microbial metabolism, and nutrient cycling. After biochar is pyrolyzed, these carbons, which also include other functional groups like oxygen and hydrogen, bond to the nutrients and minerals (Batista et al. 2018; Oh et al. 2018). Therefore, investigating the impacts of biochar features such as feedstock type, ash content, carbon content, pore and micropore volume, and surface functional group variation on Bacillus consortium adhesion to biochar may be a significant topic for future research.
4 Conclusion
This study presents the potential of Bacillus biofilm adhesion on OPKS biochar in view of the development of effective biofertilizer for soil amendment. The optimization of strain adhesion on the carrier material produced a favorable environment for consortium growth at 33 °C, 135 rpm agitation speed, neutral pH of 7.5, and 10% (w/w) sago starch as the co-carbon source in which may have assisted in the production of EPS from the Bacillus species used in this study. The optimized operating conditions from this study may be replicated for future work involving its application as biofilm-biochar biofertilizer to enhance crops growth. Bacillus biofilm-OPKS biochar may survive up to 4 months in storage and in vitro soil incubation, indicating that there is an adequate number of probiotics released by this formulation.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdullah R, Mustafa FB, Bhassu S, Azhar NAA, Bwadi BE, Ahmad NSBN, Ajeng AA (2021) Evaluation of water and soil qualities for giant freshwater prawn farming site suitability by using the AHP and GIS approaches in Jelebu, Negeri Sembilan. Malaysia Aims Geosciences 7(3):507–528
Abdulrasheed M, Zulkharnain A, Zakaria NN, Roslee AFA, Abdul Khalil K, Napis S, Convey P, Gomez-Fuentes C, Ahmad SA (2020) Response surface methodology optimization and kinetics of diesel degradation by a cold-adapted Antarctic bacterium, Arthrobacter sp. strain AQ5–05. Sustainability 12(17):6966
Abu Tawila ZM, Ismail S, Dadrasnia A, Usman MM (2018) Production and characterization of a bioflocculant produced by Bacillus salmalaya 139SI-7 and its applications in wastewater treatment. Molecules 23(10):2689
Ajeng AA, Abdullah R, Ling TC, Ismail S, Lau BF, Ong HC, Chew KW, Show PL, Chang J-S (2020) Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ Technol Innov 45:101168
Ajeng AA, Abdullah R, Junia A, Lau BF, Ling TC, Ismail S (2021a) Evaluation of palm kernel shell biochar for the adsorption of Bacillus cereus. Phys Scr 67:89
Ajeng AA, Abdullah R, Kadir WRA, Suki NIA, Saadah N (2021b) Growth of Maize and Residual Nutrients in Soil Treated with Palm Kernel Shell Biochar. Growth 52:1
Ajeng AA, Abdullah R, Ling TC, Ismail S (2021c) Adhesion of Bacillus salmalaya and Bacillus amyloliquefaciens on Oil Palm Kernel Shell Biochar: A Physicochemical Approach. J Environ Chem Eng 4:107115
Ajeng AA, Rosazlin A, Junia A, Lau BF, Ling TC, Ismail S (2021d) Evaluation of palm kernel shell biochar for the adsorption of Bacillus cereus. Phys Scr 23:45
Ajeng AA, Rosli NSM, Abdullah R, Yaacob JS, Qi NC, Loke SP (2022) Resource recovery from hydroponic wastewaters using microalgae-based biorefineries: a circular bioeconomy perspective. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2022.10.011
Aloo BN, Mbega ER, Makumba BA, Tumuhairwe JB (2022) Effects of carrier materials and storage temperatures on the viability and stability of three biofertilizer inoculants obtained from Potato (Solanum tuberosum L) Rhizosphere. Agriculture 12(2):140
Andriani D, Apriyana AY, Karina M (2020) The optimization of bacterial cellulose production and its applications: a review. Cellulose 27:6747–6766
Baker CA, Lee S, De J, Jeong KC, Schneider KR (2020) Survival of Escherichia coli O157 in autoclaved and natural sandy soil mesocosms. PLoS ONE 15(6):e0234562
Barin M, Asadzadeh F, Hosseini M, Hammer EC, Vetukuri RR, Vahedi R (2022) Optimization of Biofertilizer Formulation for Phosphorus Solubilizing by Pseudomonas fluorescens Ur21 via Response Surface Methodology. Processes 10(4):650
Batista EM, Shultz J, Matos TT, Fornari MR, Ferreira TM, Szpoganicz B, de Freitas RA, Mangrich AS (2018) Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci Rep 8(1):1–9
Bejarano A, Sauer U, Mitter B, Preininger C (2017) Parameters influencing adsorption of Paraburkholderia phytofirmans PsJN onto bentonite, silica and talc for microbial inoculants. Appl Clay Sci 141:138–145
Boyd A, Chakrabarty A (1994) Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol 60(7):2355–2359
Cheng C, Luo W, Wang Q, He L, Sheng X (2020) Combined biochar and metal-immobilizing bacteria reduces edible tissue metal uptake in vegetables by increasing amorphous Fe oxides and abundance of Fe-and Mn-oxidising Leptothrix species. Ecotoxicol Environ Saf 206:111189
Dadrasnia A, Ismail S (2015) Biosurfactant production by Bacillus salmalaya for lubricating oil solubilization and biodegradation. Int J Environ Res Public Health 12(8):9848–9863
Dalahmeh SS, Alziq N, Ahrens L (2019) Potential of biochar filters for onsite wastewater treatment: Effects of active and inactive biofilms on adsorption of per-and polyfluoroalkyl substances in laboratory column experiments. Environ Pollut 247:155–164
Davey ME, Caiazza NC, O’Toole GA (2003) Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol 185(3):1027–1036
de Kievit T (2011) 1.41 - Biofilms. In: Moo-Young M (ed) Comprehensive Biotechnology (Second Edition). Academic Press, Burlington, pp 547–558
Dervaux J, Magniez JC, Libchaber A (2014) On growth and form of Bacillus subtilis biofilms. Interface Focus 4(6):20130051
Diniz Rufino R, Moura de Luna J, de Campos Takaki GM, Asfora Sarubbo L (2014) Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron J Biotechnol 17(1):6–6
Du J, Sun P, Feng Z, Zhang X, Zhao Y (2016) The biosorption capacity of biochar for 4-bromodiphengl ether: study of its kinetics, mechanism, and use as a carrier for immobilized bacteria. Environ Sci Pollut Res 23(4):3770–3780
Egamberdieva D, Ma H, Alimov J, Reckling M, Wirth S, Bellingrath-Kimura SD (2020) Response of Soybean to Hydrochar-Based Rhizobium Inoculation in Loamy Sandy Soil. Microorganisms 8(11):1674
Feng G, Cheng Y, Wang S-Y, Hsu LC, Feliz Y, Borca-Tasciuc DA, Worobo RW, Moraru CI (2014) Alumina surfaces with nanoscale topography reduce attachment and biofilm formation by Escherichia coli and Listeria spp. Biofouling 30(10):1253–1268
Głodowska M, Schwinghamer T, Husk B, Smith D (2017) Biochar based inoculants improve soybean growth and nodulation. Agric Sci 8(9):1048–1064
Hamon MA, Lazazzera BA (2001) The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol 42(5):1199–1209
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Hong Z, Rong X, Cai P, Dai K, Liang W, Chen W, Huang Q (2012) Initial adhesion of Bacillus subtilis on soil minerals as related to their surface properties. Eur J Soil Sci 63(4):457–466
Hori K, Matsumoto S (2010) Bacterial adhesion: from mechanism to control. Biochem Eng J 48(3):424–434
Hoštacká A, Čižnár I, Štefkovičová M (2010) Temperature and pH affect the production of bacterial biofilm. Folia Microbiol 55(1):75–78
Husen E (2003) Screening of soil bacteria for plant growth promotion activities in vitro. Indonesian J Agric Sci 4(1):27–31
Ismail S, Dadrasnia A (2015) Biotechnological potential of Bacillus salmalaya 139SI: a novel strain for remediating water polluted with crude oil waste. PLoS ONE 10(4):e0120931
Ismail S, Ameen F, Bha SA, Dadrasnia A, Ajeng A (2022) Analysis and characterization of potential probiotic properties of Lactobacillus and Bacillus salmalaya 139SI. Emirates Journal of Food and Agriculture
Jayakumar A, Wurzer C, Soldatou S, Edwards C, Lawton LA, Mašek O (2021) New directions and challenges in engineering biologically-enhanced biochar for biological water treatment. Sci Total Environ 796:148977
Kamnev AA (2008) FTIR spectroscopic studies of bacterial cellular responses to environmental factors, plant-bacterial interactions and signalling. Spectroscopy 22(2–3):83–95
Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation. Routledge
Li R, Wang B, Niu A, Cheng N, Chen M, Zhang X, Yu Z, Wang S (2022) Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci Total Environ 7:155563
Mandakovic D, Maldonado J, Pulgar R, Cabrera P, Gaete A, Urtuvia V, Seeger M, Cambiazo V, González M (2018) Microbiome analysis and bacterial isolation from Lejía Lake soil in Atacama Desert. Extremophiles 22(4):665–673
Meng L, Sun T, Li M, Saleem M, Zhang Q, Wang C (2019) Soil-applied biochar increases microbial diversity and wheat plant performance under herbicide fomesafen stress. Ecotoxicol Environ Saf 171:75–83
Mitter EK, Tosi M, Obregón D, Dunfield KE, Germida JJ (2021) Rethinking crop nutrition in times of modern microbiology: innovative biofertilizer technologies. Front Sustain Food Syst 5:606815
Moradali MF, Rehm BH (2019) The role of alginate in bacterial biofilm formation. Extracellular Sugar-Based Biopolymers Matrices. Springer, Berlin, pp 517–537
Naveed M, Mustafa A, Azhar SQ-T-A, Kamran M, Zahir ZA, Núñez-Delgado A (2020) Burkholderia phytofirmans PsJN and tree twigs derived biochar together retrieved Pb-induced growth, physiological and biochemical disturbances by minimizing its uptake and translocation in mung bean (Vigna radiata L.). J Environ Manag 257:109974
Nijjer S, Rogers WE, Siemann E (2007) Negative plant–soil feedbacks may limit persistence of an invasive tree due to rapid accumulation of soil pathogens. Proc R Soc 274(1625):2621–2627
Oh JK, Yegin Y, Yang F, Zhang M, Li J, Huang S, Verkhoturov SV, Schweikert EA, Perez-Lewis K, Scholar EA (2018) The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci Rep 8(1):1–13
Otaiku AA, Soretire A, Mmom P (2022) Biofertilizer Impacts on Soybean [Glycine max (L.)] Cultivation, Humid Tropics| Biological Nitrogen Fixation, Yield, Soil Health and Smart Agriculture Framework
Pavlovsky L, Sturtevant RA, Younger JG, Solomon MJ (2015) Effects of temperature on the morphological, polymeric, and mechanical properties of Staphylococcus epidermidis bacterial biofilms. Langmuir 31(6):2036–2042
Radian R, Ichwan B, Hayat I (2022) Nanotechnology for dryland agriculture water saving: Biodegradable hydrogel application in sweet corn (Zea mays saccharate Sturt) productio. Emirates J Food Agric 78:7
Rao T (2010) Comparative effect of temperature on biofilm formation in natural and modified marine environment. Aquat Ecol 44(2):463–478
Robati D (2013) Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J Nanostruc Chem 3(1):1–6
Saleh D, Sharma M, Seguin P, Jabaji S (2020) Organic acids and root exudates of Brachypodium distachyon: Effects on chemotaxis and biofilm formation of endophytic bacteria. Can J Microbiol 66(10):562–575
Sarkar D, Datta R, Sharma S (2005) Fate and bioavailability of arsenic in organo-arsenical pesticide-applied soils: Part-I: incubation study. Chemosphere 60(2):188–195
Schoebitz M, López MD, Roldán A (2013) Bioencapsulation of microbial inoculants for better soil–plant fertilization. A Review. Agron Sustain Develop 33(4):751–765
Stancu MM (2020) Biosurfactant production by a Bacillus megaterium strain. Open Life Sci 15(1):629–637
Stanley NR, Britton RA, Grossman AD, Lazazzera BA (2003) Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J Bacteriol 185(6):1951–1957
Sun D, Hale L, Crowley D (2016) Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol Fertil Soils 52(4):515–522
Sundaramoorthy S, Raguchander T, Ragupathi N, Samiyappan R (2012) Combinatorial effect of endophytic and plant growth promoting rhizobacteria against wilt disease of Capsicum annum L. caused by Fusarium solani. Biol Control 60(1):59–67
Vanek S, Thies J, Wang B, Hanley K, Lehmann J (2016) Pore-size and water activity effects on survival of Rhizobium tropici in biochar inoculant carriers. J Microb Biochem Technol 8:296–306
Vassilev N, Vassileva M, Martos V, Garcia del Moral LF, Kowalska J, Tylkowski B, Malusá E (2020) Formulation of microbial inoculants by encapsulation in natural polysaccharides: focus on beneficial properties of carrier additives and derivatives. Front Plant Sci 11:270
Vejan P, Abdullah R, Ahmad N, Khadiran T (2022) Biochar and activated carbon derived from oil palm kernel shell as a framework for the preparation of sustainable controlled release urea fertiliser. Environ Sci Pollut Res 34:1–13
Walker VK, Palmer GR, Voordouw G (2006) Freeze-thaw tolerance and clues to the winter survival of a soil community. Appl Environ Microbiol 72(3):1784–1792
Wijesinghe G, Dilhari A, Gayani B, Kottegoda N, Samaranayake L, Weerasekera M (2019) Influence of laboratory culture media on in vitro growth, adhesion, and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus. Med Princ Pract 28(1):28–35
Willimsky G, Bang H, Fischer G, Marahiel M (1992) Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J Bacteriol 174(20):6326–6335
Xing Y, Luo X, Liu S, Wan W, Huang Q, Chen W (2021) A novel eco-friendly recycling of food waste for preparing biofilm-attached biochar to remove Cd and Pb in wastewater. J Clean Prod 311:127514
Yang W, Shang J, Li B, Flury M (2020) Surface and colloid properties of biochar and implications for transport in porous media. Crit Rev Environ Sci Technol 50(23):2484–2522
Zhang L, Gadd GM, Li Z (2021) Microbial biomodification of clay minerals. Adv Appl Microbiol 114:111–139
Acknowledgement
The authors would like to acknowledge the technical and financial support from Universiti Malaya Impact-Oriented Interdisciplinary Research Grant (IIRG) (Grant Number IIRG004-19IISS).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to writing the article. Part of the datasets in the manuscript is taken from AAA Doctoral Thesis. AAA conducted the research, RA and LTC equally contributed to the improvement of the flow and writing of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Jun Meng.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ajeng, A.A., Abdullah, R. & Ling, T.C. Biochar-Bacillus consortium for a sustainable agriculture: physicochemical and soil stability analyses. Biochar 5, 17 (2023). https://doi.org/10.1007/s42773-023-00215-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00215-z