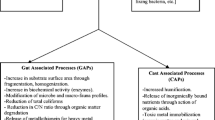
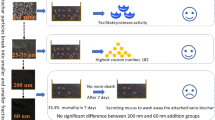
Abstract
Biochar addition has been widely used in the field to mitigate soil nitrous oxide (N2O) emissions, and can be considered as a potential method to reduce N2O emissions during vermicomposting. However, excessive biochar addition may inhibit earthworms’ activity. Thus, it is crucial to clarify the optimum addition volumes of biochar during vermicomposting. This study evaluated the impact of addition of various amounts of biochar (0, 5, 10, 15, 20 and 25% of total amount of feedstock) on earthworms’ (Eisenia fetida) activity, N2O emission and compost quality during vermicomposting. Compared with the treatment without biochar added, 5% of biochar application significantly increased earthworm total biomass (from 177.5 to 202.2 g pot−1), and cumulative burrowing activity (from 47.0% to 52.2% pixel per terrarium). The increased earthworms activity stimulated the vermicomposting process and led to the best quality of compost, which showed the highest total nutrient content (5.38%) and a significantly higher germination percentage of seeds (88%). Although N2O emissions were slightly increased by 5% biochar addition, a non-significant difference was found between the treatment with 5% biochar and the treatment without biochar added. On the contrary, 20% and 25% biochar addition not only lowered N2O emissions, but also significantly decreased the quality of compost. The results suggest that 5% biochar application is an appropriate amount to improve the quality of compost without significant N2O emissions.
Graphical Abstract

Highlights
-
Cattle dung can be effectively managed through vermicomposting
-
Biochar showed both positive and negative effects on earthworms’ performance
-
5% biochar addition promoted compost quality by stimulating earthworms’ activity
-
Higher doses of biochar were not suitable for vermicomposting
-
Available N and functional genes drove compost quality and N2O emissions
-
Biochar increased AOB but decreased nirS gene numbers in fresh cast of earthworms
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Rapid increasing demand for livestock and dairy products has stimulated livestock manure production globally, estimating about 6252 million tons per year of production worldwide (Cao et al. 2021). Improper disposal or management of manures may cause serious environmental pollution (Zubair et al. 2020). However, nutrients and organic matter in livestock manures make them a potential organic fertilizer and ameliorating agent for cultivated land (Schlegel et al. 2017).
Vermicomposting is an economical and effective technique to turn organic wastes (e.g. livestock manure) into organic fertilizers under earthworms’ activity (Garg et al. 2006). Earthworms increase air circulation to preserve an aerobic condition in the compost pile through continuous turning of substrate (Wu et al. 2015). By mixing and grinding the substrate, earthworms increase the surface area of the organic waste for microbial colonization and further decomposition (Lim et al. 2016). Moreover, earthworms’ guts stimulate specific groups of microorganisms such as bacteria, which turn complex molecules into readily available molecules (Sapkota et al. 2020). Thus, the biological activities of earthworms are crucial factors that influence the decomposition process of solid waste and the quality of vermicompost for plant growth.
The biological activities of earthworms in vermicomposting may also increase nitrous oxide (N2O) emissions, thus leading to the losses of nitrogen (N), reducing the agronomic value of the vermicompost and environmental benefits from vermicomposting (Frederickson and Howell 2003; Lv et al. 2020). Previous meta-analysis indicated that earthworm presence increased soil N2O emissions by 42% (Lubbers et al. 2013). The gut of the earthworm comprises a mobile anoxic microzone and a large concentration of available nutrient. Thus, earthworms induce N2O emissions because denitrifying bacteria are stimulated by the unique micro-conditions of earthworm gut during the ingestion and excretion process (Wu et al. 2021). In addition, enhanced organic matter mineralization by earthworms increases the substrates for N2O generation (Na et al. 2022).
Biochar is a fine-grained, degradation-resistant porous substance produced by slow pyrolysis of plant and animal biomass at low to medium temperatures under limited oxygen conditions (Shaaban et al. 2018a, b), and has been widely used in fields to mitigate soil N2O emissions (Zhang et al. 2021, 2022). The mechanisms of N2O reduction by biochar application include: (1) improvement of soil aeration, (2) suppression of denitrifiers in soil, and (3) adsorption of soil mineralized N (Wu et al. 2018; Gao et al. 2022; Wei et al. 2022). Biochar has also been proven to reduce earthworm-induced soil N2O emissions by changing the anoxic microzone of earthworms’ gut and functional gene abundance of earthworms’ casts during the short-term incubation experiment (Wu et al. 2021). Thus, biochar addition was considered as a potential method to reduce N2O emissions during the vermicomposting. However, recalcitrant C in biochar is generally not palatable to earthworms, and high recalcitrant C content in materials may inhibit earthworm growth and slow down the vermicomposting process (Sharon and Kurt 2011). The application rate of nearly 60% biochar has been found to decrease earthworms’ feeding activity in soil (Topoliantz and Ponge 2003). Therefore, an optimal biochar application amount is important for vermicomposting.
At present, however, little information is available on the literature for the comprehensive evaluation of earthworm performance, N2O emissions, and compost quality in response to various amounts of biochar addition. Thus, the objectives of this study were (1) to find the most optimal biochar application amount for vermicomposting by comparing the quality of composts and N2O emission, and (2) to investigate the main driving parameters that influence vermicomposting by the response of earthworms’ activity and the key functional genes related to nitrification and denitrification.
2 Materials and methods
2.1 Earthworm, cattle dung and biochar
Both earthworms (Eisenia fetida) and cattle dung were obtained from Hubei TianShengJia Biotechnology Co., Ltd. Adult earthworms with similar size and weight (about 0.37 ± 0.06 g individual−1) were hand collected and placed on a wet filter paper for 24 h to purge their guts. Cattle dung contained 29.5% total carbon (TC) and 1.23% total nitrogen (TN) on a dry weight basis with a C:N ratio of 24.0. The pH of cattle dung was 7.71.
Biochar used in the present study was produced from corn straw as it is cheap and easy to obtain from the field. Corn straw (Zea mays L.) was collected in the autumn, crushed, and put into a tube furnace to pyrolyze at 550 °C for 2 h under a continuous N2 environment. Then, the prepared biochar was sieved (2 mm), washed with distilled water, and dried at 80 °C. The pH and ash content of biochar were 8.54 and 7.58%, respectively. The TC, TN and C/N value of biochar 66.6%, 1.14% and 58.4, respectively. The specific surface area (BET) and total pore volume (TPV) of biochar were 114 m2 g−1 and 0.109 cm3 g−1, respectively.
2.2 Vermicomposting
The vermicomposting was designed as a single factor experiment, including six treatments: cattle dung without biochar addition (CD0), and cattle dung mixed with different doses of biochar [5% biochar (CD5), 10% biochar (CD10), 15% biochar (CD15), 20% biochar (CD20) and 25% biochar (CD25)]. Cattle dung was thoroughly mixed with different amounts of biochar, and then the distilled water was added to adjust the moisture content up to 65%. A total of 5 kg mixed material (dung, biochar and water) was added to plastic pots (40 cm × 25 cm × 25 cm), and then ~ 100 g (fresh weight) of earthworms were introduced for vermicomposting. There were six pots in each treatment: three for gas sampling, while three for compost sampling. Pots were covered with a thin black sheet with mesh to avoid earthworms escaping, and then incubated in a controlled greenhouse with temperatures ranging from 25 to 30 ℃. The moisture was maintained at 63–67% by spray-distilled water every 3 days. The duration of the experiment was 28 days, which was based on a preliminary experiment indicating more than 80% of material was ingested by earthworms within 28 days.
Closed chamber method was used for collecting gas samples to analyze N2O concentrations on a daily basis for the first fourteen days, and then every two days until the end of the experiment. The chamber was manually placed over the pots and sealed when gas sampling. Gas samples were collected from the headspace of each chamber at 0 and 60 min after the chamber closure (Shaaban et al. 2022) and analyzed immediately on a gas chromatograph (GC-7890A, Agilent Technologies, USA). The chamber was removed after sampling. Nitrous oxide fluxes were calculated assuming a linear increase of the N2O concentration over time after the chamber closed, and the cumulative gas emissions were calculated assuming linear changes between subsequent measurements.
Samples of compost were collected by a soil drill (2 cm diameter, 30 cm long) at three random locations in each pot on days 0, 7, 14 and 28. The TN and TC of samples were determined using a C/N elemental analyzer (Elementar Vario PYRO cube, Elementar, Germany). Available N (NH4+–N + NO3−–N) was analyzed using a continuous flow analyzer (SEAL Analytical GmbH, Norderstedt, Germany). The pH and electrical conductivity (EC) were measured in a 1:10 compost-deionized water mixture (Shaaban et al. 2013). After extraction with deionized water, the dissolved organic carbon (DOC) concentration of compost was measured using a total organic carbon analyzer (TOC 5000, Shimadzu, Kyoto, Japan). Germination assays (GI) for seeds of Lepidium sativum L. were conducted in a 1:1 extraction (shaken for 1 h at 25 ℃) of dry vermicompost and water according to Wu et al. (2019). The organic matter content of the final vermicompost (collected on day 28) was determined by dry combustion method. After H2SO4-H2O2 digest, the total-N, total-P, and total-K contents of final vermicompost were determined by Kjeldahl method, spectrophotometry, and flame photometry, respectively.
Compost samples collected on day 28 were subjected to the total DNA extraction by FastDNA® SPIN Kit (MP Biomedicals, Illkirch, France). Functional genes [Ammonia oxidizing archaea (AOA), ammonia oxidizing bacteria (AOB), nitrate reductase (nirS), and nitrous oxide reductase (nosZ)] that encode the key enzymes for nitrification and denitrification were determined by quantitative real-time PCR (qPCR) in Majorbio Co., Ltd. The pair of primers used for qPCR of AOA, AOB, nirS, and nosZ genes were Arch-amoAF/Arch-amoAR, amoA-1F/amoA-2R, cd3aF/R3cd, and nosZ-F/nosZ-R, respectively. The gene copy number was determined by the standard curve made with agarose gel-purified PCR products.
At the end of the experiment, the earthworm was hand sorted, and then the total earthworm biomass (fresh weight basis) and density were measured.
2.3 Earthworm behavior
Burrowing is a normal behavior of earthworms. The changes in earthworms’ burrowing behavior can be indicative of changes in the environment and the adverse effects of biochar on earthworms. Monitoring of earthworms’ burrowing behavior in cattle dung mixed with different doses of biochar was carried out using a two-dimensional terrarium (2D terrarium) according to Felten and Emmerling (2009). The experimental set-up was consistent with the vermicomposting experiment and included six treatments (CD0, CD5, CD10, CD15, CD20 and CD25). The terrarium was composed of two parallel glass plates with an external dimension of 40 × 50 cm. The distance between two glass plates was 6 mm. The mixed materials were prepared according to treatments, and then filled into the terrarium. Two individuals of earthworm were inoculated in each terrarium. Each treatment was replicated nine times. All terrariums were sealed with thin black sheets and stored in darkness at 25 °C for 120 h.
Pictures of both sides of the terrarium were captured using cameras at 12, 24, 36, 48, 60, 72, 84, 96, 108, 120 h after the commencement of the experiment. For each terrarium, data were summarized from both of the two glass plates. The pictures were imported into Adobe Photoshop 7 software package to analyze the burrowing activity of earthworms, which was shown as numbers of pixels.
At the end of the experiment, the terrarium was laid flat, and the upside of the glass plate was removed. Earthworms from each terrarium were collected and starved on wet filter papers for 12 h. Then the fresh casts left on filter paper were collected. Earthworms’ cast from 4 to 6 replicates with the same treatment was composited to one sample to meet the quantity required for total DNA extraction and determination of functional genes including AOA, AOB, nirK, and nosZ.
2.4 Statistical analysis
Statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). All data met the assumptions of normality. The effects of biochar on cumulative N2O emissions, earthworms’ performance, functional genes and temporal dynamics of chemical properties in vermicompost were analyzed using one-way analysis of variance and considered statistically different at P < 0.05. Principal component analysis (PCA) was performed with CANOCO 4.5 (Microcomputer Power, USA) to determine the relationships of chemical and biota parameters in final compost.
3 Results
3.1 Earthworms’ biomass and density
Adult Eisenia fetida individuals with similar weight (about 0.37 g individual−1) were used for inoculation. Thus, there was no obvious difference on earthworm density between treatments at the beginning of vermicomposting (Table 1). However, the change in earthworms’ biomass was significantly influenced by the application of biochar. Compared with CD0, 5% biochar addition significantly increased the total biomass of earthworms from 177.5 to 202.2 g pot−1. On the contrary, a high amount of biochar addition (CD25) exhibited adverse effects on total earthworm biomass accumulation. Interestingly, there was no significant difference in earthworm density between CD0 and CD5, which indicated that 5% biochar addition enhanced earthworm performance by growth rate instead of reproduction. Nevertheless, 25% biochar addition led to not only weight loss for individuals but also the death or escaping of earthworms during vermicomposting.
3.2 Dynamic of chemical properties during vermicomposting
Addition of biochar at doses 0%, 5%, 10% and 15% resulted in a rapid pH decrease during the vermicomposting, while the pH in CD20 and CD25 treatments kept steady (Fig. 1a). Generally, the more biochar mixed in cattle dung, the higher the pH obtained both in raw materials and final compost. The final C/N ratio of compost varied within the 17.1–29.2 range, while only CD0, CD5 and CD10 fell into 20.0 (Fig. 1b). Obvious increases in available N contents were found in the treatment of CD0, CD5, and CD10 during the vermicomposting. In contrast, a slight change in available N contents was detected in CD20 and CD25 (Fig. 1c). In the final compost, CD5 showed significantly higher available N contents than CD0, while CD20 and CD25 showed lower available N contents than other treatments. DOC content gradually decreased in all treatments during the whole vermicomposting process, and a non-significant difference was found between each treatment in the final compost (Fig. 1d). Compared with CD25 and CD20, the value of electrical conductivity (EC) in CD0, CD5, CD10, and CD15 treatments was stimulated at the initial period of vermicomposting, then decreased until the end of the study (Fig. 1e). The EC value of final compost varied within the 2.59–2.63 range for all treatments. Germination percentage (GI) gradually increased in all treatments with the vermicomposting process (Fig. 1f), while the rate of increase tended to be quicker in CD0, CD5, CD10 and CD15 treatments, resulting in a significantly higher GI value (varied from 77.2 to 88.4%) at last than the treatments of CD20 and CD25 (varied from 39.1 to 43.2%).
The nitrogen, phosphorus, potassium, and organic matter contents of the final compost were compared to Chinese national standards for agricultural organic fertilizer (NY525-2021) (Table 2). For all treatments, the total nutrient (N + P2O5 + K2O, dry basis) and organic matter content of the final compost met the requirements of national standard. However, the treatment of 5% biochar addition showed the highest total nutrient content.
3.3 N2O emissions and functional genes in the final compost
Compared with CD20 and CD25, a quick increase in N2O emissions was observed in CD0, CD5, CD10, and CD15 treatments, reaching maximum emissions on day 6 or 7, and then steadily declining with fluctuations of magnitudes until day 28 (Fig. 2a). The CD20 and CD25 treatments mitigated N2O emissions at the initial stage of vermicomposting, and resulted in significantly lower cumulative N2O emissions at last (8.32 and 7.72 mg N2O-N kg−1 dry for CD20 and CD25, respectively). The CD5 treatment showed the highest cumulative N2O emissions (15.09 mg N2O-N kg−1 dry vermicompost), but no significant difference was detected between the CD0, CD5, and CD10 treatments (Fig. 2b).
The population size of the whole microbial community and key functional genes related to nitrification and denitrification in the final compost are shown in Table 3. Compared with CD0, the gene copy number of AOB was increased, while nirS decreased by biochar addition. However, significant differences were only observed between CD0, CD5, and CD10 treatments.
3.4 Earthworm behavior and functional genes in fresh cast
In CD0, CD5, CD10 and CD15 treatments, earthworms showed continuously increased cumulative burrowing activity in the terrarium environment for 120 h, albeit with a reduced burrowing rate at the later period of the study (Fig. 3a). Compared with CD0, 5% of biochar addition significantly stimulated the burrowing activity of earthworms, while 20% and 25% of biochar addition showed inhibiting effects on earthworms’ burrowing behavior. As expected, burrowing activity of earthworms decreased with increasing materials depth (Fig. 3b). Especially for CD25, non-earthworms’ activity was found below the depth of 10 cm.
Burrowing behavior of earthworms in cattle dung mixed with different amount of biochar. a Cumulative burrowing activity of earthworms. b Vertical burrowing activity of earthworms. Different letters among treatments indicate significant (P < 0.05) differences on determined properties at the end of behavior test
The effect of biochar on earthworms’ cast copy number of AOB, AOA, nirS and nosZ genes varied in different treatments (Table 4). Non-significant differences were found for AOA gene copy number between the treatments with and without biochar addition. The gene copy number of nosZ was slightly decreased by biochar addition, while the significant difference was only observed between CD0 and CD5 treatments. Compared with the treatment without biochar, the gene copy number of AOB was significantly increased by 1.83 to 2.07 times, and the gene copy number of nirS was significantly decreased by 1.31 to 1.76 times with biochar addition. However, non-obvious difference in AOB and nirS gene copy numbers was found between the treatments with different amount of biochar addition.
The functional genes and earthworms’ performance in the final compost were used in the PCA analysis to identify the separation of biota parameters between each treatment (Fig. 4a). A noticeable isolated position was found between the treatments with low (0, 5, 10 and 15%) and high (20 and 25%) amounts of biochar addition. Treatments with 0–15% biochar addition were positively-correlated with maturity index and total nutrient content, while treatments with 20% and 25% biochar addition were negatively correlated.
Relationships of chemical and biota parameters in final compost (a) and the schematic diagram (b) illustrating the potential driving parameters that influence compost quality and N2O emissions. In b, the red or blue color indicates low (5%, 10% and 15%) or high (20% and 25%) amount of biochar addition. Circle with color indicates the positive (shown as “ + ”) or negative (shown as “−”) effects between two determined characters, and the round size of circle indicates the intensity interactions
4 Discussion
4.1 Effects of biochar on earthworms’ activity
Assuming that one earthworm consumes the feed up to half of its body weight per day (Maliå et al. 2017), the amount of the substrate (5.00 kg) was sufficient for a 28-day period of vermicomposting. In addition, all the pots were incubated in a controlled greenhouse with optimum temperatures and moisture. Thus, the decreased biomass and density of earthworms in CD25 should be attributed to the high dose of biochar addition. This adverse effect of high doses of biochar can be further confirmed by burrowing behavior of earthworms, which was significantly lower in CD20 and CD25 treatments, and mainly restricted between 0 and 10 cm. Similar results have been reported in soil by Wu et al. (2021). Firstly, biochar has been documented to be resistant to both microbial and chemical degradation, and obviously, this is not an ideal food for earthworms (Sharon and Kurt 2011). Secondly, a high amount of biochar addition may have adsorbed the readily available nutrients in vermicompost, for example, lower available N contents were observed in CD25 treatment at the beginning of vermicomposting. Thirdly, the specific surface area and total pore volume of biochar in the present study were 114 m2 g−1 and 0.109 cm3 g−1, respectively. Microbes colonized on biochar may have been protected by biochar pores (Sharon and Kurt 2011). Therefore, less food might be available to the earthworms in the treatment with a high amount of biochar addition, which then decreased the activity of earthworms.
The results showed that 5% of biochar application increased substantial growth of earthworms. Similar positive effect has been reported by Zwieten et al. (2010) and Whalen et al. (2021), which may contribute to several factors including (1) immobilization of contaminants in materials (Tammeorga et al. 2014; Zhang et al. 2019), (2) increased material total porosity and water holding capacity (Gong et al. 2018) and (3) nutrient retention and enhancement of microbial activity (Maliå et al. 2017). Sharon and Kurt (2011) suggested that the response of earthworms to biochar may be the combined effect of characteristics of soil and biochar. While the present study further implied that the dose of biochar should also be considered to elucidate the effects of biochar on earthworm population.
4.2 Effects of biochar on chemical properties of vermicomposting
Certainly, biochar's addition led to increased pH and C/N ratio of materials (Fig. 1a and b). Nevertheless, the initial pH values and C/N ratios of all treatments were within the optimal range for the growth of E. fetida (Gong et al. 2018). The pH tended to decrease during the vermicomposting in all treatments, which might be attributed to the consumption of readily available nitrogen compounds, as well as the subsequent production of phenolic compounds (Hanc and Chadimova 2014). While the continuous decreased C/N ratio may be due to the rate of organic N mineralization being lower than that of organic C (Wu et al. 2019). It should be noted that both organic matter mineralization and readily available compound consumption were dominated by earthworms’ activity. The inhibited earthworms’ activity in CD25 may help to explain the little change in pH and C/N ratio during the experiment.
The increased content of available N indicates the decomposition of organic matter. Earthworms increase N availability by enhancing microbial mineralization, and also by releasing their metabolic products (feces, urine, and mucus) (Buck et al. 1999; Blouin et al. 2013). Thus, it is not surprising that CD5 showed a quicker increase and a significant higher available N content than CD0 (Fig. 1c), as CD5 had a more active earthworm population. Since C is requisite during various metabolic activities for both earthworms and microorganisms (Devi and Khwairakpam 2020), DOC values gradually decreased in all treatments and a non-obvious difference was found between each treatment (Fig. 1d).
Electrical conductivity (EC) is a useful index to reflect salt concentration in the compost (He et al. 2016). For the treatments of CD0, CD5, CD10 and CD15, the increase in EC in the initial period of vermicomposting could be explained by the loss of organic matter by earthworm mineralization (Nayak et al. 2013). Then the volatilization of ammonia and precipitation of mineral salts may be attributed to the decrease of EC in the later period (Huang et al. 2004).
Germination tests are commonly used to assess compost phyto-toxicity (Das et al. 2011). The GI values of CD0, CD5, CD10 and CD15 gradually increased, suggesting that the inhibitors of seed germination were slowly eliminated. However, no obvious changes were found in EC and GI during vermicomposting of CD 20 and CD25, which may contribute to the limited effect of earthworms on organic matter mineralization.
In the determined indexes, C/N ratio, EC and GI are typically used in predicting the maturity of compost. The C/N ratio of compost should falls between 15 and 20 at the end of maturity (Devi and Khwairakpam 2020). Since salinity showed an adverse effect on seed germination and GI is a measure of phytotoxicity, He et al. (2016) and Das et al. (2011) recommended 4 ms cm−1 of EC and 80% of GI as the threshold for the field application of compost. In this view, only the treatments of CD0, CD5 and CD10 can meet the desired quality of compost, and 5% of biochar addition resulted in the highest total nutrient of products.
4.3 Effects of biochar on N2O emissions from vermicomposting
Existing research indicates that biochar addition is an effective way to reduce N2O emissions during thermophilic composting for example, Wang et al. (2013) found that total N2O emissions from compost were lower by 25.9% with biochar addition. Those phenomenons may result from: (1) capture of nitrates by the biochar; (2) increasing pH; (3) improving aeration, and (4) changing the denitrifier community composition (Awasthi et al. 2016; Wang et al. 2021; Ravindran et al. 2022). However, it should be noted that earthworms may increase N2O emissions by a similar pathway: (1) stimulating the activity of denitrifiers by inoculating gut-derived microorganisms through casting and (2) increasing the substrates for N2O generation by stimulated organic material mineralization (Wu et al. 2015, 2021; Sun et al. 2020). In the treatment of 5% biochar addition, it seems that stimulated earthworms’ activity magnified the promoting effect of earthworms on N2O generation, and overlapped the mitigation effect of biochar on N2O emission. On the contrary, biochar influence N2O emission more in the treatments of CD20 and CD25. This view can be evidenced by (1) dynamic available N content during the vermicompost, as well as (2) the difference in functional gene numbers between final compost and fresh cast.
-
(1)
Available N content: Available N is an important substance for N2O generation (Wu et al. 2015; Shaaban et al. 2019; Shaaban et al. 2018a, b). At day 0 of vermicomposting, available N contents in materials were gradually decreased with the increasing amount of biochar addition, indicating the nitrate capture effects of biochar. However, the available N content in CD5 increased quickly during the vermicomposting and exceeded CD0 on day 14, indicating that earthworms stimulated more N release than biochar capture. On the contrary, 20% and 25% of biochar addition limited the activity of earthworms, and showed the lowest available N content during the whole vermicomposting process.
-
(2)
Functional genes: Earthworm gut is ideally suited for N2O-producing bacteria, as it provides an anoxic microzone and a large amount of organic substrates (Horn et al. 2003; Wang et al. 2016). Obviously, biochar addition significantly changed the response of fresh cast bacteria, as shown in Table 3, a significant increased AOB and significantly decreased nirS gene copy numbers in all biochar-added treatments. This is because the specific surface and pore of biochar may serve as air (O2) reservoirs when earthworms ingest biochar particles, which may slightly increase O2 concentrations in earthworm guts (Wu et al. 2021). On the contrary, the significant difference in AOB and nirS gene copy numbers in final compost was only detected between CD0 and CD5, instead of between treatments with and without biochar added. These results implied a negligible effect of gut-stimulated bacteria on vermicomposting microbe community with high amounts of biochar addition, which may explain the decreased earthworms’ burrowing activity, since earthworms’ activity is a crucial factor influencing the spreading of microorganisms.
Figure 4b illustrates the potential driving parameters that influence compost quality and N2O emissions. Low amount of biochar addition stimulated earthworms’ activity, improved the mature process of cattle dung, and thus resulted in a higher compost quality. Although available N content increased, the final N2O emission was mitigated by the change in compost microbial community, which was driven by earthworm gut associated microbes. No matter how much biochar was added, biochar showed the consistent effects on cast functional genes. However, the change in cast microbial community showed a limited effect on vermicomposting when a high amount of biochar mixed, which may be attributed to the inhabited earthworms’ activity. Instead, the adsorption and dilution effect of high amount of biochar addition has sharply decreased the available N contents, which then significantly decreased N2O emissions.
5 Conclusion
Although slightly increased N2O emissions during the vermicomposting, 5% biochar addition clearly promoted the quality of final compost. On the contrary, 20% and 25% biochar additions lowered N2O emissions, but greatly decreased the quality of compost. The present study highlighted that low amount of biochar application is a potential method to improve the efficiency of vermicomposting during the practices. However, the parameters of biochar varied when different feedstocks were used for biochar production or biochar pyrolyzed under different conditions. Therefore, it is necessary to further investigate the role of different types of biochar on vermicomposting.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Awasthi MK, Wang Q, Huang H et al (2016) Effect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J Clean Prod 135:829–835. https://doi.org/10.1016/j.jclepro.2016.07.008
Blouin M, Hodson ME, Delgado EA et al (2013) A review of earthworm impact on soil function and ecosystem services. Eur J Soil Sci 64:161–182. https://doi.org/10.1111/ejss.12025
Buck C, Langmaack M, Schrader S (1999) Nutrient content of earthworm casts influenced by different mulch types. Eur J Soil Biol 35:23–30. https://doi.org/10.1016/S1164-5563(99)00102-8
Cao Y, Tian Y, Wu Q et al (2021) Vermicomposting of livestock manure as affected by carbon-rich additives (straw, biochar and nanocarbon): a comprehensive evaluation of earthworm performance, microbial activities, metabolic functions and vermicompost quality. Bioresour Technol 320:124404. https://doi.org/10.1016/j.biortech.2020.124404
Das M, Uppal HS, Singh R et al (2011) Co-composting of physic nut (Jatropha curcas) deoiled cake with rice straw and different animal dung. Bioresour Technol 102:6541–6546. https://doi.org/10.1016/j.biortech.2011.03.058
Devi C, Khwairakpam M (2020) Bioconversion of Lantana camara by vermicomposting with two different earthworm species in monoculture. Bioresour Technol 296:122308. https://doi.org/10.1016/j.biortech.2019.122308
Felten D, Emmerling C (2009) Earthworm burrowing behaviour in 2D terraria with single- and multi-species assemblages. Biol Fert Soils 45:789–797. https://doi.org/10.1007/s00374-009-0393-8
Frederickson J, Howell G (2003) Large-scale vermicomposting: emission of nitrous oxide and effects of temperature on earthworm populations. Pedobiologia 47:724–730. https://doi.org/10.1078/0031-4056-00250
Gao Y, Fang Z, Zwieten VL et al (2022) A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. Biochar 4:36. https://doi.org/10.1007/s42773-022-00160-3
Garg P, Gupta A, Satya S (2006) Vermicomposting of different types of waste using Eisenia foetida: a comparative study. Bioresour Technol 97:391–395. https://doi.org/10.1016/j.biortech.2005.03.009
Gong X, Cai L, Li S et al (2018) Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost. Ecotox Environ Safe 156:197–204. https://doi.org/10.1016/j.ecoenv.2018.03.023
Hanc A, Chadimova Z (2014) Nutrient recovery from apple pomace waste by vermicomposting technology. Bioresour Technol 168:240–244. https://doi.org/10.1016/j.biortech.2014.02.031
He X, Zhang Y, Shen M et al (2016) Effect of vermicomposting on concentration and speciation of heavy metals in sewage sludge with additive materials. Bioresour Technol 218:867–873. https://doi.org/10.1016/j.biortech.2016.07.045
Horn MA, Schramm A, Drake HL (2003) The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl Environ Microb 69:1662–1669. https://doi.org/10.1128/AEM.69.3.1662-1669.2003
Huang GF, Wong J, Wu QT et al (2004) Effect of C/N on composting of pig manure with sawdust. Waste Manage 24:805–813. https://doi.org/10.1016/j.wasman.2004.03.011
Lim SL, Lee LH, Wu TY (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J Clean Prod 111:262–278. https://doi.org/10.1016/j.jclepro.2015.08.083
Lubbers IM, Van Groenigen K, Fonte S et al (2013) Greenhouse-gas emissions from soils increased by earthworms. Nat Clim Change 3:187–194. https://doi.org/10.1038/nclimate1692
Lv B, Cui Y, Wei H et al (2020) Elucidating the role of earthworms in N2O emission and production pathway during vermicomposting of sewage sludge and rice straw. J Hazard Mater 400:123215. https://doi.org/10.1016/j.jhazmat.2020.123215
Maliå S, Golaå S, Caceres R et al (2017) Biochar amendment for integrated composting and vermicomposting of sewage sludge - The effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour Technol 225:206–214
Na L, Hu C, Jiang Y et al (2022) Earthworms promote the transfer of 15N-urea to lettuce while limit appreciably increase 15N losing to environment. Environ Res 212:113423. https://doi.org/10.1016/j.envres.2022.113423
Nayak AK, Varma VS, Kalamdhad AS (2013) Effect of various C/N ratios during vermicomposting of sewage sludge using Eesenea fetida. J Environ Sci Technol 6:63–78. https://doi.org/10.3923/jest.2013.63.78
Ravindran B, Awasthi MK, Karmegam N et al (2022) Co-composting of food waste and swine manure augmenting biochar and salts: nutrient dynamics, gaseous emissions and microbial activity. Bioresour Technol 344:126300. https://doi.org/10.1016/j.biortech.2021.126300
Sapkota R, Santos S, Farias P et al (2020) Insights into the earthworm gut multi-kingdom microbial communities. Sci Total Environ 727:138301. https://doi.org/10.1016/j.scitotenv.2020.138301
Schlegel AJ, Assefa Y, Bond HD et al (2017) Changes in soil nutrients after 10 years of cattle manure and swine effluent application. Soil till Res 172:48–58. https://doi.org/10.1016/j.still.2017.05.004
Shaaban M, Abid M, Abou-Shanab R (2013) Amelioration of salt affected soils in rice paddy system by application of organic and inorganic amendments. Plant Soil Environ 59(5):227–233
Shaaban M, Wu Y, Peng Q, Wu L, Van Zwieten L, Khalid M, Younas A, Lin S, Zhao J, Bashir S (2018a) The interactive effects of dolomite application and straw incorporation on soil N2O emissions. Eur J Soil Sci 69(3):502–511
Shaaban M, Van Zwieten L, Bashir S, Younas A, Núñez-Delgado A, Chhajro MA, Kubar KA, Ali U, Rana MS, Mehmood MA (2018b) A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J Environ Manag 228:429–440
Shaaban M, Hu R, Wu Y, Younas A, Xu X, Sun Z, Jiang Y, Lin S (2019) Mitigation of N2O emissions from urine treated acidic soils by liming. Environ Pollut 255:113237
Shaaban M et al (2022) Effects of water regimes on soil N2O, CH4 and CO2 emissions following addition of dicyandiamide and N fertilizer. Environ Res 212:113544. https://doi.org/10.1016/j.envres.2022.113544
Sharon LW, Kurt AS (2011) Impact of biochar on earthworm populations: a review. Appl Environ Soil Sci 2011:541592. https://doi.org/10.1155/2011/541592
Sun M, Chao H, Zheng X et al (2020) Ecological role of earthworm intestinal bacteria in terrestrial environments: a review. Sci Total Environ 740:140008. https://doi.org/10.1016/j.scitotenv.2020.140008
Tammeorga P, Parviainen T, Nuutinen V et al (2014) Effects of biochar on earthworms in arable soil: avoidance test and field trial in boreal loamy sand. Agric Ecosyst Environ 191:150–157. https://doi.org/10.1016/j.agee.2014.02.023
Topoliantz S, Ponge JF (2003) Burrowing activity of the geophagous earthworm Pontoscolex corethrurus (Oligochaeta : Glossoscolecidae) in the presence of charcoal. Appl Soil Ecol 23:267–271. https://doi.org/10.1016/S0929-1393(03)00063-5
Wang C, Lu H, Dong D et al (2013) Insight into the effects of biochar on manure composting: evidence supporting the relationship between N2O emission and denitrifying community. Environ Sci Technol 47:7341–7349. https://doi.org/10.1021/es305293h
Wang Z, Peng S, Sun Y et al (2016) How gut-stimulated denitrifiers influence soil N2O emission without earthworm activity. Eur J Soil Biol 76:70–73. https://doi.org/10.1016/j.ejsobi.2016.08.001
Wang SP, Wang L, Sun ZY et al (2021) Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour Technol 337:125492. https://doi.org/10.1016/j.biortech.2021.125492
Wei Z, Senbayram M, Zhao X et al (2022) Biochar amendment alters the partitioning of nitrate reduction by significantly enhancing DNRA in a paddy field. Biochar 4:44. https://doi.org/10.1007/s42773-022-00166-x
Whalen JK, Benslim H, Elmi AA et al (2021) Earthworm populations are stable in temperate agricultural soils receiving wood-based biochar. Pedosphere 31:398–404. https://doi.org/10.1016/S1002-0160(20)60080-7
Wu Y, Muhammad S, Hao R et al (2015) Effect of the earthworm gut-stimulated denitrifiers on soil nitrous oxide emissions. Eur J Soil Biol 70:104–110. https://doi.org/10.1016/j.ejsobi.2015.08.001
Wu D, Senbayram M, Zang H et al (2018) Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Appl Soil Ecol 129:121–127. https://doi.org/10.1016/j.apsoil.2018.05.009
Wu Y, Chen Y, Shaaban M et al (2019) Evaluation of microbial inoculants pretreatment in straw and manure co-composting process enhancement. J Clean Prod 239:118078. https://doi.org/10.1016/j.jclepro.2019.118078
Wu Y, Liu J, Shaaban M et al (2021) Dynamics of soil N2O emissions and functional gene abundance in response to biochar application in the presence of earthworms. Environ Pollut 268:115670. https://doi.org/10.1016/j.envpol.2020.115670
Zhang Q, Saleem M, Wang C (2019) Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci Total Environ 671:52–58. https://doi.org/10.1016/j.scitotenv.2019.03.364
Zhang Q, Zhang X, Duan P et al (2021) The effect of long-term biochar amendment on N2O emissions: experiments with N15–O18 isotopes combined with specific inhibition approaches. Sci Total Environ 769:144533. https://doi.org/10.1016/j.scitotenv.2020.144533
Zhang Y, He M, Wang L et al (2022) Biochar as construction materials for achieving carbon neutrality. Biochar 4:59. https://doi.org/10.1007/s42773-022-00182-x
Zubair M, Wang S, Zhang P et al (2020) Biological nutrient removal and recovery from solid and liquid livestock manure: recent advance and perspective. Bioresour Technol 301:122823. https://doi.org/10.1016/j.biortech.2020.122823
Zwieten LV, Kimber S, Morris S et al (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246. https://doi.org/10.1007/s11104-009-0050-x
Acknowledgements
Not applicable.
Funding
This work was supported by Major Science and Technology Program of Hainan Province (ZDKJ2021009), National Natural Science Foundation of China (32171638), and Yunan Science and Technology Talents and Platform Program (202205AF150004).
Author information
Authors and Affiliations
Contributions
The first draft was written by YW. Supervision and project administration were addressed by MS and RH. Experiment was conducted by YW, YZ and XX. Review was performed by QL, YC, MS and RH. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Li, Q., Zheng, Y. et al. Optimizing biochar addition for vermicomposting: a comprehensive evaluation of earthworms’ activity, N2O emissions and compost quality. Biochar 5, 4 (2023). https://doi.org/10.1007/s42773-022-00203-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00203-9